Distribution Pattern of Coral Reef Fishes in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
2.3.1. Coral Fish Diversity Index (CFDI)
2.3.2. Cluster Analysis
3. Results
3.1. Species Composition
3.2. Community Characteristics of Coral Reef Species
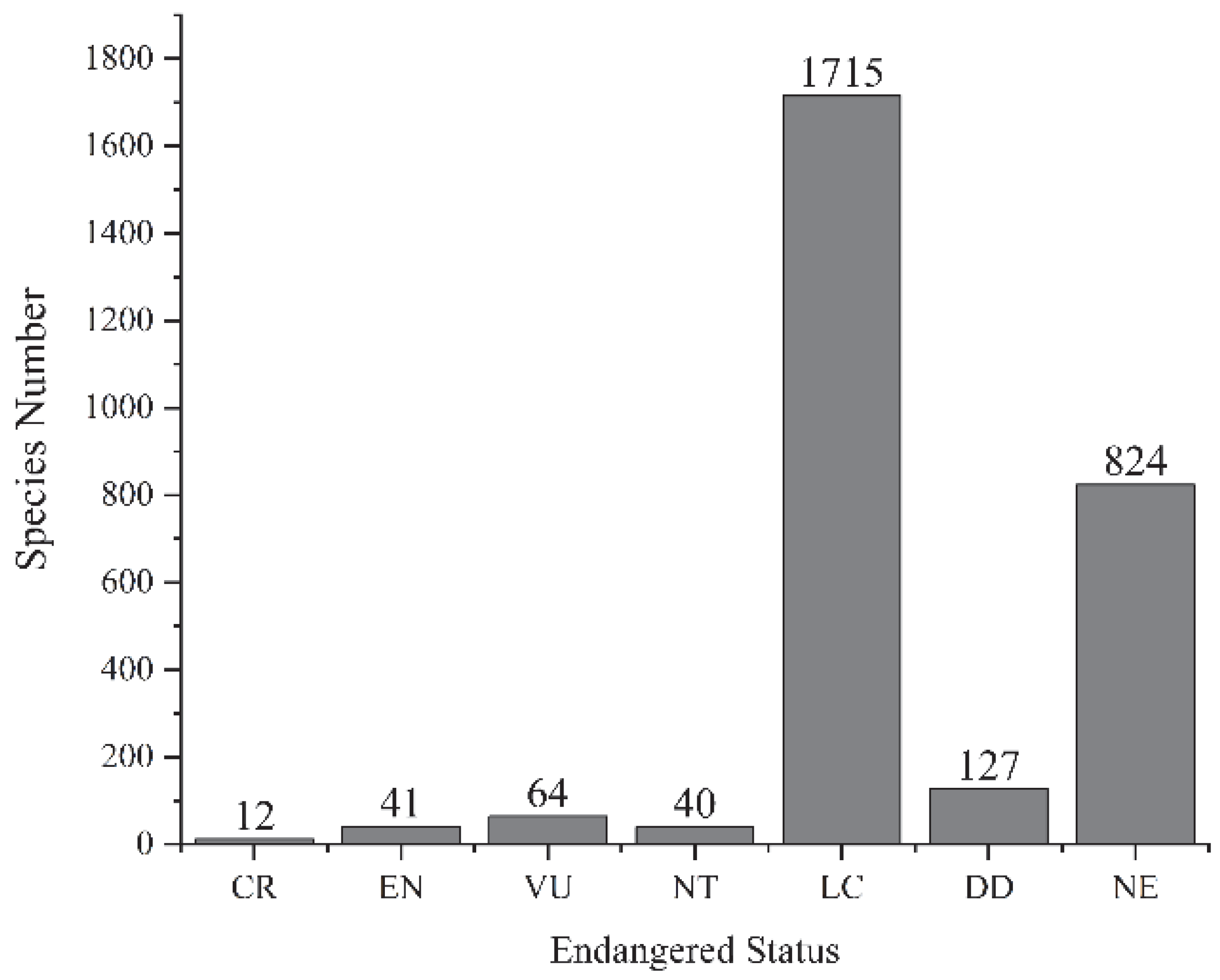
3.2.1. Endangered Status
3.2.2. Types of Feeding Habit
3.2.3. Type of Habitat
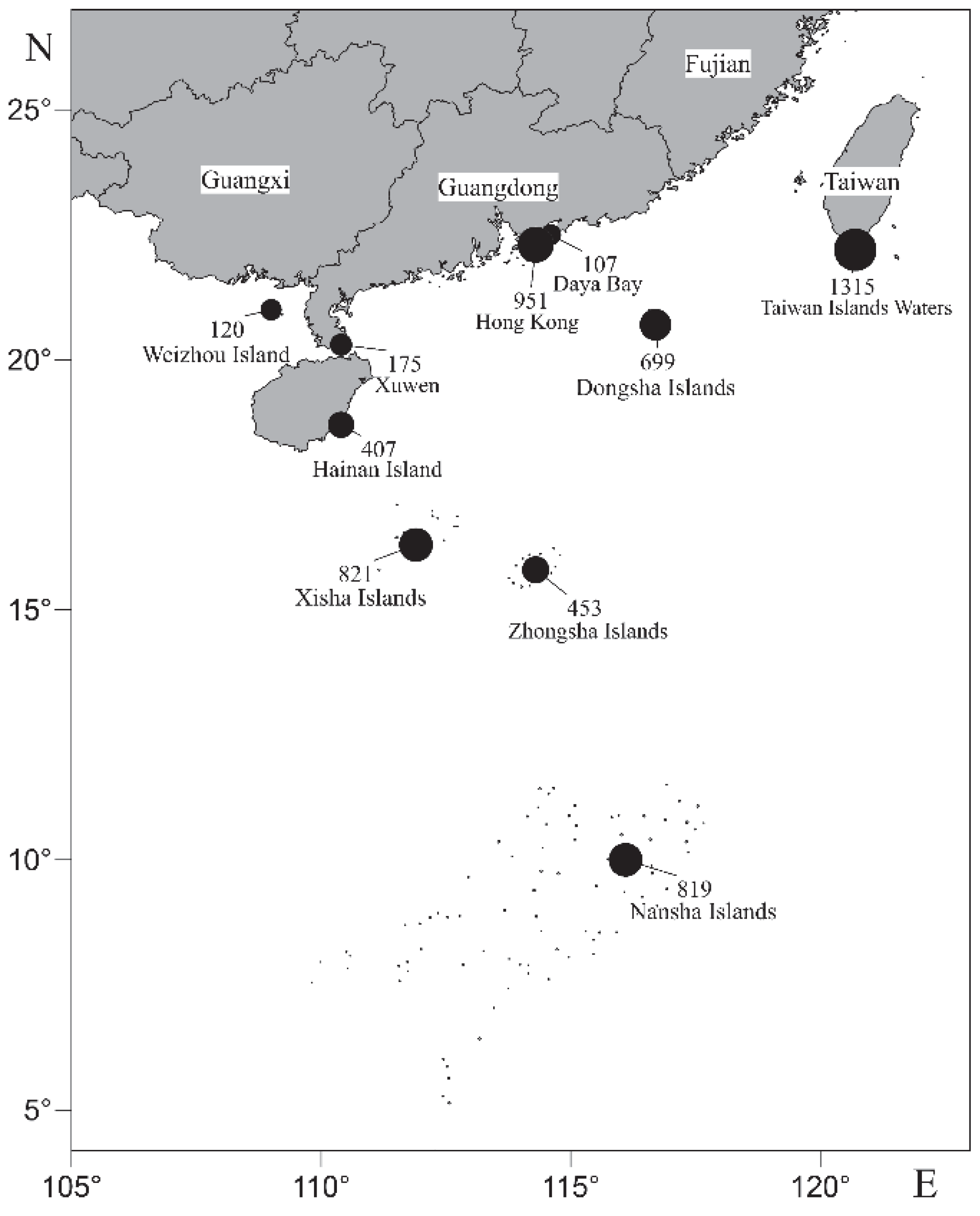
3.3. Distribution Patterns of Coral Reef Fish Species
3.4. Correlation between Coral and Coral Reef Fishes
4. Discussion
4.1. Coral Reef Fish Species Composition
4.2. Distribution Patterns of Coral Reef Fish Species
4.3. Relationship between the Species of Coral and Coral Reef Fishes
4.4. Endangered Status of Coral Reef Fish in China
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Jin, H.; Cai, Z.H. A review of the role and function of microbes in coral reef ecosystem. Chin. J. Appl. Ecol. 2014, 25, 919–930. [Google Scholar] [CrossRef]
- Zhang, M.R. Confirmation of global biomass distribution of coral reef fish. Sci. Technol. Dly. 2016, 2. [Google Scholar] [CrossRef]
- Samoilys, M.; Alvarez-Filip, L.; Myers, R.; Chabanet, P. Diversity of Coral Reef Fishes in the Western Indian Ocean: Implications for Conservation. Diversity 2022, 14, 102. [Google Scholar] [CrossRef]
- Ceccarelli, D.M.; Lestari, A.P.; Rudyanto; White, A.T. Emerging marine protected areas of eastern Indonesia: Coral reef trends and priorities for management. Mar. Policy 2022, 141, 105091. [Google Scholar] [CrossRef]
- Pan, Y.F.; Li, H.X.; Lin, L.; Liu, S.; He, W.H.; Xu, X.R. Progress on microplastics pollution in coral reef ecosystems and its ecological effects. Environ. Ecol. 2019, 01, 15–25. [Google Scholar]
- Cao, W.; Wong, M.H. Current status of coastal zone issues and management in China: A review. Environ. Int. 2007, 33, 985–992. [Google Scholar] [CrossRef]
- Hughes, T.P.; Huang, H.; Young, M.A.L. The Wicked Problem of China’s Disappearing Coral Reefs. Conserv. Biol. 2013, 27, 261–269. [Google Scholar] [CrossRef]
- Burke, L.; Selig, L.; Spalding, M. Reefs at Risk in Southeast Asia. World Resour. Inst. 2001, 83, 2010. [Google Scholar]
- Muis; Kurnia, R.; Sulistiono; Taryono. Diversity of coral reef fish in the coastal water of Spelman Straits, Southeast Sulawesi. IOP Conf. Ser. Earth Environ. Sci. 2020, 420, 012021. [Google Scholar] [CrossRef]
- Arai, T. Diversity and conservation of coral reef fishes in the Malaysian South China Sea. Rev. Fish Biol. Fish. 2015, 25, 85–101. [Google Scholar] [CrossRef]
- Eddy, T.D.; Lam, V.W.Y.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Cheung, W.W.L. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]
- Razak, T.B.; Boström-Einarsson, L.; Alisa, C.A.G.; Vida, R.T.; Lamont, T.A.C. Coral reef restoration in Indonesia: A review of policies and projects. Mar. Policy 2022, 137, 104940. [Google Scholar] [CrossRef]
- Bostrm-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Mcleod, I.M. Coral restoration—A systematic review of current methods, successes, failures and future directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef] [PubMed]
- Seraphim, M.J.; Sloman, K.A.; Alexander, M.E.; Janetski, N.; Jompa, J.; Ambo-Rappe, R.; Snellgrove, D.; Mars, F.; Harborne, A.R. Interactions between coral restoration and fish assemblages: Implications for reef management. J. Fish Biol. 2020, 97, 633–655. [Google Scholar] [CrossRef]
