Promotion of Soil Microbial Community Restoration in the Mu Us Desert (China) by Aerial Seeding
Abstract
1. Introduction
2. Materials and Methods
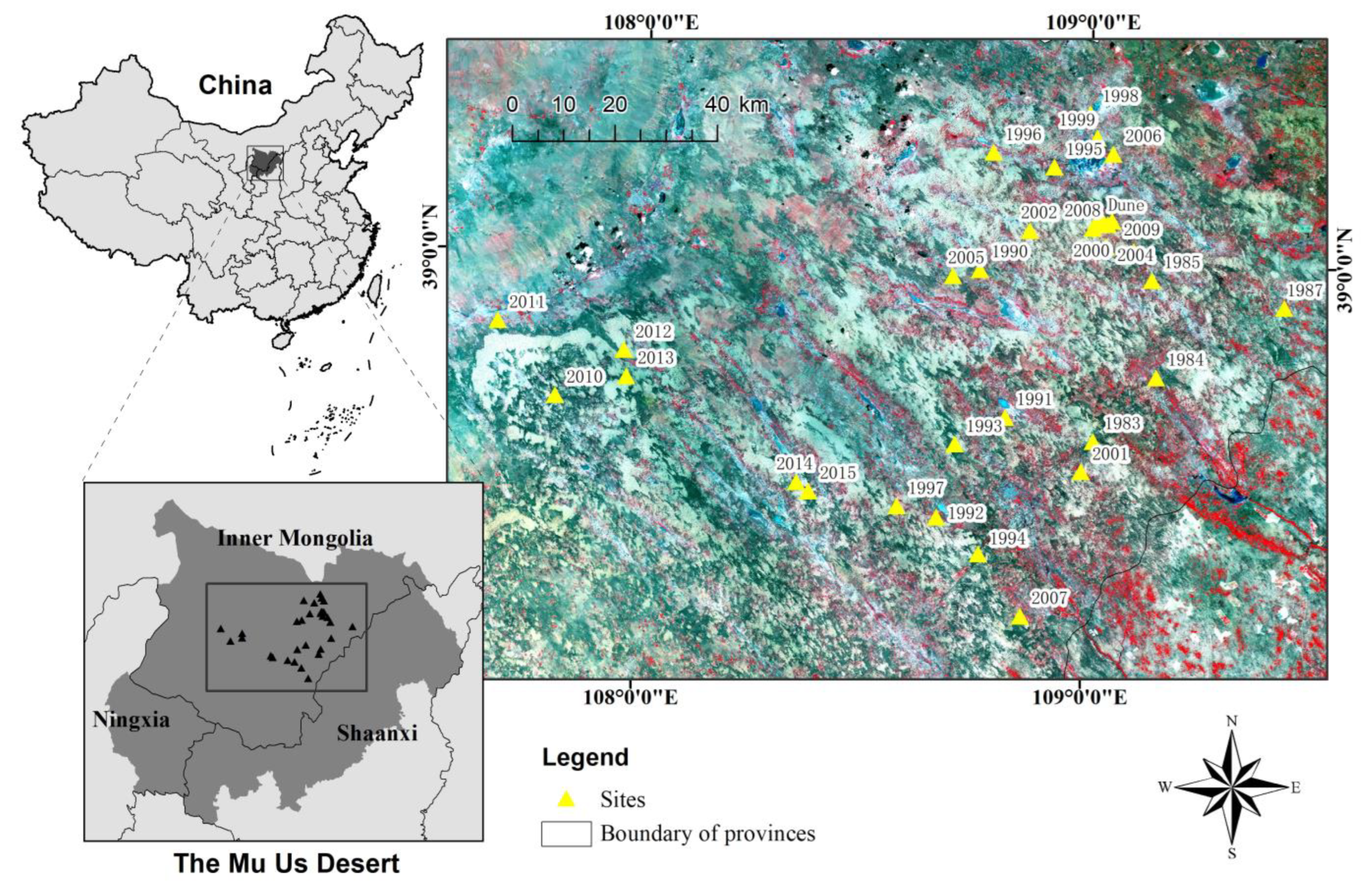
2.1. Study Area
2.2. Sites and Sampling
2.3. Determination of Soil Nutrient Indexes
2.4. Soil Microbial Sequencing
2.5. Data Analyses
3. Results
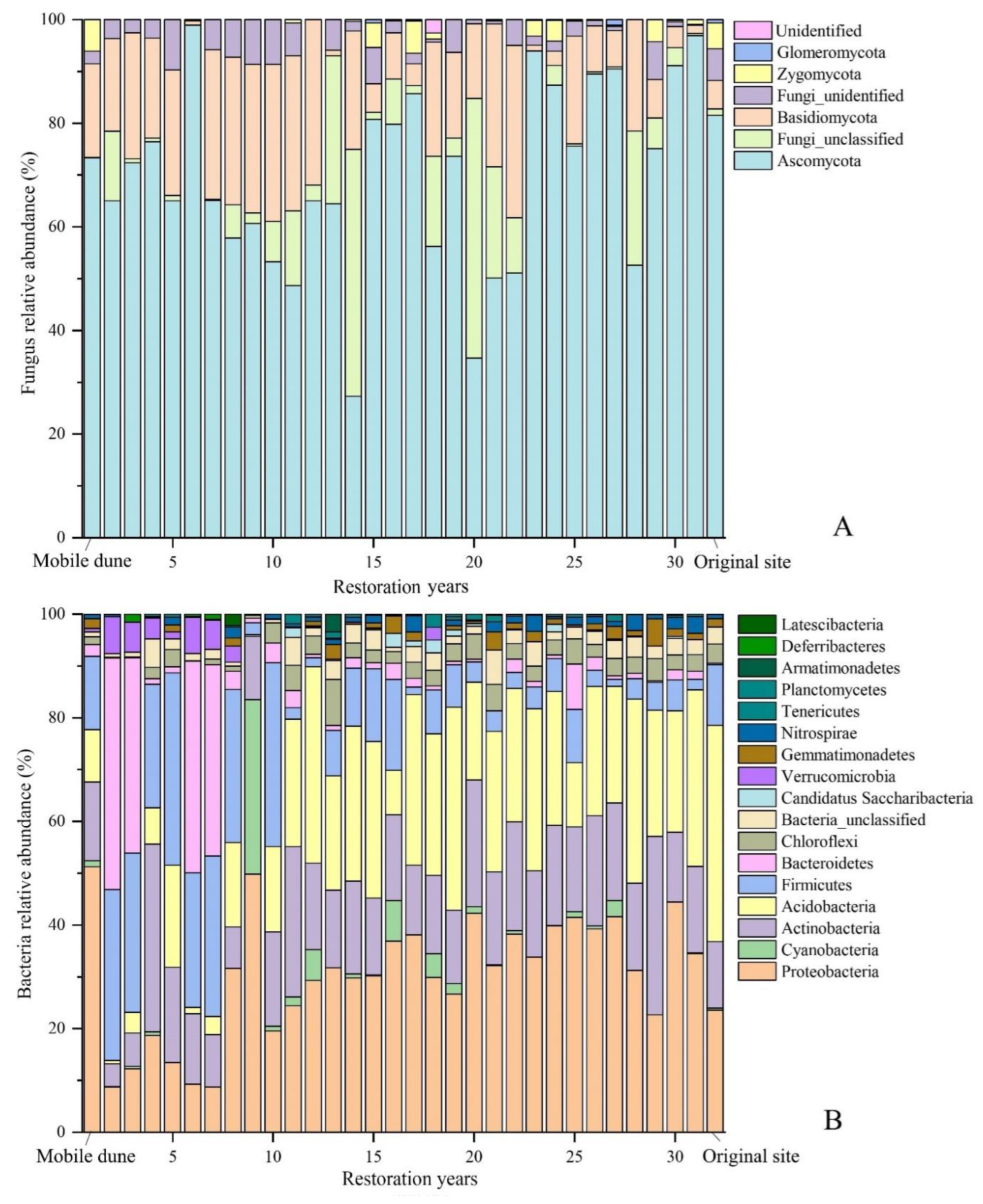
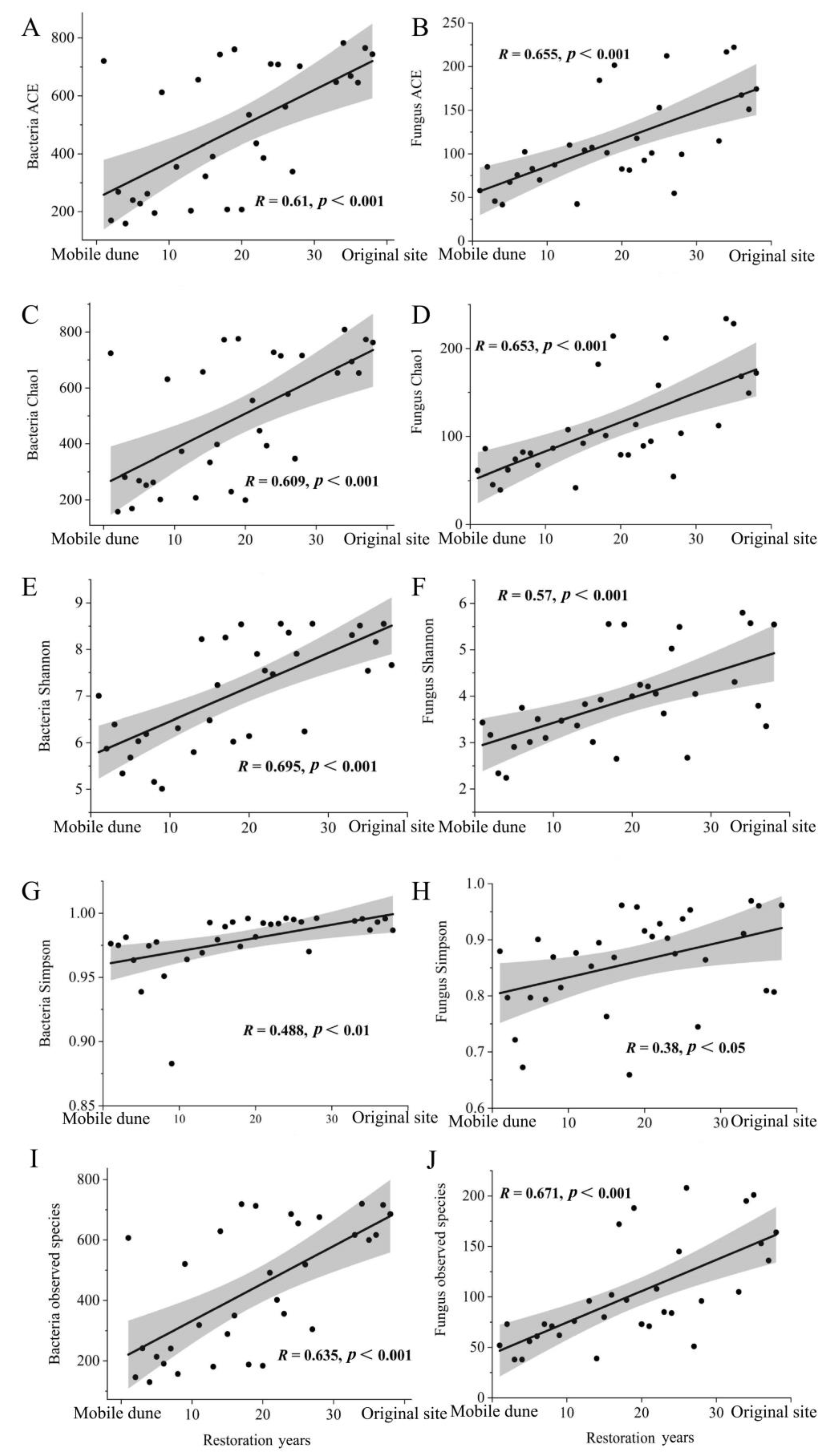
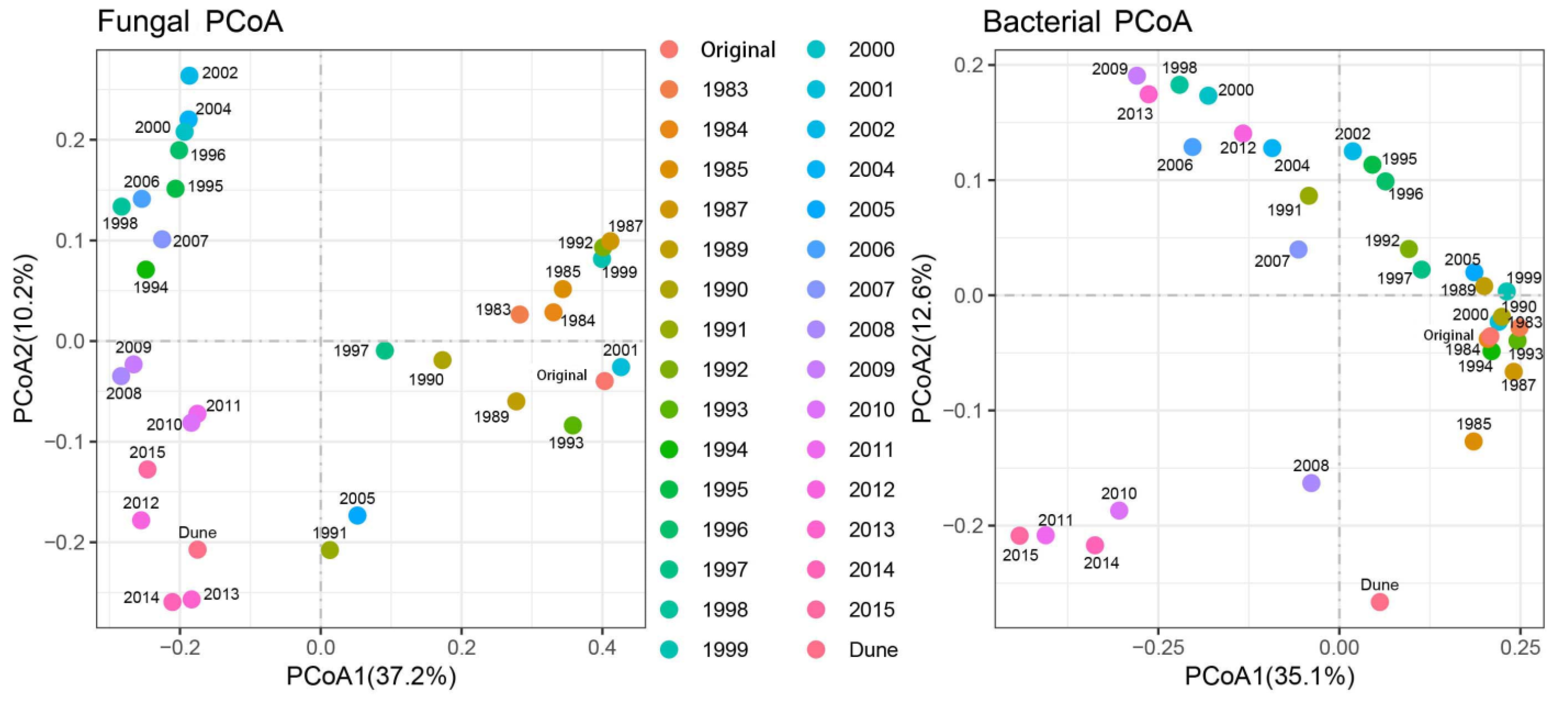
3.1. Changes in Soil Microbial Diversity with Restoration Period
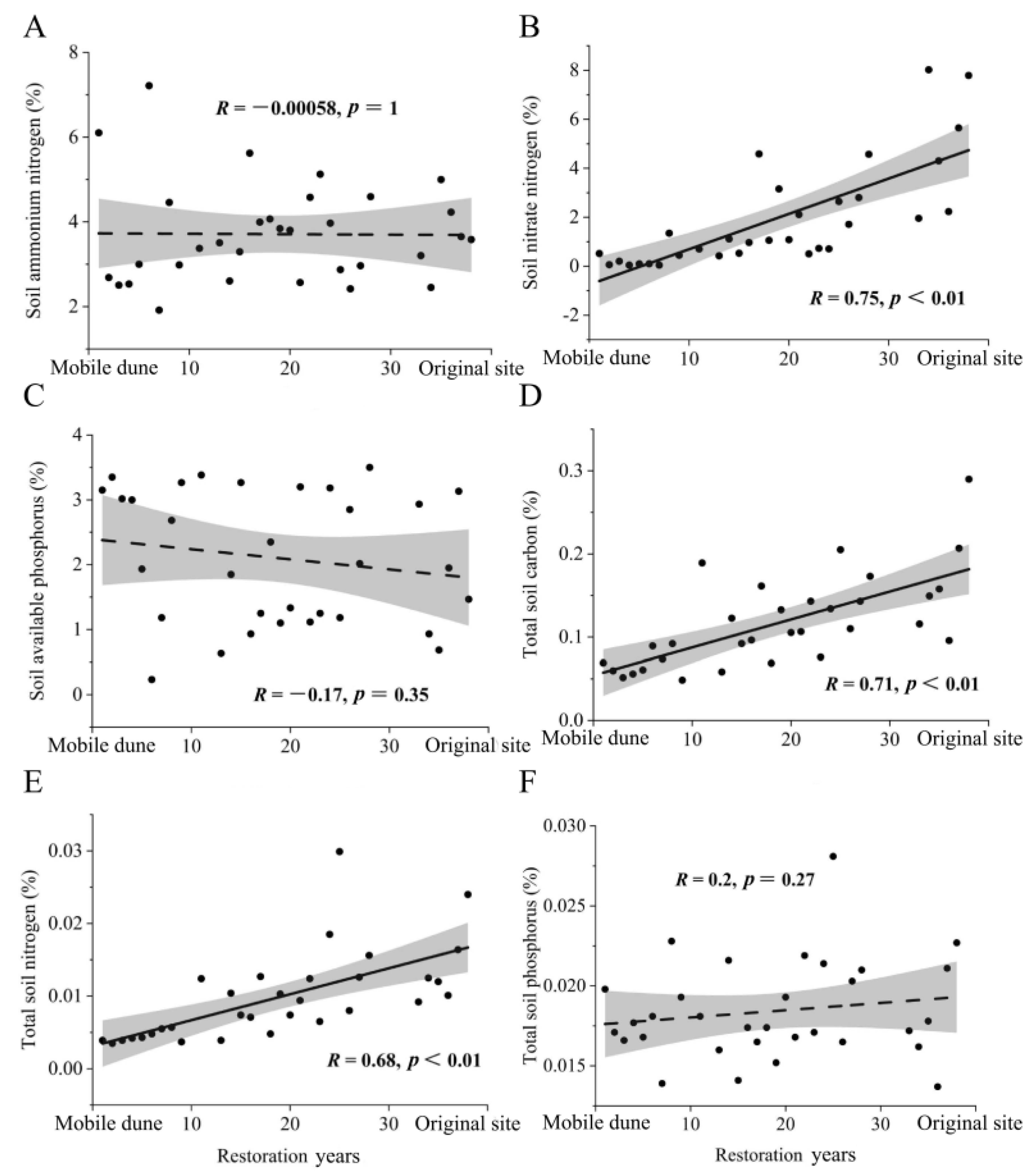
3.2. Changes in Soil Nutrient Indexes with Restoration Period
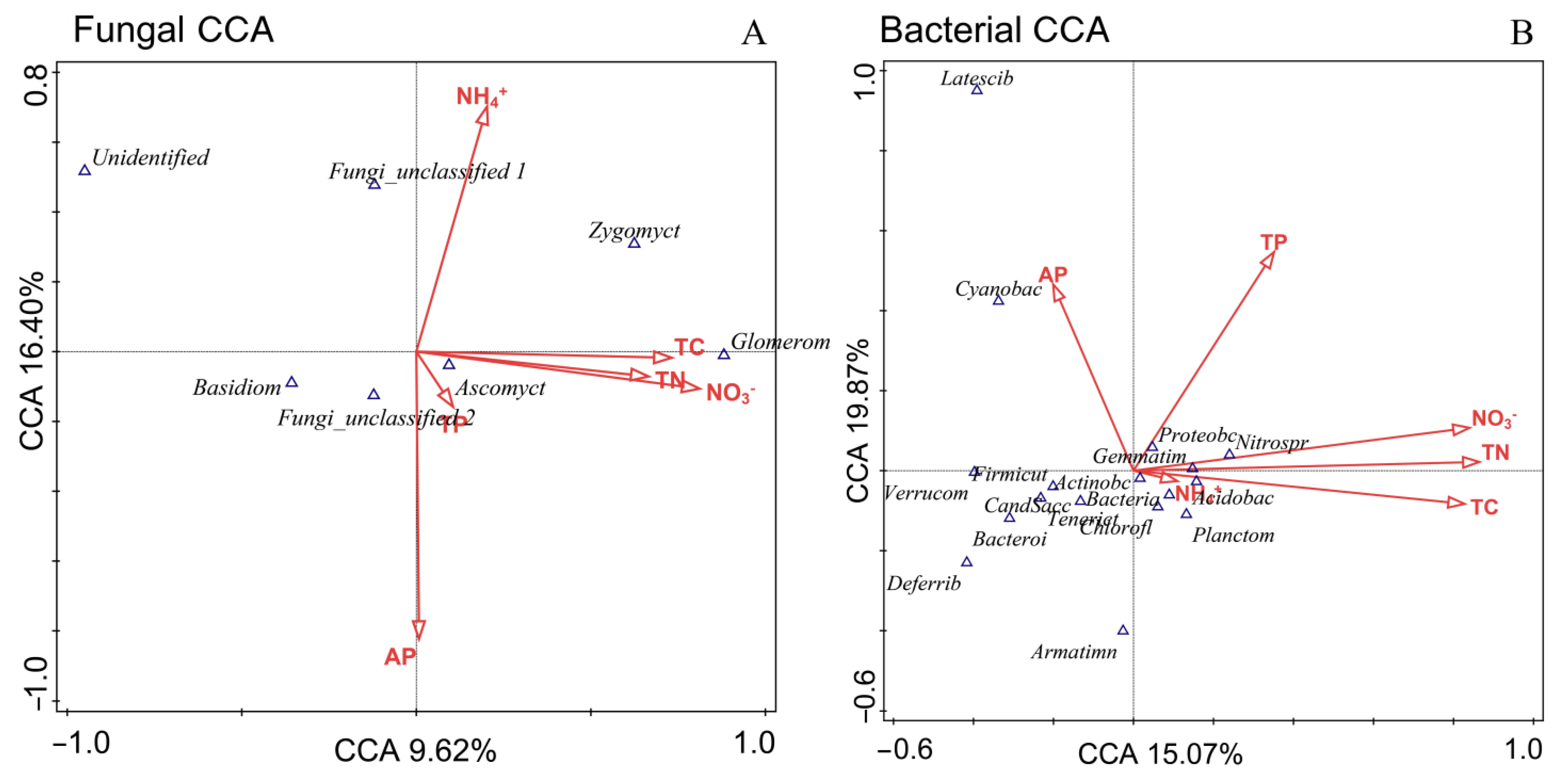
3.3. Relationship between Soil Microbial Diversity and Soil Nutrient Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, A.; Huangfu, C.; Li, J.; Wang, H.; Yang, D. Effects of Increasing Nitrogen Deposition on Soil Microbial Community Structure of Stipa Baicalensis Steppe in Inner Mongolia, China. Ecol. Environ. Sci. 2017, 26, 1100–1106. [Google Scholar]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Dowd, S.; Sun, Y.; Allen, V. Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol. Biochem. 2008, 40, 2762–2770. [Google Scholar] [CrossRef]
