Productive and Sustainable H2 Production from Waste Aluminum Using Copper Oxides-Based Graphene Nanocatalysts: A Techno-Economic Analysis
Abstract
:1. Introduction
2. Methodology
2.1. Synthesis of CuO NPs
2.2. Synthesis of Cu2O NPs
2.3. Synthesis of rGO and MLG
2.4. Synthesis of CuO-rGO Composites
2.5. Synthesis of Cu2O-MLG Composite
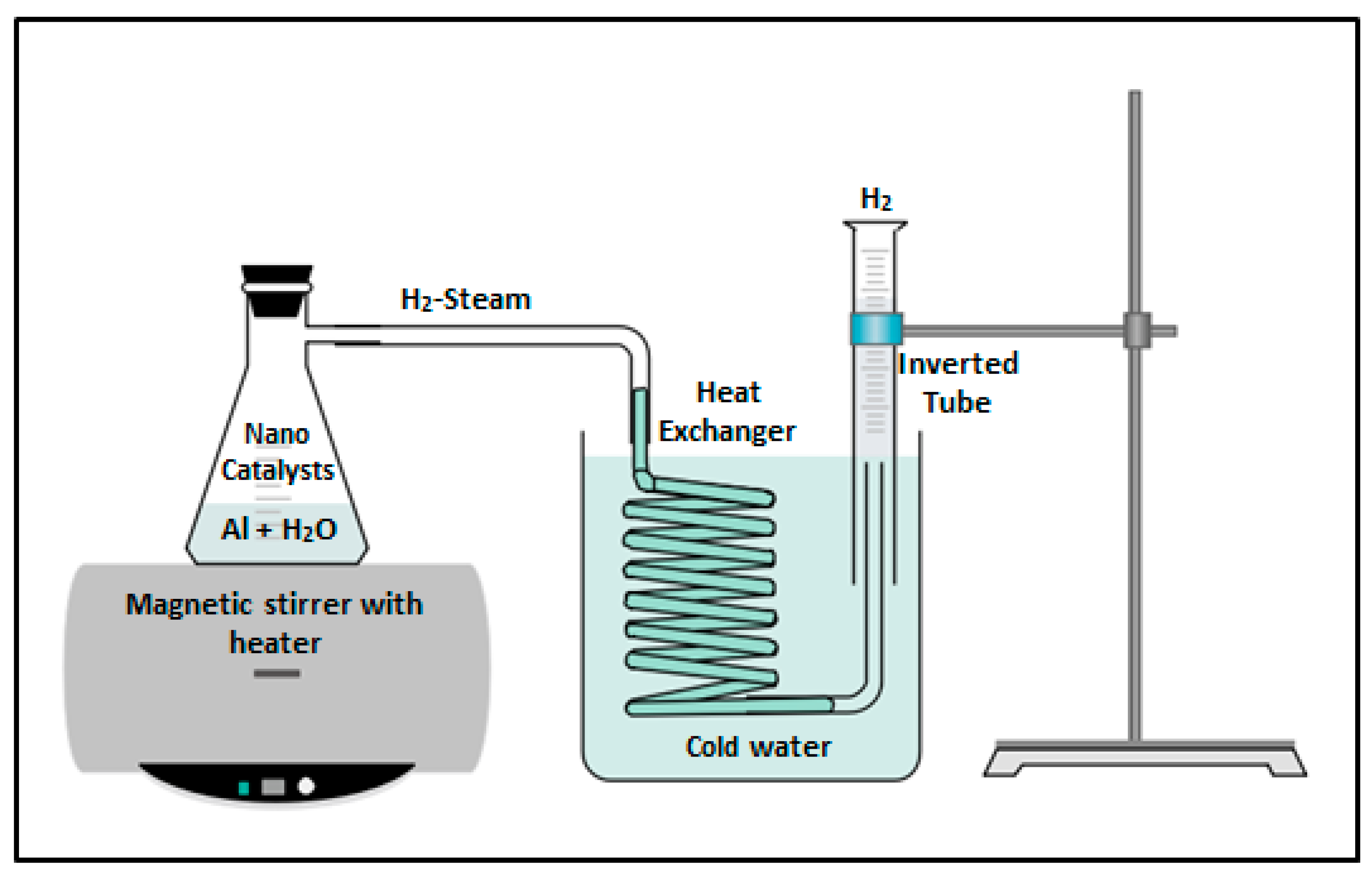
2.6. Catalytic Experiments