- Hixon, M.A. 60 Years of Coral Reef Fish Ecology: Past, Present, Future. Bull. Mar. Sci. 2011, 87, 727–765. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Renema, W.; Rosen, B.R. Biodiversity hotspots, evolution and coral reef biogeography: A review. Biot. Evol. Environ. Chang. Southeast Asia 2012, 82. [Google Scholar] [CrossRef]
- Coker, D.J.; Wilson, S.K.; Pratchett, M.S. Importance of live coral habitat for reef fishes. Rev. Fish Biol. Fish. 2014, 24, 89–126. [Google Scholar] [CrossRef]
- Robinson, J.P.W.; Wilson, S.K.; Robinson, J.; Gerry, C.; Lucas, J.; Assan, C.; Govinden, R.; Jennings, S.; Graham, N.A.J. Productive instability of coral reef fisheries after climate-driven regime shifts. Nat. Ecol. Evol. 2019, 3, 183–190. [Google Scholar] [CrossRef]
- Huang, H.L.; Feng, C.; Li, L.; Rao, X.; Chen, S.; Yang, J.L. The development status and prospect of contemporary marine fisheries. J. Fish. Sci. China 2022, 29, 938–949. [Google Scholar] [CrossRef]
- Ault, J.S.; Smith, S.G.; Johnson, M.W.; Grove, L.; Bohnsack, J.A.; Dinardo, G.T.; Mclaughlin, C.; Ehrhardt, N.M.; Mcdonough, V.; Seki, M.P. Length-based risk analysis of management options for the southern Florida USA multispecies coral reef fish fishery. Fish. Res. 2022, 249, 106210. [Google Scholar] [CrossRef]
- Allgeier, J.E.; Valdivia, A.; Cox, C.; Layman, C.A. Fishing down nutrients on coral reefs. Nat. Commun. 2016, 7, 12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Laboute, P. Coral Reef Fish Diversity. BioOne Complet. 2022. [Google Scholar] [CrossRef]
- Dwita, N.; Idris; Widjoyo, N.S. Diversity of reef fish on Lembeh Island as an indicator of the coral reef health condition. IOP Conf. Ser. Earth Environ. Sci. 2022, 967, 012006. [Google Scholar] [CrossRef]
- Leahy, S.M. Habitat Determinants of Chaetodon Butterflyfish and Fishery-Targeted Coral Reef Fish Assemblages in the Central Philippines. Ph.D. Thesis, James Cook University, Douglas, QLD, Australia, 2016. [Google Scholar]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Fisheries productivity under progressive coral reef degradation. J. Appl. Ecol. 2018, 55, 1041–1049. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Kochzius, M. Changes in trophic community structure of shore fishes at an industrial site in the Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. 2002, 239, 287–299. [Google Scholar] [CrossRef]
- Chen, Q.C. The protection of biodiversity in the South China Sea. Biodivers. Sci. 2011, 19, 834–836. [Google Scholar]
- Strona, G.; Lafferty, K.D.; Fattorini, S.; Beck, P.S.A.; Guilhaumon, F.; Arrigoni, R.; Montano, S.; Seveso, D.; Galli, P.; Planes, S.; et al. Global tropical reef fish richness could decline by around half if corals are lost. Proc. R. Soc. B-Biol. Sci. 2021, 288, 20210274. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, Z.Z.; Zhang, J. Fish Composition and Diversity of Four Coral Reefs in the South China Sea Based on Hand-Line Catch. J. Mar. Sci. Eng. 2022, 10, 38. [Google Scholar] [CrossRef]
- Richards, B.L.; Williams, L.D.; Vetter, O.J.; Williams, G.J. Environmental Factors Affecting Large-Bodied Coral Reef Fish Assemblages in the Mariana Archipelago. PLoS ONE 2012, 7, e31374. [Google Scholar] [CrossRef]
- Carreónpalau, L. Organic Carbon Sources and their Transfer in a Gulf of Mexico Coral Reef Ecosystem. Ph.D. Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2015. [Google Scholar]
- Akita, Y.; Kurihara, T.; Uehara, M.; Shiwa, T.; Iwai, K. Impacts of overfishing and sedimentation on the feeding behavior and ecological function of herbivorous fishes in coral reefs. Mar. Ecol. Prog. Ser. 2022, 686, 141–157. [Google Scholar]