- Maron, P.-A.; Lejon, D.P.; Carvalho, E.; Bizet, K.; Lemanceau, P.; Ranjard, L.; Mougel, C. Assessing genetic structure and diversity of airborne bacterial communities by DNA fingerprinting and 16S rDNA clone library. Atmos. Environ. 2005, 39, 3687–3695. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Jarvie, S.; Yan, Y.; Han, P.; Liu, T.; Guo, K.; Ren, L.; Yue, K.; Wu, H.; et al. Ecosystem restoration through aerial seeding: Interacting plant–soil microbiome effects on soil multifunctionality. Land Degrad. Dev. 2021, 32, 5334–5347. [Google Scholar] [CrossRef]
- Li, M.-m.; Liu, A.-t.; Zou, C.-j.; Xu, W.-d.; Shimizu, H.; Wang, K.-y. An overview of the “Three-North” Shelterbelt project in China. For. Stud. China 2012, 14, 70–79. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, J.; Liu, J.; He, B.; Lei, T.; Wang, Q. Increasing terrestrial vegetation activity of ecological restoration program in the Beijing–Tianjin Sand Source Region of China. Ecol. Eng. 2013, 52, 37–50. [Google Scholar] [CrossRef]
- Jia, X.; Fu, B.; Feng, X.; Hou, G.; Liu, Y.; Wang, X. The tradeoff and synergy between ecosystem services in the Grain-for-Green areas in Northern Shaanxi, China. Ecol. Indic. 2014, 43, 103–113. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Wang, J.; Van, C.; Li, Z. Analysis on desertification dynamics based on remote sensing and GIS in zone along the Great Wall in northern Shaanxi province. J. Desert Res. 2005, 25, 63–67. [Google Scholar]
- Liu, Y.; Zhang, H.; Xiong, M.; Li, F.; Zhang, X.; Pan, G.; Wang, G. Effect of climate change on soil microbial diversity and function. China Environ. Sci. 2016, 36, 3793–3799. [Google Scholar]