3. Characterization and Measurement Tools
4. Results and Discussions
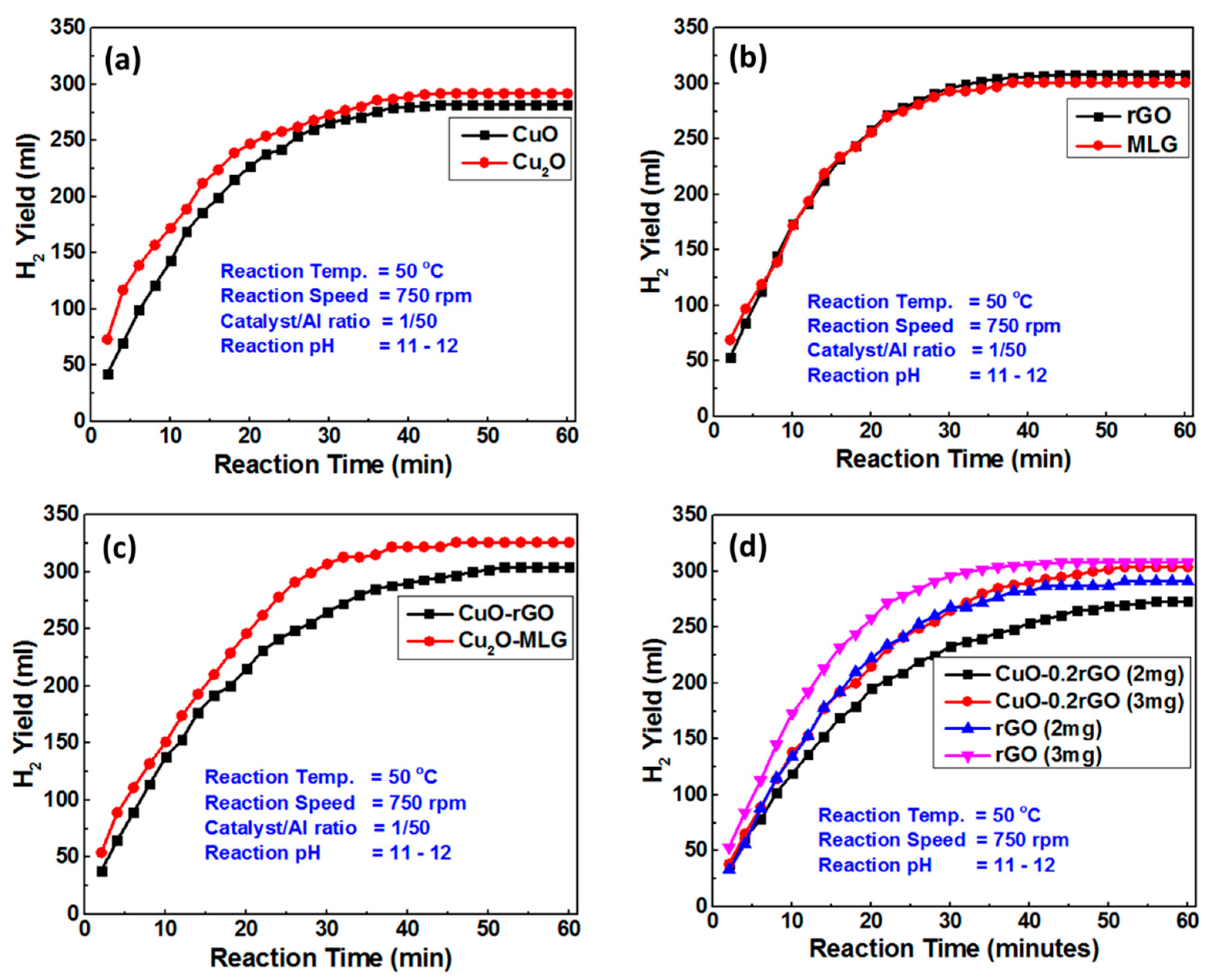
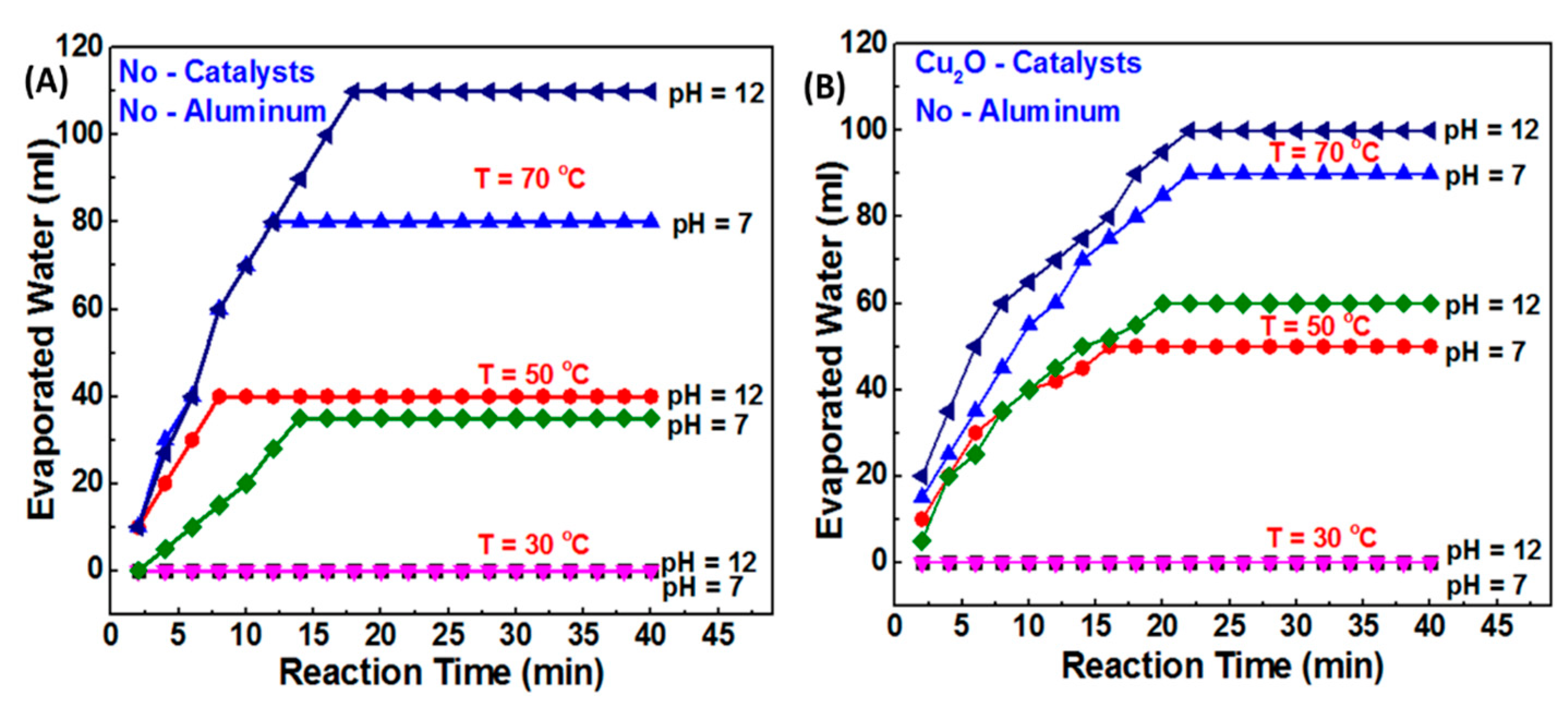
4.1. Hydrogen Production
4.2. Economic Feasibility Study
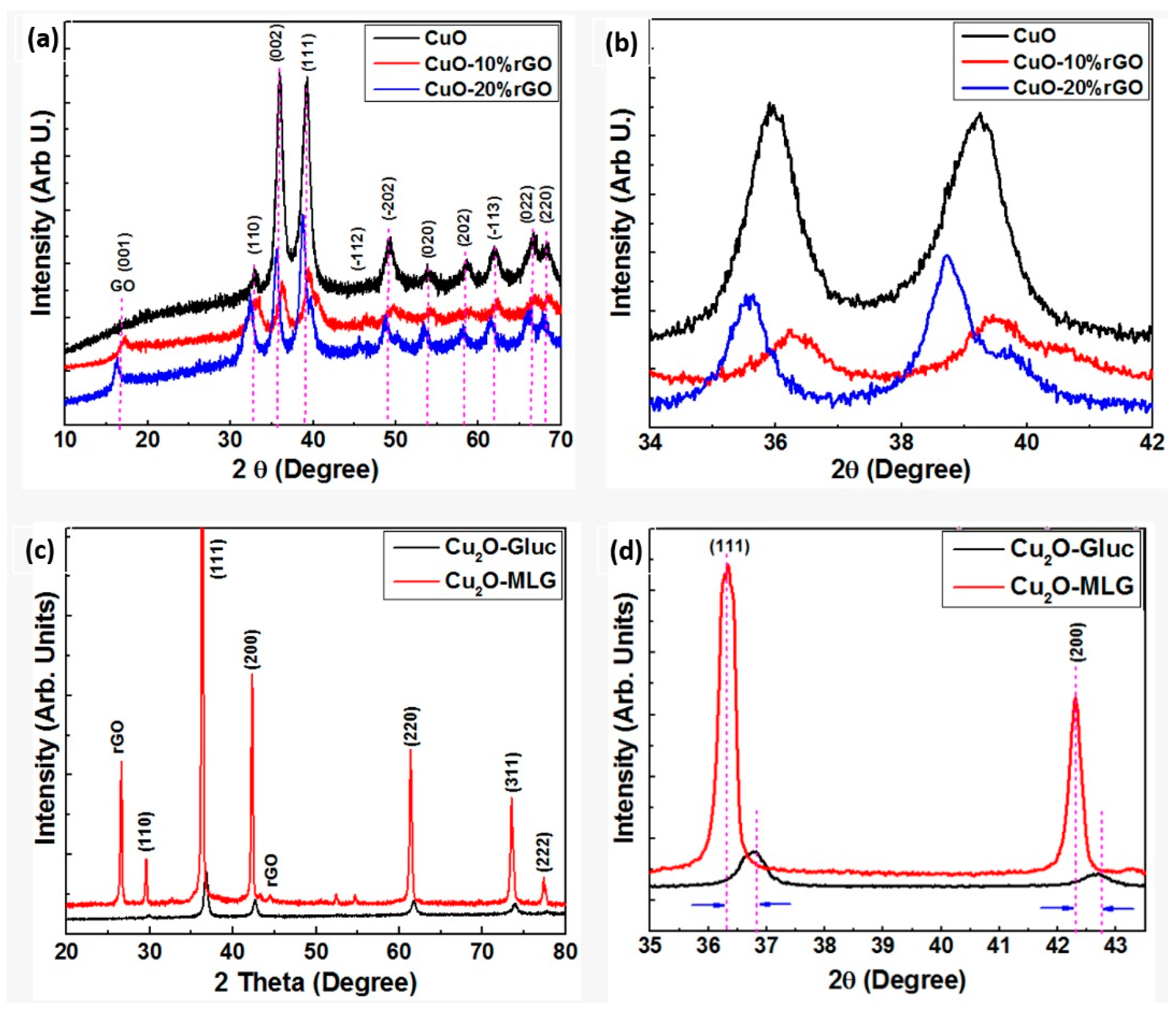
4.3. The Phase Structure and Surface Properties of Nanocomposites
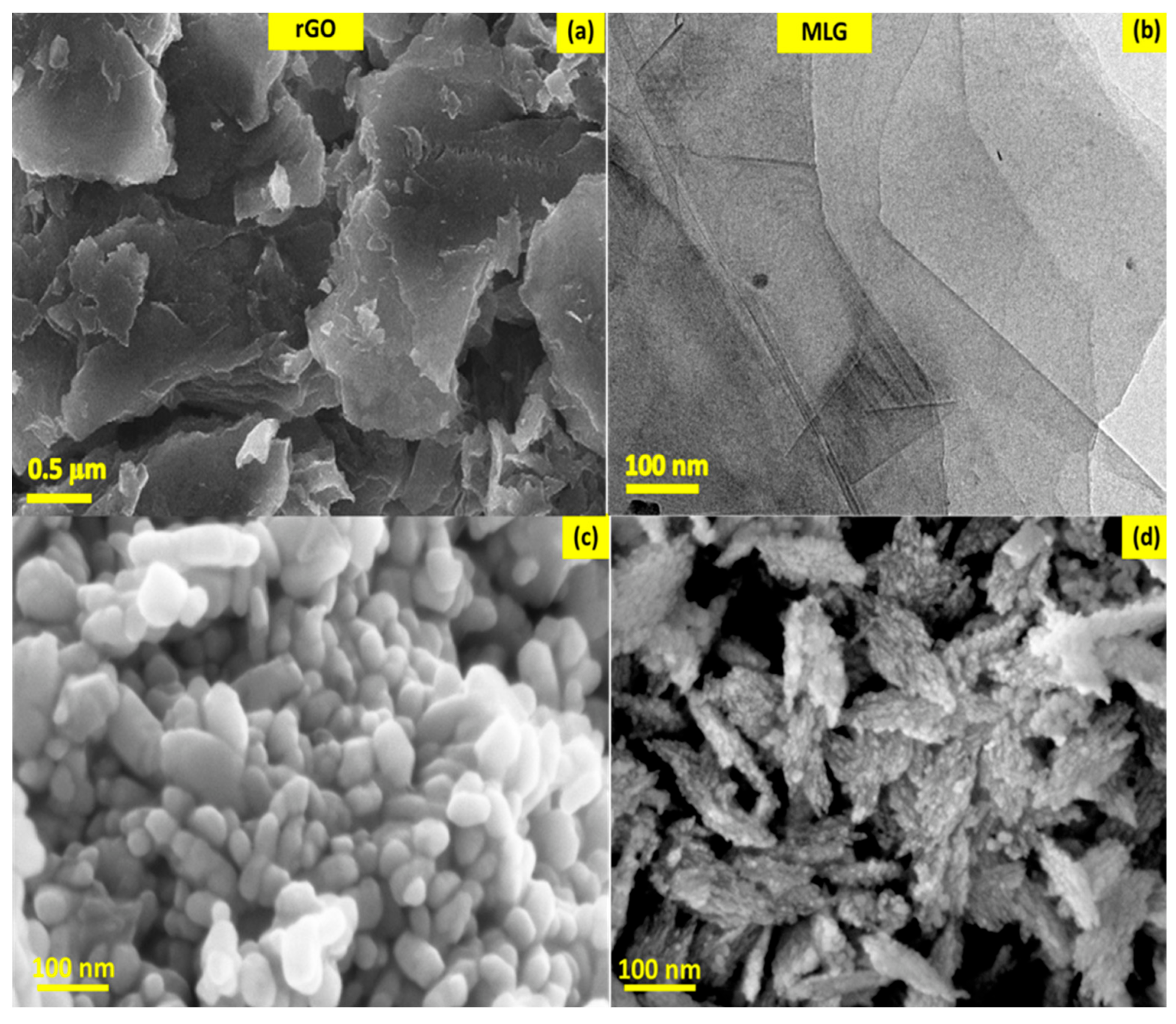
4.4. Morphology of the Nanocatalysts