- Zhang, J.; Zhang, K.; Chen, Z.Z.; Jiang, Y.N.; Cai, Y.C.; Gong, Y.Y.; Yu, W.M. Length-Weight Relationship Parameters of Tropical Coral Reef Fishes in the South China Sea. Pak. J. Zool. 2020, 52, 821–824. [Google Scholar] [CrossRef]
- Yu, K.F. Introduction to the Science of Coral Reefs; Science Press: Beijing, China, 2018. [Google Scholar]
- Lian, C.; Luo, M.Y. Analysis of endangered reasons and protection measures of freshwater endangered fish in China. China Fish. 2010, 3, 28–30. [Google Scholar] [CrossRef]
- Allen, G.R. A rapid biodiversity assessment of the coral reefs of Milne Bay Province, Papua New Guinea. RAP Bull. Biol. Assess. 1988. [Google Scholar]
- Huang, H. Status of Coral Reefs in China (2010–2019); Science Press: Beijing, China, 2021. [Google Scholar]
- McManus, J.W. Offshore Coral Reef Damage, Overfishing, and Paths to Peace in the South China Sea. Int. J. Mar. Coast. Law 2017, 32, 199–237. [Google Scholar] [CrossRef]
- Allen, G.R.; Adrim, M. Coral reef fishes of Indonesia. Zool. Stud. 2003, 42, 1–72. [Google Scholar]
- Nguyen, L.V.; Mai, D.X. Reef fish fauna in the coastal waters of Vietnam. Mar. Biodivers. 2020, 50, 100. [Google Scholar] [CrossRef]
- Larson, H.K.; Williams, R.S.; Hammer, M.P. An annotated checklist of the fishes of the Northern Territory, Australia. Zootaxa 2013, 3696, 1–293. [Google Scholar] [CrossRef] [Green Version]
- Rajan, P.T.; Sreeraj, C.R.; Immanuel, T. Fishes of Andaman Andaman and Nicobar Islands: A Checklist. J. Sci. Assoc. 2021, 26, 95–130. [Google Scholar]
- Kulbicki, M.; Williams, J.T. Checklist of the shorefishes of Ouvea Atoll New Caledonia. Atoll Res. Bull. 1997, 443. [Google Scholar] [CrossRef]
- Galzin, R.; Lecchini, D.; Williams, J.T.; Planes, S.; Menou, J.L. Diversity of coral reef fish at Rapa Island (French Polynesia). Cybium 2006, 30, 221–234. [Google Scholar]
- Drew, J.A.; Buxman, C.L.; Holmes, D.D.; Mandecki, J.L.; Mungkaje, A.J.; Richardson, A.C.; Westneat, M.W. Biodiversity inventories and conservation of the marine fishes of Bootless Bay, Papua New Guinea. BMC Ecol. 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Z.H. Spatial Distribution of Coral Community and Benthic Algae and Their Ecological Impacts across the South China Sea. Ph.D. Thesis, Guangxi University, Nanning, China, 2021. [Google Scholar]
- Allen, G.R. Indo-Pacific coral-reef fishes as indicators of conservation hotspots. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; Volume 2, pp. 23–27. [Google Scholar]
- Du, J.; Loh, K.-H.; Hu, W.; Zheng, X.; Affendi, Y.A.; Ooi, J.L.S.; Ma, Z.; Rizman-Idid, M.; Chan, A.A. An updated checklist of the marine fish fauna of Redang Islands, Malaysia. Biodivers. Data J. 2019, 7, e47537. [Google Scholar] [CrossRef] [Green Version]
- Wickel, J.; Jamon, A.; Pinault, M.; Durville, P.; Chabanet, P. Species composition and structure of marine fish communities in Mayotte Island (south-western Indian Ocean). Cybium Int. J. Ichthyol. 2014, 38, 179–203. [Google Scholar]
- Jayaprabha, N.; Purusothaman, S.; Srinivasan, M. Biodiversity of coral reef associated fishes along southeast coast of India. Reg. Stud. Mar. Sci. 2018, 18, 97–105. [Google Scholar] [CrossRef]
- Yuan, M.; Tang, Y.; Xu, S.N.; Chen, Z.Z.; Yang, Y.T.; Jiang, Y.E. Community structure of fishery resources from the Nansha waters of Pearl River Estuary in autumn. South China Fish. Sci. 2017, 13, 18–25. [Google Scholar] [CrossRef]
