Sustainable and Health-Protecting Food Ingredients from Bioprocessed Food by-Products and Wastes
Abstract
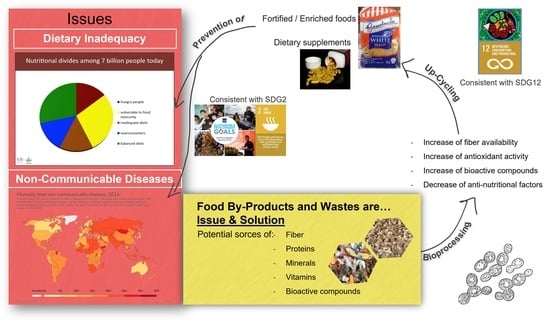
1. Introduction
2. Amounts, Costs, and Environmental Impacts of Disposal
2.1. FW Quantification at European Union (EU) Scale
2.2. FW Economic Assessment and Costs
2.3. FBPW Environmental Aspects
2.4. Social Issues
3. Microorganisms for Bioprocessing Food by-Products and Wastes
3.1. Involvement of Yeasts during FBPW Valorization
3.1.1. Dietary Fiber
3.1.2. Enzymes
3.1.3. Pigments
3.1.4. Other Bioactive Compounds
3.2. Involvement of Molds in FBPW Management
3.3. Involvement of Lactic Acid Bacteria in FBPW Management
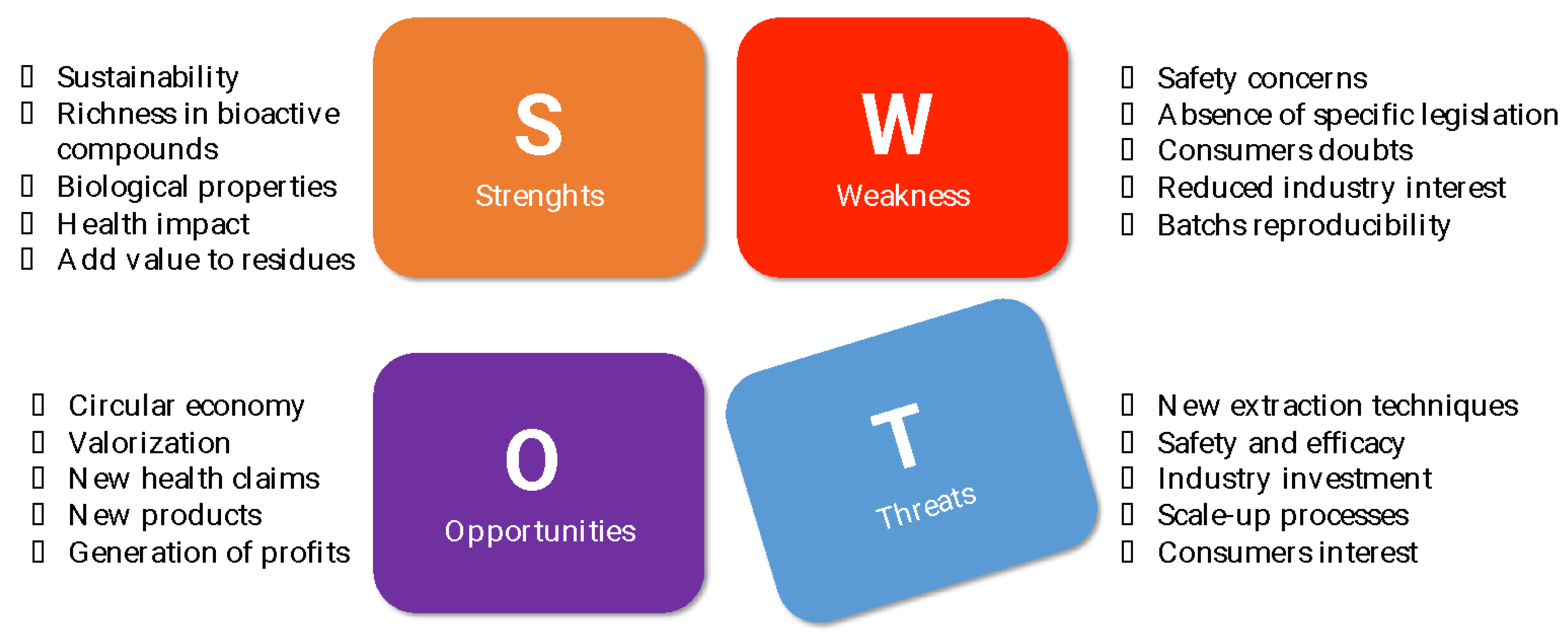
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popkin, B.M. Relationship between shifts in food system dynamics and acceleration of the global nutrition transition. Nutr. Rev. 2017, 75, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fanzo, J. Healthy and Sustainable Diets and Food Systems: The Key to Achieving Sustainable Development Goal 2? Food Ethics 2019, 4, 159–174. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W., Jr.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Shafiee, S.I.; Omar, N.; Ibrahim, Z. Prevalence and Factors Associated with Geriatric Malnutrition in Healthcare Institutions: A Systematic Review. Malays. J. Med. Health Sci. 2022, 18, 140–149. [Google Scholar]
- Mudryj, A.N.; Waugh, A.K.; Slater, J.J.; Duerksen, D.R.; Bernstein, C.N.; Riediger, N.D. Nutritional implications of dietary gluten avoidance among Canadians: Results from the 2015 Canadian Community Health Survey. Br. J. Nutr. 2021, 126, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, M.; Shalini, T.; Reddy, P.Y.; Seshacharyulu, M.; Madhavi, G.; Kumar, B.N.; Reddy, G.B. Prevalence of vitamin deficiencies in an apparently healthy urban adult population: Assessed by subclinical status and dietary intakes. Nutrition 2019, 63–64, 106–113. [Google Scholar] [CrossRef]
- O’Connell, M.L.; Coppinger, T.; Lacey, S.; Arsenic, T.; McCarthy, A.L. The nutritional status and dietary intake of free-living seniors: A cross-sectional study. Clin. Nutr. ESPEN 2021, 43, 478–486. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. 11–Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products; Academic Press: Cambridge, MA, USA, 2017; pp. 267–292. [Google Scholar]
- Pinto, D.; Braga, N.; Silva, A.M.; Delereu-Matos, C.; Rodrigues, F. Chestnut. In Valorization of Fruit Processing By-Products; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 127–144. [Google Scholar]
- Rodrigues, F.; Nunes, M.A.d.M.; Oliveira, M.B.P.P. Chapter 12—Applications of Recovered Bioactive Compounds in Cosmetics and Health Care Products In Olive Mill Waste; Academic Press: Cambridge, MA, USA, 2017; pp. 255–274. [Google Scholar]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Bioactivity, phytochemical profile and pro-healthy properties of Actinidia arguta: A review. Food Res. Int. 2020, 136, 109449. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Frühbeck, G.; De Angelis, M.; de Bari, O.; Wang, D.Q.; Lammert, F.; Portincasa, P. The Role of Diet in the Pathogenesis of Cholesterol Gallstones. Curr. Med. Chem. 2019, 26, 3620–3638. [Google Scholar] [CrossRef]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef]