5. Conclusions
- (1)
- For the first time, the present study investigated the critical design considerations that are important for performing the H2 elaboration efficiently, and recommended the optimum conditions (temperatures, pressure, and speed) for high yield and quality of H2 gas.
- (2)
- The present work introduces a technology to indirectly measure the amount of water vapor in the produced H2.
- (3)
- The current study proposes a feasible method for separating the H2–steam mixture before storage by using a water basin to condense the evaporated water molecules.
- (4)
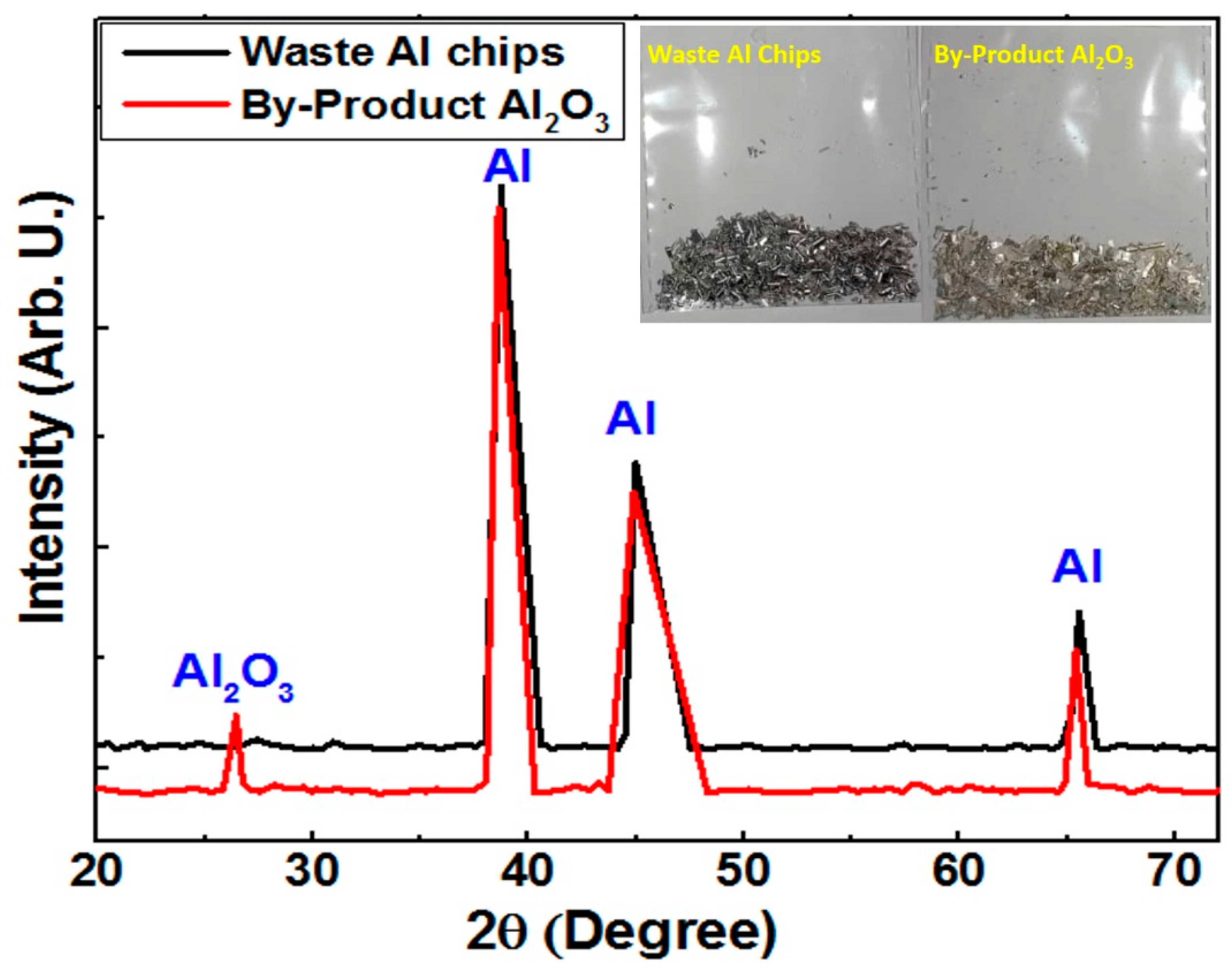
- For the first time, the phase structure of waste aluminum chips and the by-product of Al2O3 are investigated using the XRD technique and color variations.