- Low, J.K.Y.; Chou, L.M. Distribution of coral reef fish in Singapore. In Proceedings of the Third ASEAN Science and Technology Week Conference Proceedings, Singapore, 21–23 September 1992; Volume 6, pp. 39–144. [Google Scholar]
- Keith, P. Biology and ecology of amphidromous Gobiidae of the Indo-Pacific and the Caribbean regions. J. Fish Biol. 2003, 63, 831–847. [Google Scholar] [CrossRef]
- Yu, Y.N. Research on Species Identification and Distribution of Gobiidae Early Resource in the Yangtze Estuary. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Tornabene, L.; Valdez, S.; Erdmann, M.; Pezold, F. Support for a ’Center of Origin’ in the Coral Triangle: Cryptic diversity, recent speciation, and local endemism in a diverse lineage of reef fishes (Gobiidae: Eviota). Mol. Phylogenet. Evol. 2015, 82, 200–210. [Google Scholar] [CrossRef]
- Andradi-Brown, D.A.; Beer, A.J.E.; Colin, L.; Hastuti; Head, C.E.I.; Hidayat, N.I.; Lindfield, S.J.; Mitchell, C.R.; Pada, D.N.; Piesinger, N.M.; et al. Highly diverse mesophotic reef fish communities in Raja Ampat, West Papua. Coral Reefs 2021, 40, 111–130. [Google Scholar] [CrossRef]
- Nur, F.M.; Batubara, A.S.; Perdana, A.W.; Eriani, K.; Muchlisin, Z.A. Checklist of coral fishes in Lhoknga and Lhok Mata Ie Beaches, Aceh Besar, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 348, 012104. [Google Scholar] [CrossRef]
- Muallil, R.N.; Tambihasan, A.M.; Enojario, M.J.; Ong, Y.N.; Nañola, C.L. Inventory of commercially important coral reef fishes in Tawi-Tawi Islands, Southern Philippines: The Heart of the Coral Triangle. Fish. Res. 2020, 230, 105640. [Google Scholar] [CrossRef]
- Satapoomin, U. A preliminary checklist of coral reef fishes of the Gulf of Thailand, South China Sea. Raffles Bull. Zool. 2000, 48, 31–53. [Google Scholar]
- Lu, Y.; Ding, Z.; Li, W.; Chen, X.; Yu, Y.; Zhao, X.; Lian, X.; Wang, Y. The effect of seawater environmental factors on the corals of Wailingding Island in the Pearl River Estuary. Cont. Shelf Res. 2020, 197, 104076. [Google Scholar] [CrossRef]
- Duprey, N.N.; Wang, T.X.; Kim, T.; Cybulski, J.D.; Vonhof, H.B.; Crutzen, P.J.; Haug, G.H.; Sigman, D.M.; Martinez-Garcia, A.; Baker, D.M. Megacity development and the demise of coastal coral communities: Evidence from coral skeleton delta N-15 records in the Pearl River estuary. Glob. Chang. Biol. 2020, 26, 1338–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadovy, Y.; Cornish, A.S. Reef Fishes of Hong Kong; Hong Kong University Press: Hong Kong, China, 2000. [Google Scholar]
- Bellwood, D.R.; Wainwright, P.C. The History and Biogeography of Fishes on Coral Reefs. Coral Reef Fishes 2002, 5, 32. [Google Scholar]
- Weng, L.N. Hong Kong coral reef census: Coral growth and healthy stable biological variety rich in biological varieties. Hong Kong Wenwei Rep. 2015. Available online: https://www.chinanews.com.cn/ga/2015/12-06/7658018.shtml (accessed on 10 November 2022).
- Ditzel, P.; König, S.; Musembi, P.; Peters, M.K. Correlation between Coral Reef Condition and the Diversity and Abundance of Fishes and Sea Urchins on an East African Coral Reef. Oceans 2022, 3, 1–14. [Google Scholar] [CrossRef]
- Komyakova, V.; Munday, P.L.; Jones, G.P. Relative Importance of Coral Cover, Habitat Complexity and Diversity in Determining the Structure of Reef Fish Communities. PLoS ONE 2013, 8, e83178. [Google Scholar] [CrossRef]
- Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Resistance and resilience of a coral reef fish community to changes in coral cover. Mar. Ecol. Prog. Ser. 2008, 371, 263–271. [Google Scholar] [CrossRef]