- Eriksson, M.; Persson Osowski, C.; Björkman, J.; Hansson, E.; Malefors, C.; Eriksson, E.; Ghosh, R. Challenge application in cosmetic industry. Resour. Conserv. Recycl. 2018, 130, 140–151. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: Global Food Losses and Food Waste—Extent, Causes and Prevention–FAO, 2011. In Biotechnology; FAO: Rome, Italy, 2013; p. 70. [Google Scholar]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef] [PubMed]
- FAO. SAVE FOOD: Global Initiative on Food Loss and Waste Reduction—Definitional Framework of Food Loss. In Food And Agriculture Organization Of The United Nations; FAO: Rome, Italy, 2014. [Google Scholar]
- Food and Agriculture Organization of the United States. The State of Food and Agriculture 2019; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of food waste per product group along the food supply chain in the European Union: A mass flow analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef]
- Corrado, S.; Sala, S. Food waste accounting along global and European food supply chains: State of the art and outlook. Waste Manag. 2018, 79, 120–131. [Google Scholar] [CrossRef]
- Perito, M.A.; Di Fonzo, A.; Sansone, M.; Russo, C. Consumer acceptance of food obtained from olive by-products. Br. Food J. 2020, 122, 212–226. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- European Union. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 On Waste and Repealing Certain Directives. Off. J. Eur. Union 2008, 312, 3–30. [Google Scholar]
- Patinha Caldeira, C.; Corrado, S.; Sala, S. Food waste accounting—Methodologies, challenges and opportunities. Publ. Off. Eur. Union 2017, 20, 93–100. [Google Scholar]
- Lipinski, B.; Hanson, C.; Waite, R.; Searchinger, T.; Lomax, J. Reducing Food Loss and Waste; World Resources Institute: Washington DC, USA, 2013. [Google Scholar]
- Östergren, K.; Gustavsson, J.; Bos-Brouwers, H.; Timmermans, T.; Hansen, O.; Møller, H.; Anderson, G.; O’Connor, C.; Soethoudt, H.; Quested, T.; et al. FUSIONS Definitional Framework for Food Waste. In FUSIONS Coordinators: Toine Timmermans; Timmermans, T., Bos-Brouwers, H., Eds.; European Commission (FP7), Coordination and Support Action—CSA: Wageningen UR, The Netherlands, 2014. [Google Scholar]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. In Communication from the Commission to the European Parliament, the Council, the European Economic And Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, inefficiencies and waste in the global food system. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Monier, V. Preparatory Study on Food Waste across EU 27; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Bräutigam, K.-R.; Jörissen, J.; Priefer, C. The extent of food waste generation across EU-27: Different calculation methods and the reliability of their results. Waste Manag. Res. 2014, 32, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Holsteijn, F.V.; Kemna, R. Minimizing food waste by improving storage conditions in household refrigeration. Resour. Conserv. Recycl. 2018, 128, 25–31. [Google Scholar] [CrossRef]
- Segrè, A.F.L.; Politano, A.; Vittuari, M. Background Paper on the Economics of Food Loss and Waste 2014; FAO: Rome, Italy, 2014. [Google Scholar]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef]
- Otles, S.; Despoudi, S.; Bucatariu, C.; Kartal, C. Chapter 1—Food waste management, valorization, and sustainability in the food industry. In Food Waste Recovery; Galanakis, C.M., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 3–23. [Google Scholar]