- (5)
- An economic feasibility study was performed for a small town, Taiz, in Yemen, with a daily collection of waste aluminum of up to 432 kg/day.
- (6)
- The cost of H2 produced by this method is competitive with water-splitting technologies, and the reaction was completed in 20 min. Furthermore, an increase in the production capacity can steeply reduce the production cost of H2 gas.
- (a)
- Images from electron microscopes showed how CuO and Cu2O NPs were well-distributed on the graphene sheet, while the XRD and Raman spectroscopy analysis proved their phase structure.
- (b)
- The surface functionality of the particles represents a constant source for destroying the aluminum laminar oxide layer, without needing an external basic/acidic medium.
- (c)
- A comparison of rGO and MLG indicated prompt catalytic activity for the H2 production yield and kinetics.
- (d)
- A comparison of CuO and Cu2O NPs showed that both samples were good enough for the efficient and feasible production of H2, with better performance for Cu2O NPs than CuO NPs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, A.; Peu, S.D. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Iten, M.; Matos, H.A. Review of Thermochemical Technologies for Water and Energy Integration Systems: Energy Storage and Recovery. Sustainability 2022, 14, 7506. [Google Scholar] [CrossRef]
- Chau, K.; Djire, A.; Khan, F. Review and analysis of the hydrogen production technologies from a safety perspective. Int. J. Hydrogen Energy 2022, 47, 13990–14007. [Google Scholar] [CrossRef]
- Shi, R.; Chen, X.; Qin, J.; Wu, P.; Jia, L. The State-of-the-Art Progress on the Forms and Modes of Hydrogen and Ammonia Energy Utilization in Road Transportation. Sustainability 2022, 14, 11904. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Özcan, H. Comprehensive review on the techno-economics of sustainable large-scale clean hydrogen production. J. Clean. Prod. 2019, 220, 593–609. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Shapovalov, Y.; Tokpayev, R.; Khavaza, T.; Nauryzbayev, M. Generation of Hydrogen and Oxygen from Water by Solar Energy Conversion. Sustainability 2021, 13, 13941. [Google Scholar] [CrossRef]
- Shah, N.R.A.M.; Yunus, R.M.; Rosman, N.N.; Wong, W.Y.; Arifin, K.; Minggu, L.J. Current progress on 3D graphene-based photocatalysts: From synthesis to photocatalytic hydrogen production. Int. J. Hydrogen Energy 2021, 46, 9324–9340. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic water splitting hydrogen production via environmental benign carbon based nanomaterials. Int. J. Hydrogen Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Epelle, E.I.; Desongu, K.S.; Obande, W.; Adeleke, A.A.; Ikubanni, P.P.; Okolie, J.A.; Gunes, B. A comprehensive review of hydrogen production and storage: A focus on the role of nanomaterials. Int. J. Hydrogen Energy 2022, 47, 20398–20431. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Masudy-Panah, S.; Moakhar, R.S.; Chakrabortty, S.; Ghosh, S.; Kushwaha, A.; Katal, R.; Chua, C.S.; Xiao, G.; Tripathy, S.; et al. Nanoengineered Advanced Materials for Enabling Hydrogen Economy: Functionalized Graphene–Incorporated Cupric Oxide Catalyst for Efficient Solar Hydrogen Production. Glob. Chall. 2020, 4, 1900087. [Google Scholar] [CrossRef]
- Wang, H.; Leung, D.; Leung, M.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Kurbatova, A.I.; Vlaskin, M.S.; Valyano, G.E.; Grigorenko, A.V.; Ambaryan, G.N.; Dudoladov, A.O. Waste to Hydrogen: Elaboration of Hydroreactive Materials from Magnesium-Aluminum Scrap. Sustainability 2022, 14, 4496. [Google Scholar] [CrossRef]
- Trowell, K.; Goroshin, S.; Frost, D.; Bergthorson, J. Hydrogen production rates of aluminum reacting with varying densities of supercritical water. RSC Adv. 2022, 12, 12335–12343. [Google Scholar] [CrossRef]
- Kumar, D.; Muthukumar, K. An overview on activation of aluminium-water reaction for enhanced hydrogen production. J. Alloys Compd. 2020, 835, 155189. [Google Scholar] [CrossRef]
- Setiani, P.; Watanabe, N.; Sondari, R.R.; Tsuchiya, N. Mechanisms and kinetic model of hydrogen production in the hydrothermal treatment of waste aluminum. Mater. Renew. Sustain. Energy 2018, 7, 10. [Google Scholar] [CrossRef]
- Salueña-Berna, X.; Marín-Genescà, M.; Massagués Vidal, L.; Dagà-Monmany, J.M. Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials 2021, 14, 7323. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, R.; Orlando, A.; Borrini, D.; Tassi, F.; Bicocchi, G.; Renzulli, A. Hydrogen-Rich Gas Produced by the Chemical Neutralization of Reactive By-Products from the Screening Processes of the Secondary Aluminum Industry. Sustainability 2021, 13, 12261. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Huang, C.-H. Enhancement of hydrogen generation using waste aluminum cans hydrolysis in low alkaline de-ionized water. Int. J. Hydrogen Energy 2016, 41, 3741–3747. [Google Scholar] [CrossRef]