- Graham, N.A.J. Habitat Complexity: Coral Structural Loss Leads to Fisheries Declines. Curr. Biol. 2014, 24, R359–R361. [Google Scholar] [CrossRef] [Green Version]
- Hein, M.Y.; Beeden, R.; Birtles, R.A.; Chase, T.J.; Couture, F.; Haskin, E.; Marshall, N.; Ripple, K.; Terry, L.; Willis, B.L.; et al. Effects of coral restoration on fish communities: Snapshots of long-term, multiregional responses and implications for practice. Restor. Ecol. 2020, 28, 1158–1171. [Google Scholar] [CrossRef]
- Cole, A.J.; Pratchett, M.S.; Jones, G.P. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish. 2008, 9, 286–307. [Google Scholar] [CrossRef]
- Diazpulido, G.; Mccook, L. The fate of bleached corals: Patterns and dynamics of algal recruitment. Mar. Ecol. Prog. Ser. 2002, 232, 115–128. [Google Scholar] [CrossRef]
- Jones, G.P.; McCormick, M.I.; Srinivasan, M.; Eagle, J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA 2004, 101, 8251–8253. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.K.; Dolman, A.M.; Cheal, A.J.; Emslie, M.J.; Pratchett, M.S.; Sweatman, H.P.A. Maintenance of fish diversity on disturbed coral reefs. Coral Reefs 2009, 28, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Garpe, K.C.; Yahya, S.A.S.; Lindahl, U.; Ohman, M.C. Long-term effects of the 1998 coral bleaching event on reef fish assemblages. Mar. Ecol. Prog. Ser. 2006, 315, 237–247. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Bijoux, J.P.; Robinson, J. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 8425–8429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, I.H.; Kwok, K.Y. Marine Fish Fauna in Hong Kong Waters. Zool. Stud. 1999, 38, 130–152. [Google Scholar] [CrossRef]
- Liu, Z.L.; Yang, L.L.; Yan, L.P.; Yuan, X.W.; Chen, J.H. Fish assemblages and environmental interpretation in the northern Taiwan Strait and its adjacent waters in summer. J. Fish. Sci. China 2016, 23, 1399–1416. [Google Scholar] [CrossRef]
- Komyakova, V.; Jones, G.P.; Monday, P.L. Strong effects of coral species on the diversity and structure of reef fish communities: A multi-scale analysis. PLoS ONE 2018, 13, e0202206. [Google Scholar] [CrossRef] [Green Version]
- Ismail, F.; Akbar, N.; Tahir, I.; Paembonan, R.E.; Marus, I.; Wibowo, E.S. An assessment of small islands coral cover and coral-reef fish diversity at Oba Sub-district, Halmahera Island. IOP Conf. Ser. Earth Environ. Sci. 2021, 890, 012060. [Google Scholar] [CrossRef]
- Belmaker, J. Species richness of resident and transient coral-dwelling fish responds differentially to regional diversity. Glob. Ecol. Biogeogr. 2009, 18, 426–436. [Google Scholar] [CrossRef]
- Messmer, V.; Jones, G.P.; Munday, P.L.; Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 2011, 92, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Anthony, K.; Berkelmans, R.; Dove, S.; Willis, B. Vulnerability of Reef-Building Corals on the Great Barrier Reef to Climate Change; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2007. [Google Scholar]
- Sheppard, C.; Spalding, M.; Bradshaw, C.; Wilson, S. Erosion vs. recovery of coral reefs after 1998 El Nio: Chagos reefs, Indian Ocean. Ambio 2002, 31, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W. Fish utilization of simulated coral reef frameworks versus eroded rubble substrates off Panama, eastern Pacific. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2003. [Google Scholar]