- Pfaltzgraff, L.A.; De bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Lundqvist, J.d.F.C.; Molden, D. Saving Water: From Field to Fork Curbing Losses and Wastage in the Food Chain; Food and Agriculture Organization of the United Nations: Stockholm, Sweden, 2008; p. 67. [Google Scholar]
- Aiello, G.; Enea, M.; Muriana, C. Economic benefits from food recovery at the retail stage: An application to Italian food chains. Waste Manag. 2014, 34, 1306–1316. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Campoy-Muñoz, P.; Cardenete, M.A.; Delgado, M.C. Economic impact assessment of food waste reduction on European countries through social accounting matrices. Resour. Conserv. Recycl. 2017, 122, 202–209. [Google Scholar] [CrossRef]
- Stenmarck, A.J.C.; Quested, T.; Moates, G. FUSIONS: Estimates of European food waste levels—Reducing Food Waste Through Social Innovation; Fusions: Stockholm, Sweden, 2016. [Google Scholar]
- Koester, U. Food Loss and Waste as an Economic and Policy Problem. In World Agricultural Resources and Food Security; Emerald Publishing: Bingley, UK, 2017; Volume 17, pp. 275–288. [Google Scholar]
- Anriquez, G.; Foster, W.; Ortega, J.; Santos Rocha, J. In search of economically significant food losses: Evidence from Tunisia and Egypt. Food Policy 2021, 98, 101912. [Google Scholar] [CrossRef]
- Chegere, M.J. Post-harvest losses reduction by small-scale maize farmers: The role of handling practices. Food Policy 2018, 77, 103–115. [Google Scholar] [CrossRef]
- Galanakis, C.M. Chapter 3—The universal recovery strategy. In Food Waste Recovery, 2nd ed.; Galanakis, C.M., Ed.; Academic Press: San Diego, CA, USA, 2021; pp. 51–68. [Google Scholar]
- Vanham, D.; Bouraoui, F.; Leip, A.; Grizzetti, G.; Bidoglio, G. Lost water and nitrogen resources due to EU consumer food waste. Environ. Res. Lett. 2015, 10, 084008. [Google Scholar] [CrossRef]
- Beretta, C.; Stoessel, F.; Baier, U.; Hellweg, S. Quantifying food losses and the potential for reduction in Switzerland. Waste Manag. 2013, 33, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.D.; Reay, D.S.; Higgins, P.; Bomberg, E. A half-century of production-phase greenhouse gas emissions from food loss & waste in the global food supply chain. Sci. Total Environ. 2016, 571, 721–729. [Google Scholar]
- Kummu, M.; de Moel, H.; Porkka, M.; Siebert, S.; Varis, O.; Ward, P.J. Lost food, wasted resources: Global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci. Total Environ. 2012, 438, 477–489. [Google Scholar] [CrossRef]
- FAO. Food Wastage Footprint: Impacts on Natural Resources: Summary Report; FAO: Rome, Italy, 2013. [Google Scholar]
- Notarnicola, B.; Sala, S.; Anton, A.; McLaren, S.J.; Saouter, E.; Sonesson, U. The role of life cycle assessment in supporting sustainable agri-food systems: A review of the challenges. J. Clean. Prod. 2017, 140, 399–409. [Google Scholar] [CrossRef]
- Piras, S.; Pancotto, F.; Righi, S.; Vittuari, M.; Setti, M. Community social capital and status: The social dilemma of food waste. Ecol. Econ. 2021, 183, 106954. [Google Scholar] [CrossRef]
- Schanes, K.; Dobernig, K.; Gözet, B. Food waste matters—A systematic review of household food waste practices and their policy implications. J. Clean. Prod. 2018, 182, 978–991. [Google Scholar] [CrossRef]