- Aylikci, N.K.; Mert, S.O.; Aylikci, V.; Bahceci, E.; Depci, T.; Oruç, Ö. Microhydrogen production with water splitting from daily used waste aluminum. Int. J. Hydrogen Energy 2021, 46, 28912–28924. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, M.; Mousa, M.; Hussein, A.; Hammouti, B.; Hadda, T.B.; Warad, I. Copper(II)-Oxide Nanostructures: Synthesis, Characterizations and their Applications–Review. J. Mater. Environ. Sci. 2013, 4, 792–797. [Google Scholar]
- Bhanushali, S.; Ghosh, P.; Ganesh, A.; Cheng, W. 1D Copper Nanostructures: Progress, Challenges and Opportunities. Small 2014, 11, 1232–1252. [Google Scholar] [CrossRef]
- Cuong, H.N.; Pansambal, S.; Ghotekar, S.; Oza, R.; Hai, N.T.T.; Viet, N.M.; Nguyen, V.-H. New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: A review. Environ. Res. 2021, 203, 111858. [Google Scholar] [CrossRef]
- Yam, K.; Guo, N.; Jiang, Z.; Li, S.; Zhang, C. Graphene-Based Heterogeneous Catalysis: Role of Graphene. Catalysts 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Yao, Z.; Wang, X. Graphene-Based Nanomaterials for Catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Jain, V.; Kandasubramanian, B. Functionalized graphene materials for hydrogen storage. J. Mater. Sci. 2020, 55, 1865–1903. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; He, Y.; Huang, H.; Wu, Y.; Hou, L.; Liu, X.; Yang, T.; Zou, J.; Huang, B. Synthesis, growth mechanism and thermal stability of copper nanoparticles encapsulated by multi-layer graphene. Carbon 2012, 50, 2119–2125. [Google Scholar] [CrossRef]
- Hidalgo-Manrique, P.; Lei, X.; Xu, R.; Zhou, M.; Kinloch, I.A.; Young, R.J. Copper/graphene composites: A review. J. Mater. Sci. 2019, 54, 12236–12289. [Google Scholar] [CrossRef] [Green Version]
- Sehar, S.; Sher, F.; Zhang, S.; Khalid, U.; Sulejmanović, J.; Lima, E.C. Thermodynamic and kinetic study of synthesised graphene oxide-CuO nanocomposites: A way forward to fuel additive and photocatalytic potentials. J. Mol. Liq. 2020, 313, 113494. [Google Scholar] [CrossRef]
- Mondal, P.; Sinha, A.; Salam, N.; Roy, A.S.; Jana, N.R.; Islam, S.M. Enhanced catalytic performance by copper nanoparticle–graphene based composite. RSC Adv. 2013, 3, 5615–5623. [Google Scholar] [CrossRef]
- Xin, S.; Shen, J.; Liu, G.; Chen, Q.; Xiao, Z.; Zhang, G.; Xin, Y. High electricity generation and COD removal from cattle wastewater in microbial fuel cells with 3D air cathode employed non-precious Cu2O/reduced graphene oxide as cathode catalyst. Energy 2020, 196, 117123. [Google Scholar] [CrossRef]
- Xin, S.; Shen, J.; Liu, G.; Chen, Q.; Xiao, Z.; Zhang, G.; Xin, Y. Electricity generation and microbial community of single-chamber microbial fuel cells in response to Cu2O nanoparticles/reduced graphene oxide as cathode catalyst. Chem. Eng. J. 2020, 380, 122446. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Tong, J.; Liu, Y.; Xu, S.; Cao, Y.; Cao, S. Photocatalytic CO2 conversion to methanol by Cu2O/graphene/TNA heterostructure catalyst in a visible-light-driven dual-chamber reactor. Nano Energy 2016, 27, 320–329. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, H.; Lu, S.; Yang, Z.; Bin Xu, B.; Xing, L.; Liu, T.X. Cu2O nano-flowers/graphene enabled scaffolding structure catalyst layer for enhanced CO2 electrochemical reduction. Appl. Catal. B Environ. 2022, 305, 121022. [Google Scholar] [CrossRef]
- Moghanlou, A.O.; Bezaatpour, A.; Sadr, M.H.; Yosefi, M.; Salimi, F. Cu2O/rGO as an efficient photocatalyst for transferring of nitro group to amine group under visible light irradiation. Mater. Sci. Semicond. Process. 2021, 130, 105838. [Google Scholar] [CrossRef]
- Ali, M.; Remalli, N.; Yehya, F.; Chaudhary, A.K.; Srikanth, V.V. Picosecond laser induced fragmentation of coarse Cu2O particles into nanoparticles in liquid media. Appl. Surf. Sci. 2015, 357, 1601–1605. [Google Scholar] [CrossRef]
- Ali, M.; Rotte, N.K.; Srikanth, V.V. Honey aided solution synthesis of polycrystalline Cu2O particles. Mater. Lett. 2014, 128, 253–255. [Google Scholar] [CrossRef]
- Amrani, A.M.; Srikanth, V.V.S.S.; Labhsetwar, N.K.; Al-Fatesh, A.S.; Shaikh, H. Phoenix dactylifera F. mediated green synthesis of Cu2O particles for Arsenite uptake from water. Sci. Technol. Adv. Mater. 2016, 17, 760–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Yehya, F.; Chaudhary, A.K.; Srikanth, V.V.S.S. A novel top-down reduction approach for Cu2O nanoparticles using nanosecond and picosecond lasers. AIP Conf. Proc. 2014, 1620, 327–330. [Google Scholar]
- Amrani, M.A.; Alrafai, H.A.; Al-Nami, S.Y.; Obeidat, F.; Alwahbani, F.; Alhammadi, M.A.; Qasem, A. Green synthesis of Size-Controlled copper oxide nanoparticles as catalysts for H2 production from industrial waste aluminum. Int. J. Energy Res. 2022, 46, 14023–14035. [Google Scholar] [CrossRef]