- Duffy, J.E.; Lefcheck, J.S.; Stuart-Smith, R.D.; Navarrete, S.A.; Edgar, G.J. Biodiversity enhances reef fish biomass and resistance to climate change. Proc. Natl. Acad. Sci. USA 2016, 113, 6230–6235. [Google Scholar] [CrossRef] [Green Version]
- Go, K.T.B.; Anticamara, J.A.; de Ramos, J.A.; Gabona, S.F.; Agao, D.F.; Hererra, E.C.; Bitara, A.U. Species richness and abundance of non-cryptic fish species in the Philippines: A global center of reef fish diversity. Biodivers. Conserv. 2015, 24, 2475–2495. [Google Scholar] [CrossRef]
- Zgliczynski, B.J.; Williams, I.D.; Schroeder, R.E.; Nadon, M.O.; Richards, B.L.; Sandin, S.A. The IUCN Red List of Threatened Species: An assessment of coral reef fishes in the US Pacific Islands. Coral Reefs 2013, 32, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.J.; Zhang, J.; Chen, Z.Z. Research advances on coral reef fish biodiversity and conservation. Chin. J. Ecol. 2021, 40, 2996–3006. [Google Scholar] [CrossRef]
- Buchanan, J.R.; Krupp, F.; Burt, J.A.; Feary, D.A.; Ralph, G.M.; Carpenter, K.E. Living on the edge: Vulnerability of coral-dependent fishes in the Gulf. Mar. Pollut. Bull. 2016, 105, 480–488. [Google Scholar] [CrossRef]
- Miller, R.M.; RODRíGUEZ, J.P.; Aniskowicz-Fowler, T.; Bambaradeniya, C.; Boles, R.; Eaton, M.A.; GÄRdenfors, U.; Keller, V.; Molur, S.; Walker, S. National Threatened Species Listing Based on IUCN Criteria and Regional Guidelines: Current Status and Future Perspectives. Conserv. Biol. 2010, 21, 684–696. [Google Scholar] [CrossRef]
- Shi, Y.R.; Li, Y.Z.; Sun, D.F.; Lu, W.H. Analysis on effect of summer closed fishing for ten years in South China Sea from resource change, ecological protection, economic benefit and social influence. China Fish. 2008, 14–16. [Google Scholar] [CrossRef]
- Anonymous. China Marine Protection Industry Report: China has established 271 Marine reserves. Fish. Sci. Technol. Inf. 2020, 47, 350. [Google Scholar]
- Corrales, C.M.; Delan, G.G.; Rica, R.L.V.; Piquero, A.S.; Monte, I.A. A Baseline Study on Coral Reef Fishes in the Marine Protected Areas in Southern Cebu, Philippines. Trop. Technol. J. 2015, 19, 4. [Google Scholar] [CrossRef]
- Bradley, P.; Jessup, B.; Pittman, S.J.; Jeffrey, C.F.G.; Ault, J.S.; Carrubba, L.; Lilyestrom, C.; Appeldoorn, R.S.; Schärer, M.T.; Walker, B.K.; et al. Development of a reef fish biological condition gradient model with quantitative decision rules for the protection and restoration of coral reef ecosystems. Mar. Pollut. Bull. 2020, 159, 111387. [Google Scholar] [CrossRef] [PubMed]
- Song, B.L. The Addendum of Coastal Fishes of Zhanjiang City. J. Zhanjiang Fish. Coll. 1992, 12, 30–33. [Google Scholar]
- Song, B.L.; Yang, P. Preliminary Exploration on the Fish Fauna of Zhanjiang Coast. J. Guangdong Ocean Univ. 1984, 95–106. [Google Scholar]
- Huang, H.; Lian, J.S.; Wang, H.J.; Chen, Y.H. Xuwen Coral Reef and Its Biodiversity; Ocean Press: Beijing, China, 2007. [Google Scholar]
- Yang, G.H.; Hou, X.Q.; Chen, C.L.; Sun, S.L. Fish Species Composition in the Coral Reef of Coastal Xuwen County. Fish. Sci. 2008, 27, 533–538. [Google Scholar] [CrossRef]
- Liang, Y.B. Investigation and Evaluation of Red Tide Hazards in China; Ocean Press: Beijing, China, 2012. [Google Scholar]
- Jia, X.P.; Chen, P.M.; Tang, Z.C.; Cai, W.G.; Li, C.H.; Qin, C.X. Research and Demonstration of Key Technologies for Artificial Reefs; Ocean Press: Beijing, China, 2011. [Google Scholar]
- Du, W.L.; She, G.H. Report of a Rapid Survey of Coral Fish in Daya Bay, Guangdong Province, China. 2015. Available online: https://www.docin.com/p-2008133718.html (accessed on 13 August 2022).