- Mondéjar-Jiménez, J.-A.; Ferrari, G.; Secondi, L.; Principato, L. From the table to waste: An exploratory study on behaviour towards food waste of Spanish and Italian youths. J. Clean. Prod. 2016, 138, 8–18. [Google Scholar] [CrossRef]
- Hebrok, M.; Boks, C. Household food waste: Drivers and potential intervention points for design—An extensive review. J. Clean. Prod. 2017, 151, 380–392. [Google Scholar] [CrossRef]
- Carus, M.; Dammer, L. The Circular Bioeconomy—Concepts, Opportunities, and Limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- Grasso, S.; Asioli, D. Consumer preferences for upcycled ingredients: A case study with biscuits. Food Qual. Prefer. 2020, 84, 103951. [Google Scholar] [CrossRef]
- Choi, K.R.; Yu, H.E.; Lee, S.Y. Microbial food: Microorganisms repurposed for our food. Microb. Biotechnol. 2022, 15, 18–25. [Google Scholar] [CrossRef]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Siragusa, S.; De Angelis, M.; Paradiso, A.; Minervini, F.; De Gara, L.; Gobbetti, M. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows. Int. J. Food. Microbiol. 2008, 127, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.T.; Khong, N.M.H.; Lim, S.S.; Hee, Y.Y.; Sim, B.I.; Lau, K.Y.; Lai, O.M. A review: Modified agricultural by-products for the development and fortification of food products and nutraceuticals. Trends Food Sci. Technol. 2017, 59, 148–160. [Google Scholar] [CrossRef]
- Kaur, R.; Wani, S.P.; Singh, A.; Lal, K. Wastewater production, treatment and use in India. In Proceedings of the National Report Presented at the 2nd Regional Workshop on Safe Use of Wastewater in Agriculture 2012, New Delhi, India, 16–18 May 2012. [Google Scholar]
- Prabhu, A.A.; Gadela, R.; Bharali, B.; Deshavath, N.N.; Dasu, V.V. Development of high biomass and lipid yielding medium for newly isolated Rhodotorula mucilaginosa. Fuel 2019, 239, 874–885. [Google Scholar] [CrossRef]
- Lateef, A.; Oloke, J.K.; Gueguim Kana, E.B.; Oyeniyi, S.O.; Onifade, O.R.; Oyeleye, A.O.; Oladosu, O.C.; Oyelami, A.O. Improving the quality of agro-wastes by solid-state fermentation: Enhanced antioxidant activities and nutritional qualities. World J. Microbiol. Biotechnol. 2008, 24, 2369–2374. [Google Scholar] [CrossRef]
- Han, Z.; Park, A.; Su, W.W. Valorization of papaya fruit waste through low-cost fractionation and microbial conversion of both juice and seed lipids. RSC Adv. 2018, 8, 27963–27972. [Google Scholar] [CrossRef]
- Salgado, V.; Fonseca, C.; Lopes da Silva, T.; Roseiro, J.C.; Eusébio, A. Isolation and Identification of Magnusiomyces capitatus as a Lipase-Producing Yeast from Olive Mill Wastewater. Waste Biomass Val. 2020, 11, 3207–3221. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, A.; Singh, J.; Singh, N.; Singh, S.; Tomar, G.S.; Nain, P.K.S.; Khare, S.K.; Nain, L. Co-production of gamma amino butyric acid (GABA) and lactic acid using Lactobacillus plantarum LP-9 from agro-residues. Environ. Technol. Innov. 2021, 23, 101650. [Google Scholar] [CrossRef]
- Pontonio, E.; Dingeo, C.; Gobbetti, M.; Rizzello, C.G. Maize Milling By-Products: From Food Wastes to Functional Ingredients Through Lactic Acid Bacteria Fermentation. Front. Microbiol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of bioactive compounds in barley industry by-products during submerged and solid state fermentation with antimicrobial Pediococcus acidilactici strain LUHS29. Food Sci. Nutr. 2020, 8, 340–350. [Google Scholar] [CrossRef]