- Amrani, M.A.; Bagash, M.; Yehya, F. An Efficient Green Synthesis Method for the Synthesis of Silver Nanoparticles Using the Extraction of Catha Edulis Leaves. Albaydha Univ. J. 2019, 1, 214–223. [Google Scholar] [CrossRef]
- Jammula, R.K.; Tumuluri, A.; Rotte, N.K.; James Raju, K.C.; Srikanth, V.V.S.S. Cupric oxide decked few-layered graphene: Synthesis and dielectric. Carbon 2014, 78, 374–383. [Google Scholar] [CrossRef]
- Ali, M.; Abu-Taleb, A.; Remalli, N.; Abdullah, M.; Vadali, S.V.S.S.; Labhsetwar, N. Dragon’s blood aided synthesis of Ag/Ag2O core/shell nanostructures and Ag/Ag2O decked multi-layered graphene for efficient As(III) uptake from water and antibacterial activity. RSC Adv. 2016, 6, 44145–44153. [Google Scholar]
- Bolt, A.; Dincer, I.; Agelin-Chaab, M. A Review of Unique Aluminum–Water Based Hydrogen Production Options. Energy Fuels 2021, 35, 1024–1040. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lyu, L.-M.; Yang, Y.-C.; Huang, M.H. Synthesis of Cu2O Nanocrystals from Cubic to Rhombic Dodecahedral Structures and Their Comparative Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef]
- Ho, J.-Y.; Huang, M.H. Synthesis of Submicrometer-Sized Cu2O Crystals with Morphological Evolution from Cubic to Hexapod Structures and Their Comparative Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 14159–14164. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, B.; Zhang, T.; Gao, D.; Xu, A.-W. Shape Effects of Cu2O Polyhedral Microcrystals on Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 5073–5079. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2: H Embedding and Kinetic Effects in the Formation of Suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef]
- Yamukyan, M.; Manukyan, K.; Kharatyan, S. Copper oxide reduction by hydrogen under the self-propagation reaction mode. J. Alloy. Compd. 2009, 473, 546–549. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis, and Design of Chemical Process; Pearson Education: London, UK, 2012. [Google Scholar]
- Dinesh, G.K.; Chauhan, R.; Chakma, S. Influence and strategies for enhanced biohydrogen production from food waste. Renew. Sustain. Energy Rev. 2018, 92, 807–822. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef]
- Han, W.; Hu, Y.Y.; Li, S.Y.; Li, F.F.; Tang, J.H. Biohydrogen production from waste bread in a continuous stirred tank reactor: A techno-economic analysis. Bioresour. Technol. 2016, 221, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Liu, Z.; Fang, J.; Huang, J.; Zhao, H.; Li, Y. Techno-economic analysis of dark fermentative hydrogen production from molasses in a continuous mixed immobilized sludge reactor. J. Clean. Prod. 2016, 127, 567–572. [Google Scholar] [CrossRef]
- Han, W.; Yan, Y.; Gu, J.; Shi, Y.; Tang, J.; Li, Y. Techno-economic analysis of a novel bioprocess combining solid state fermentation and dark fermentation for H2 production from food waste. Int. J. Hydrogen Energy 2016, 41, 22619–22625. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-C.; Liu, Y.-F.; Chu, C.-Y.; Chang, P.-L.; Hsu, C.-W.; Lin, P.-J.; Wu, S.-Y. Techno-economic evaluation of biohydrogen production from wastewater and agricultural waste. Int. J. Hydrogen Energy 2012, 37, 15704–15710. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.; Roopmani, P.; Gumpu, M.B.; Krishnan, U.M.; Sastikumar, D. Synthesis, characterization and bioimaging application of laser-ablated graphene-oxide nanoparticles (nGOs). Diam. Relat. Mater. 2020, 104, 107733. [Google Scholar] [CrossRef]
- Ganesan, K.; Jothi, V.K.; Natarajan, A.; Rajaram, A.; Ravichandran, S.; Ramalingam, S. Green synthesis of Copper oxide nanoparticles decorated with graphene oxide for anticancer activity and catalytic applications. Arab. J. Chem. 2020, 13, 6802–6814. [Google Scholar] [CrossRef]
- Kottappara, R.; Palantavida, S.; Pillai, S.C.; Vijayan, B.K. Composition tuning in copper-oxide decorated reduced graphene oxide yields efficient photo- and reduction catalysts. Surf. Interfaces 2020, 22, 100792. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Z.; Zhang, H.; Cao, G.; Sun, Q.; Cao, J. CuO Nanorods-Decorated Reduced Graphene Oxide Nanocatalysts for Catalytic Oxidation of CO. Catalysts 2016, 6, 214. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, X.-L.; Shu, C.-Y.; Guo, Y.-G.; Wang, C.-R. Synthesis of CuO/graphene nanocomposite as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2010, 20, 10661–10664. [Google Scholar] [CrossRef]
- Bu, I.Y.Y.; Huang, R. Fabrication of CuO-decorated reduced graphene oxide nanosheets for supercapacitor applications. Ceram. Int. 2017, 34, 45–50. [Google Scholar] [CrossRef]