- Zou, Q.; Wu, Z.Q.; Huang, L.L.; Ding, Y.; Huang, X. Coral reef fish species composition in Weizhou Island, Guangxi. J. South. Agric. 2020, 51, 1–10. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zhan, R.G. Marine Reef Fish; Kenting National Park Administration: Pingtung Hsieng, Taiwan, 1986. [Google Scholar]
- Taiwan Marine National Park Management Office(TMNPMO). 2022. Available online: https://www.marine.gov.tw (accessed on 13 October 2021).
- Li, C.L.; Lin, X.Z. Comparisons of Fish Assemblages in the Tropical Seagrass Beds in Kenting and Tongsha Island. Biol. Sci. 2009, 51, 1–14. [Google Scholar]
- Chen, Z.Q. Green Wild Fish Trace—Green Island Marine Fish Ecological Guide; Marine National Park Management Office: Hongkong, China, 2010. [Google Scholar]
- Guo, S.J.; Yang, D.Q.; Su, Y.; Lin, Y.J. Secret LAN Yu—LAN Yu Ecological Sketch; Marine National Park Management Office: Hongkong, China, 2010. [Google Scholar]
- Fu, Y.C. Plasticity of Feeding Habits of Two Plectroglyphidodon Damselfishes on Coral Reefs at Southern Taiwan, Evidence from Stomach Content and Stable Isotope Analyses. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2007. [Google Scholar]
- Wu, Z.J.; Cai, Z.F.; Chen, S.Q.; Zhang, G.X.; Li, X.M.; Wang, D.R.; Yao, H.J. Species distribution and diversity of the coral reef fishes in the shallow reefs along the east and south coasts of Hainan Island. J. Fish. China 2015, 39, 1203–1217. [Google Scholar] [CrossRef]
- Liu, J.; Tian, M.C. A Preliminary Study and Review of the Coral Reef Fishes from Hainan Island. Mar. Sci. 1995, 5, 28–32. [Google Scholar]
- Xie, M.L. Fish Connectivity in Mangrove-Seagrass-Coral Reef Continuum—A Case Study in Wenchang, Hainan Province. Master’s Thesis, Shantou University, Shantou, China, 2019. [Google Scholar]
- Lian, J.S.; Huang, H.; Huang, L.M.; Wang, D.R. Coral Reefs and Their Biodiversity in Sanya; Ocean Press: Beijing, China, 2010. [Google Scholar]
- Li, X.B. The Present Situation, Ecological Restoration and Protection of the Coral Reef at Sanya Island; Science Press: Beijing, China, 2019. [Google Scholar]
- Du, J.G.; Xie, M.L.; Wang, Y.Y.; Chen, Z.H.; Liu, W.H.; Liao, J.J.; Chen, B. Connectivity of fish assemblages along the mangrove-seagrass-coral reef continuum in Wenchang, China. Acta Oceanol. Sin. 2020, 39, 43–52. [Google Scholar] [CrossRef]
- Huang, D.Y.; Wang, J.J.; Chen, G.L.; Zhen, X.Q. Community structure and ecological warning of reef-associated fish macrobenthos in the Yalong Bay. Chin. J. Ecol. 2021, 40, 412–426. [Google Scholar] [CrossRef]
- Wang, D.R.; Wu, R.; Li, Y.C.; Wu, Z.J. A Study on Typical Tropical Marine Ecosystem in Hainan Province; Ocean Press: Beijing, China, 2013. [Google Scholar]
- Wu, Z.J.; Li, Y.C.; Chen, S.Q. Ecological Environment and Biological Resources in the Western Sea Area of Hainan Island; Ocean Press: Beijing, China, 2019. [Google Scholar]
- Shao, G.Z.; Chen, Z.P.; Chen, J.Y.; Huang, J.H.; Guo, R.W. Species composition and geographical distribution of fishes in Tungsha Island and Taiping Island in the South China Sea. Biodivers. Sci. 2011, 19, 737–763. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Liu, J.X.; Meng, Q.W.; Yang, Y.R.; Cheng, Q.T. The Fishes of the Islands in the South China Sea; Science Press: Beijing, China, 1979. [Google Scholar]
- Wu, N.; Zhang, N.; Cao, M.; Guo, H.Y.; Zhu, K.C.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. The identification analysis of fish eggs from lagoon of Yongshu Reef based on DNA barcoding technology. Freshw. Fish. 2018, 48, 51–57. [Google Scholar] [CrossRef]
- Li, Y.J. Biodiversity and Biological Characteristics of Dominant Fish Species of Typical Reef Fishes in South China Sea. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Phung, N.H. The species composition of coral reef fishes in the Spratly Islands, Central South China Sea. Mar. Biol. South China Sea 1998, 113–127. [Google Scholar]
- Fang, H.D.; Lv, X.L. Guide to Corals of Nansha Islands; China Ocean University Press: Qingdao, China, 2020. [Google Scholar]
- Liu, S.; Lin, X.Z.; Zhang, L.; Huang, H. Coral Reef Fish Ecology Atlas of Nansha Islands; Science Press: Beijing, China, 2021. [Google Scholar]
- Chen, J.P.; Jan, R.Q.; Shao, K.T. Checklist of Reef Fishes from Taiping Island (Itu Aba Island), Spratly Islands, South China Sea; University Hawaii Press: Honolulu, HI, USA, 1997; Volume 51, pp. 143–166. [Google Scholar]