- Wang, Y.; Li, J.; Jing, L.; Zhang, Y.; Zhang, J. Effects of different precipitation treatments on soil ecological chemistry and microbial diversity in the Loess Plateau. Acta Ecol. Sin. 2020, 40, 1517–1531. [Google Scholar]
- Kuramae, E.; Gamper, H.; van Veen, J.; Kowalchuk, G. Soil and plant factors driving the community of soil-borne microorganisms across chronosequences of secondary succession of chalk grasslands with a neutral pH. FEMS Microbiol. Ecol. 2011, 77, 285–294. [Google Scholar] [CrossRef]
- Dai, J.; Yan, R.; Wei, Z.; Bai, Y.; Zhang, S.; Wang, T.; Sun, S. Effects of short-term fertilization on soil microorganisms in a mown Leymus chinensis meadow. Chin. J. Ecol. 2017, 36, 2431–2437. [Google Scholar]
- Xu, F.; Zhang, T.; Huai, B.-D.; Sui, W.-Z.; Yang, X. Effects of land use changes on soil fungal community structure and function in the riparian wetland along the downstream of the songhua river. Environ. Sci. 2021, 42, 2531–2540. [Google Scholar]
- Oliver, A.K.; Callaham, M.A., Jr.; Jumpponen, A. Soil fungal communities respond compositionally to recurring frequent prescribed burning in a managed southeastern US forest ecosystem. For. Ecol. Manag. 2015, 345, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Hong, G.; Li, Z.; Gao, X.; Wu, Y.; Wang, X.; Wang, P.; Yang, J. Assessment of the ecosystem service function of Sandy Lands at different times following aerial seeding of an endemic species. Sustainability 2018, 10, 902. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Yan, Y.; Zhang, X.; Niu, J.; Svenning, J.-C. Ecological restoration is the dominant driver of the recent reversal of desertification in the Mu Us Desert (China). J. Clean. Prod. 2020, 268, 122241. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Havrilla, C.A.; Liu, Y.; Wu, G.L. Plant litter crust role in nutrients cycling potentials by bacterial communities in a sandy land ecosystem. Land Degrad. Dev. 2021, 32, 3194–3203. [Google Scholar] [CrossRef]
- Wang, G.; Su, Z.; Mao, L.; Zhang, Q.; Ma, Y. Characteristics of Soil Surface Grain Size in Ordos Plateau along the Agro-pastoral Ecotone of North China. J. Desert Res. 2019, 39, 183. [Google Scholar]
- Corre, M.D.; Beese, F.O.; Brumme, R. Soil nitrogen cycle in high nitrogen deposition forest: Changes under nitrogen saturation and liming. Ecol. Appl. 2003, 13, 287–298. [Google Scholar] [CrossRef]
- Zhang, Q.; Buyantuev, A.; Li, F.Y.; Jiang, L.; Niu, J.; Ding, Y.; Kang, S.; Ma, W. Functional dominance rather than taxonomic diversity and functional diversity mainly affects community aboveground biomass in the Inner Mongolia grassland. Ecol. Evol. 2017, 7, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Whitton, C.; Floyd, R.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1935–1943. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Bell, T.; Newman, J.A.; Silverman, B.W.; Turner, S.L.; Lilley, A.K. The contribution of species richness and composition to bacterial services. Nature 2005, 436, 1157–1160. [Google Scholar] [CrossRef]
- Cao, C.; Yao, J.; Han, X.; Zhang, Y. Soil microbes functional diversity in sand-fixing Caragana microphylla communities in Horqin Sandy Land. Chin. J. Appl. Ecol. 2011, 22, 2309–2315. [Google Scholar]
- Abakumov, E.; Zverev, A.; Kichko, A.; Kimeklis, A.; Andronov, E. Soil microbiome of different-aged stages of self-restoration of ecosystems on the mining heaps of limestone quarry (Elizavetino, Leningrad region). Open Agric. 2021, 6, 57–66. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Hooper, D.U.; Bignell, D.E.; Brown, V.K.; Brussard, L.; Dangerfield, J.M.; Wall, D.H.; Wardle, D.A.; Coleman, D.C.; Giller, K.E.; Lavelle, P. Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: Patterns, mechanisms, and feedbacks. Bioscience 2000, 50, 1049–1061. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef]
- Cai, Q.; Ding, G. A discussion on evaluation indexes of forestry soil eco-environment quality. J. Mt. Agric. Biol. 2006, 25, 256–261. [Google Scholar]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Esmaeilzadeh-Salestani, K.; Bahram, M.; Ghanbari Moheb Seraj, R.; Gohar, D.; Tohidfar, M.; Eremeev, V.; Talgre, L.; Khaleghdoust, B.; Mirmajlessi, S.M.; Luik, A.; et al. Cropping systems with higher organic carbon promote soil microbial diversity. Agric. Ecosyst. Environ. 