- Xie, C.; Coda, R.; Chamlagain, B.; Edelmann, M.; Deptula, P.; Varmanen, P.; Piironen, V.; Katina, K. In situ fortification of vitamin B12 in wheat flour and wheat bran by fermentation with Propionibacterium freudenreichii. J. Cereal Sci. 2018, 81, 133–139. [Google Scholar] [CrossRef]
- Pontonio, E.; Lorusso, A.; Gobbetti, M.; Rizzello, C.G. Use of fermented milling by-products as functional ingredient to develop a low-glycaemic index bread. J. Cereal Sci. 2017, 77, 235–242. [Google Scholar] [CrossRef]
- Katina, K.; Juvonen, R.; Laitila, A.; Flander, L.; Nordlund, E.; Kariluoto, S.; Piironen, V.; Poutanen, K. Fermented Wheat Bran as a Functional Ingredient in Baking. Cereal Chem. 2012, 89, 126–134. [Google Scholar] [CrossRef]
- Coda, R.; Kärki, I.; Nordlund, E.; Heiniö, R.L.; Poutanen, K.; Katina, K. Influence of particle size on bioprocess induced changes on technological functionality of wheat bran. Food Microbiol. 2014, 37, 69–77. [Google Scholar] [CrossRef]
- Kim, D.; Han, G.D. Ameliorating effects of fermented rice bran extract on oxidative stress induced by high glucose and hydrogen peroxide in 3T3-L1 adipocytes. Plant Foods Hum. Nutr. 2011, 66, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Jirasatid, S.; Limroongreungrat, K.; Nopharatana, M.; Monacolin, K. Pigments and citrinin of rice pasta by-products fermented by Monascus purpureus. Int. Food Res. J. 2019, 26, 1279–1284. [Google Scholar]
- Shin, H.-Y.; Kim, S.-M.; Lee, J.H.; Lim, S.-T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Papapostolou, H.; Dimitrellou, D.; Kopsahelis, N.; Katechaki, E.; Bekatorou, A.; Bosnea, L.A. Whey valorisation: A complete and novel technology development for dairy industry starter culture production. Bioresour. Technol. 2009, 100, 3734–3739. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Luz, C.; Ritieni, A.; Mañes, J.; Meca, G. Whey fermented by using Lactobacillus plantarum strains: A promising approach to increase the shelf life of pita bread. J. Dairy Sci. 2020, 103, 5906–5915. [Google Scholar] [CrossRef]
- Alonso, S.; Herrero, M.; Rendueles, M.; Díaz, M. Residual yoghurt whey for lactic acid production. Biomass Bioener. 2010, 34, 931–938. [Google Scholar] [CrossRef]
- Virtanen, T.; Pihlanto, A.; Akkanen, S.; Korhonen, H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J. Appl. Microbiol. 2007, 102, 106–115. [Google Scholar] [CrossRef]
- Daverey, A.; Pakshirajan, K.; Sumalatha, S. Sophorolipids production by Candida bombicola using dairy industry wastewater. Clean. Technol. Environ. Policy 2011, 13, 481–488. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Rydin, Y.; Home, R.; Taylor, K. Making the Most of the Planning Appeals System; Association of District Councils: London, UK, 1990. [Google Scholar]
- Jain, S.; Anal, A.K. Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J. Food Sci. Technol. 2017, 54, 1062–1072. [Google Scholar] [CrossRef]
- Ruthu; Murthy, P.S.; Rai, A.K.; Bhaskar, N. Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. J. Food Sci. Technol. 2014, 51, 1884–1892. [Google Scholar]