- Li, Y.Z.; Jia, X.P.; Chen, G.B.; Chen, P.M.; Shu, L.M.; Zeng, X.G. South China Sea Coral Reef Fish Stocks; Ocean Press: Beijing, China, 2007. [Google Scholar]
- Gao, Y.L.; Huang, H.; Lian, J.S.; Yang, J.H. The species diversity and trophic structure of reef fishes in the waters of the Xisha Archipelago. Biodivers. Sci. 2014, 22, 618–623. [Google Scholar] [CrossRef]
- Qiu, S.T.; Chen, B.; Du, J.G.; Loh, K.H.; Liao, J.J.; Liu, X.M.; Yang, W. Checklist of the coral fish fauna of Xisha Islands, China. Biodivers. Data J. 2021, 9, e63945. [Google Scholar] [CrossRef]
- Huang, H. Coral Reef Atlas of Xisha Islands; Science Press: Beijing, China, 2018. [Google Scholar]
- Fu, L. Coral Reef fishes of the South China Sea—The Xisha, Nansha, and Zhongsha Islands; China Citic Press: Beijing, China, 2014. [Google Scholar]
- Chen, Q.C.; Cai, Y.Z. Coral reef fish—Nansha Islands and Tropical Ornamental Fishes; Science Press: Beijing, China, 1994. [Google Scholar]
- Shao, K.T.; Ho, H.C.; Lin, P.L.; Lee, P.F.; Lee, M.Y.; Tsai, C.Y.; Liao, Y.C.; Lin, Y.C. A Checklist of the Fishes of Southern Taiwan, Northern South China Sea. Raffles Bull. Zool. 2008, 19, 233–271. [Google Scholar]
- Sun, J.Y. New record for fish in the Gulf of Tonkin. J. Guangxi Acad. Sci. 1990, 6, 7. [Google Scholar]





| References | Areas | Family |
|---|---|---|
| This study | Coral reefs of China waters | Gobiidae (212), Labridae (153), Pomacentridae (129), Serranidae (125), Apogonidae (96), Scorpaenidae (90), Blenniidae (68), Carangidae (63), Macrouridae (56), Muraenidae (56) |
| Samoilys et al. (2022) [3] | Madagascar, Comoros, Mozambique, and Tanzania | Labridae (6), Chaetodontidae (4), Pomacentridae (3), Scaridae (2), Epinephelidae (2), Acanthuridae (1), Lutjanidae (1), Haemulidae (1), Pomacanthidae (1), Ostraciidae (1) |
| Andradi-Brown et al. (2021) [57] | Raja Ampat, West Papua | Labridae (5), Nemipteridae (3), Chaetodontidae (3), Acanthuridae (2), Balistidae (2), Pomacanthidae (2), Pomacentridae (2), Serranidae (2), Lethrinidae (2), Lutjanidae (1) |
| Muallil et al. (2020) [59] | Tawi-Tawi Islands, south Philippines | Epinephelinae (48), Lutjanidae (40), Acanthuridae (33), Scarinae (31), Lethrinidae (26), Nemipteridae (19), Balistidae (16), Mullidae (16), Siganidae (14), Haemulidae (13) |
| Nguyen and Mai (2020) [41] | Coastal waters of Vietnam | Pomacentridae (110), Gobiidae (107), Labridae (100), Apogonidae (60), Serranidae (47), Blenniidae (46), Chaetodontidae (41), Scaridae (36), Scorpaenidae (32), Acanthuridae (30) |
| Jayaprabha et al. (2018) [51] | Southeast coast of India | Carangidae (22), Serranidae (16), Lutjanidae (13), Mullidae (7), Haemulidae (6), Acanthuridae (5), Apogonidae (5), Siganidae (4), Nemipteridae (4), Synodontidae (4) |
| Arai (2015) [10] | Malaysian South China Sea | Gobiidae (133), Pomacentridae (108), Labridae (85), Serranidae (65), Apogonidae (57), Carangidae (53), Chaetodontidae (45), Lutjanidae (38), Blenniidae (36), Tetraodontidae (34) |
| Satapoomin (2000) [60] | Gulf of Thailand | Pomacentridae (37), Gobiidae (28), Labridae (26), Serranidae (17), Apogonidae (16), Scaridae (10), Carangidae (9), Lutjanidae (9), Nemipteridae (8), Siganidae (7) |
| Low and Chou (1992) [53] | Coral reefs of Singapore | Pomacentridae (28), Labridae (16), Apogonidae (8), Nemipteridae (7), Serranidae (6), Chaetodontidae (4), Blenniidae (3), Lutjanidae (3), Monacanthidae (3), Pomacanthidae (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Li, C.; Wang, T.; Zhao, J.; Liu, Y.; Xiao, Y. Distribution Pattern of Coral Reef Fishes in China. Sustainability 2022, 14, 15107. https://doi.org/10.3390/su142215107
Shi J, Li C, Wang T, Zhao J, Liu Y, Xiao Y. Distribution Pattern of Coral Reef Fishes in China. Sustainability. 2022; 14(22):15107. https://doi.org/10.3390/su142215107
Chicago/Turabian StyleShi, Juan, Chunhou Li, Teng Wang, Jinfa Zhao, Yong Liu, and Yayuan Xiao. 2022. "Distribution Pattern of Coral Reef Fishes in China" Sustainability 14, no. 22: 15107. https://doi.org/10.3390/su142215107
APA StyleShi, J., Li, C., Wang, T., Zhao, J., Liu, Y., & Xiao, Y. (2022). Distribution Pattern of Coral Reef Fishes in China. Sustainability, 14(22), 15107. https://doi.org/10.3390/su142215107