2021, 319, 107521. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Zhou, Z.; Huang, G.; Wang, X.; Han, Q.; Liu, G. The Application of Mixed Organic and Inorganic Fertilizers Drives Soil Nutrient and Bacterial Community Changes in Teak Plantations. Microorganisms 2022, 10, 958. [Google Scholar] [CrossRef]
- Gao, Y.; He, Z. Study on Soils Effect Factors to Fungi Diversity in Hebei Province. Chin. Agric. Sci. Bull. 2010, 26, 177–181. [Google Scholar]
- Su, N.; Jarvie, S.; Yan, Y.; Gong, X.; Li, F.; Han, P.; Zhang, Q. Landscape context determines soil fungal diversity in a fragmented habitat. CATENA 2022, 213, 106163. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, D.; Zang, S.; Zhang, D.; Sun, H. Characteristics of soil bacterial and fungal community of typical forest in the Greater Khingan Mountains based on high-throughput sequencing. Microbiol. China 2021, 48, 1093–1105. [Google Scholar]
- Chen, X.; Zhu, D.; Zhao, C.; Zhang, L.; Chen, L.; Duan, W. Community Composition and Diversity of Fungi in Soils under Different Types of Pinus koraiensis Forests. Acta Pedol. Sin. 2019, 56, 1221–1234. [Google Scholar]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Dörfelt, H.; Gube, M.; Jackson, D.J.; Reitner, J.; Seyfullah, L.J. Estimating the Phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Mol. Phylogenetics Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Brabcová, V.