- Solorzano-Ojeda, E.D.; Sánchez-Valdes, S.; Ramos-Devalle, L.F.; Betancourt-Galindo, R.; da Silva, L.; Fernández-Tavizón, S.; Hernández-Gámez, J.F.; Pérez-Camacho, O.; Ramírez-Vargas, E.; Morales-Acosta, D.; et al. Effect of ionic liquid on graphene decorated with copper nanostructure dispersion towards silicon/graphene/copper composites with enhanced thermal, electrical and antimicrobial properties. Iran. Polym. J. 2021, 30, 1339–1349. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Skakalova, V.; Kaiser, A.B. Graphene: Properties, Preparation, Characterization and Devices, 2nd ed.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. J. Carbon Res. C 2015, 1, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Hunge, Y.; Kang, S.-W. Spongy ball-like copper oxide nanostructure modified by reduced graphene oxide for enhanced photocatalytic hydrogen production. Mater. Res. Bull. 2020, 133, 111026. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Huang, H.; Chen, T.; Zhang, M.; Wang, Y. Synthesis and photocatalytic properties of CuO nanostructures. J. Nanosci. Nanotechnol. 2014, 14, 3428–3432. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Ji, L.; Chen, Z. Preparation of nanostructured Cu(OH)2 and CuO electrocatalysts for water oxidation by electrophoresis deposition. J. Mater. Res. 2018, 33, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Song, X.; Song, Q.; Yin, Z. A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. CrystEngComm 2012, 14, 6710–6719. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, P.; Zhuang, H.; Jiao, Z.; Fang, T.; Xu, W.; Lu, B.; Jiang, Y. Hierarchical self-assembly of microscale leaf-like CuO on graphene sheets for high-performance electrochemical capacitors. J. Mater. Chem. A 2013, 1, 367–373. [Google Scholar] [CrossRef]
- Gulati, U.; Rajesh, U.C.; Rawat, D.S. RGO@CuO Nanocomposites from a Renewable Copper Mineral Precursor: A Green Approach for Decarboxylative C(sp3)-H Activation of Proli ne Amino Acid To Afford Value-Added Synthons. ACS Sustain. Chem. Eng. 2018, 6, 10039–10051. [Google Scholar] [CrossRef]
- Yang, S.T.; Chang, Y.; Wang, H.; Liu, G.; Chen, S.; Wang, Y.; Liu, Y.; Cao, A. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J. Colloid Interface Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef]






| Raw Material | Cost (USD/kg) | Quantity (Kg/Cycle) | Cost (USD/Cycle) | Cost (USD/Cycle) | Cycle Time (h) |
|---|---|---|---|---|---|
| Aluminum (Chips) | 0.5 | 20 | 10 | ---- | 1 h |
| Aluminum (Blocks) | 1.0 | 20 | ---- | 20 | |
| Al2O3 byproduct | 0.5 | 30 | −15 | −15 | |
| Sodium hydroxide | 2 | 2.5 | 5 | 5 | |
| Copper chloride | 5 | 1 | 5 | 5 | |
| Distilled water | 0.01 | 100 | 1 | 1 | |
| Total cost (USD/cycle) | ----- | ---- | 06 | 16 |
| Item | Data | Waste Aluminum Chips | Waste Aluminum Blocks | Note |
|---|---|---|---|---|
| Cost | Cost | |||
| Capital | Total fixed cost | USD 120,000 | USD 150,000 | Depreciation period: 10 years |
| Operation | Raw material Utilities * Labor cost | USD 42,000/y USD 12,000/y USD 39,200/y ** | USD 112,000/y USD 18,000/y USD 39,200/y | Year = 360 days * Electricity ** 2800 (USD/employer year) (14 employers, 2 batches) |
| Interest Maintenance Other | 10% of capital cost 5% of utilities 1% of capital cost | USD 12,000/y USD 1515/y USD 1200/y | USD 15,000/y USD 2000/y USD 1500/y | |
| H2 product cost | USD 108/day (USD 0.000516 /liter) | USD 521.4/day (USD 0.0009 /liter) | ||
| Feedstock | Cost of H2 Production | Reference |
|---|---|---|
| Food waste | 3.20 USD/kg | [57] |
| Food waste | 11.35 USD/kg * | [58] |
| Food waste | 14.91 USD/kg * | [59] |
| Molasses | 30.04 USD/kg * | [60] |
| Food waste | 25.48 USD/kg * | [61] |
| Wastewater | 30.04 USD/kg * | [62] |
| Agricultural waste | 30.04 USD/kg * | [62] |
| Aluminum waste (chips) | 6.71 USD/kg | This study |
| Aluminum waste (blocks) | 11.66 USD/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrani, M.A.; Haddad, Y.; Obeidat, F.; Ghaleb, A.M.; Mejjaouli, S.; Rahoma, I.; Galil, M.S.A.; Shameeri, M.; Alsofi, A.A.; Saif, A. Productive and Sustainable H2 Production from Waste Aluminum Using Copper Oxides-Based Graphene Nanocatalysts: A Techno-Economic Analysis. Sustainability 2022, 14, 15256. https://doi.org/10.3390/su142215256
Amrani MA, Haddad Y, Obeidat F, Ghaleb AM, Mejjaouli S, Rahoma I, Galil MSA, Shameeri M, Alsofi AA, Saif A. Productive and Sustainable H2 Production from Waste Aluminum Using Copper Oxides-Based Graphene Nanocatalysts: A Techno-Economic Analysis. Sustainability. 2022; 14(22):15256. https://doi.org/10.3390/su142215256
Chicago/Turabian StyleAmrani, Mokhtar Ali, Yara Haddad, Firas Obeidat, Atef M. Ghaleb, Sobhi Mejjaouli, Ibrahim Rahoma, Mansour S. A. Galil, Mutahar Shameeri, Ahmed A. Alsofi, and Amin Saif. 2022. "Productive and Sustainable H2 Production from Waste Aluminum Using Copper Oxides-Based Graphene Nanocatalysts: A Techno-Economic Analysis" Sustainability 14, no. 22: 15256. https://doi.org/10.3390/su142215256