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Vaněk, D.; Frouz, J.; Šantrůčková, H.; Baldrian, P. Litter decomposition along a primary post-mining chronosequence. Biol. Fertil. Soils 2014, 50, 827–837. [Google Scholar] [CrossRef]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Purahong, W.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Däumlich, V.; Mital, S.; Buscot, F.; Hofrichter, M.; Gutknecht, J.L.; Krüger, D. Uncoupling of microbial community structure and function in decomposing litter across beech forest ecosystems in Central Europe. Sci. Rep. 2014, 4, 7014. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Kuske, C.R.; Schmidt, T.M. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum Acidobacteria. Appl. Environ. Microbiol. 2011, 77, 586–596. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Attard, E.; Poly, F.; Commeaux, C.; Laurent, F.; Terada, A.; Smets, B.F.; Recous, S.; Roux, X.L. Shifts between Nitrospira-and Nitrobacter-like nitrite oxidizers underlie the response of soil potential nitrite oxidation to changes in tillage practices. Environ. Microbiol. 2010, 12, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, C.; Zeng, X.; Feng, Y.; Weng, J.; Lin, X.; Zhu, J.; Xiong, Z.; Xu, J.; Cai, Z. Autotrophic growth of nitrifying community in an agricultural soil. ISME J. 2011, 5, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Canbolat, M.Y.; Bilen, S.; Çakmakçı, R.; Şahin, F.; Aydın, A. Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol. Fertil. Soils 2006, 42, 350–357. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zu, L.; Long, F.; Yang, X.; Wang, S.; Zhang, Q.; He, Y.; Chen, D.; Sui, M.; Zhang, G.; et al. Promotion of Soil Microbial Community Restoration in the Mu Us Desert (China) by Aerial Seeding. Sustainability 2022, 14, 15241. https://doi.org/10.3390/su142215241
Ma Y, Zu L, Long F, Yang X, Wang S, Zhang Q, He Y, Chen D, Sui M, Zhang G, et al. Promotion of Soil Microbial Community Restoration in the Mu Us Desert (China) by Aerial Seeding. Sustainability. 2022; 14(22):15241. https://doi.org/10.3390/su142215241
Chicago/Turabian StyleMa, Yina, Lei Zu, Fayu Long, Xiaofan Yang, Shixiong Wang, Qing Zhang, Yuejun He, Danmei Chen, Mingzhen Sui, Guangqi Zhang, and et al. 2022. "Promotion of Soil Microbial Community Restoration in the Mu Us Desert (China) by Aerial Seeding" Sustainability 14, no. 22: 15241. https://doi.org/10.3390/su142215241
APA StyleMa, Y., Zu, L., Long, F., Yang, X., Wang, S., Zhang, Q., He, Y., Chen, D., Sui, M., Zhang, G., Zang, L., & Liu, Q. (2022). Promotion of Soil Microbial Community Restoration in the Mu Us Desert (China) by Aerial Seeding. Sustainability, 14(22), 15241. https://doi.org/10.3390/su142215241