- Radha, P.; Narayanan, S.; Chaudhuri, A.; Anjum, S.; Thomas, D.L.; Pandey, R.; Ramani, K. Synthesis of single-cell oil by Yarrowia lipolytica MTCC 9520 utilizing slaughterhouse lipid waste for biodiesel production. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Thirulogachandar, A.; Priyadharshni, V.S.; Anbarasan, T.; Saraswathy, S.; Jayanthi, S. Production of Microbial Lipid using Slaughterhouse Wastewater as Substrate. Int. J. Appl. Eng. Res. 2015, 10, 324–327. [Google Scholar]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries—Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Steyn, A.; Viljoen-Bloom, M.; van Zyl, W.H. Valorization of apple and grape wastes with malic acid-degrading yeasts. Folia. Microbiol. 2021, 66, 341–354. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, N.; Yadav, A.N.; Singh, J.; Rastegari, A.A.; Saxena, A.K. Agriculturally and Industrially Important Fungi: Current Developments and Potential Biotechnological Applications. In Recent Advancement in White Biotechnology Through Fungi: Volume 2: Perspective for Value-Added Products and Environments; Yadav, A.N., Singh, S., Mishra, S., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–64. [Google Scholar]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Singh, M.; Liu, S.X.; Vaughn, S.F. Effect of corn bran as dietary fiber addition on baking and sensory quality. Biocatal. Agric. Biotechnol. 2012, 1, 348–352. [Google Scholar] [CrossRef]
- Wambogo, E.A.; Ansai, N.; Ahluwalia, N.; Ogden, C.L. The Contribution of Discrete Vegetables, Mixed Dishes, and Other Foods to Total Vegetable Consumption: US Ages 2 Years and Over, 2017–2018. J. Acad. Nutr. Diet 2022, 122, 2115–2126.e2. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and Atherosclerosis: Human Trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [CrossRef]
- Ferrannini, E.; Buzzigoli, G.; Bonadonna, R.; Giorico, M.A.; Oleggini, M.; Graziadei, L.; Pedrinelli, R.; Brandi, L.; Bevilacqua, S. Insulin resistance in essential hypertension. N. Engl. J. Med. 1987, 317, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G. Insulin Resistance, Hypertension, and Coronary Heart Disease. J. Clin. Hypert. 2003, 5, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Edel, A.L.; Rodriguez-Leyva, D.; Maddaford, T.G.; Caligiuri, S.P.; Austria, J.A.; Weighell, W.; Guzman, R.; Aliani, M.; Pierce, G.N. Dietary flaxseed independently lowers circulating cholesterol and lowers it beyond the effects of cholesterol-lowering medications alone in patients with peripheral artery disease. J. Nutr. 2015, 145, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Min, S.-L. Functional Fermented Food Additive and Functionality Health Food Manufacturing Method. KR101955775B1, 7 March 2019. [Google Scholar]
- Koletta, P.; Irakli, M.; Papageorgiou, M.; Skendi, A. Physicochemical and technological properties of highly enriched wheat breads with wholegrain non wheat flours. J. Cereal Sci. 2014, 60, 561–568. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Pobiega, K.; Błażejak, S.; Kieliszek, M. The scale-up cultivation of Candida utilis in waste potato juice water with glycerol affects biomass and β(1,3)/(1,6)-glucan characteristic and yield. Appl. Microbiol. Biotechnol. 2018, 102, 9131–9145. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Sangkharak, K. Utilization of Waste Glycerol from Biodiesel Process as a Substrate for Mono-, Di-, and Triacylglycerol Production. Energy Procedia 2017, 138, 895–900. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Broda, M.; Żywicka, A.; Styburski, D.; Sobolewski, P.; Gorący, K.; Migdał, P.; Junka, A.; Fijałkowski, K. Potato Juice, a Starch Industry Waste, as a Cost-Effective Medium for the Biosynthesis of Bacterial Cellulose. Int. J. Mol. Sci. 2021, 22, 10807. [Google Scholar] [CrossRef]
- Chotigavin, N.; Sriphochanart, W.; Yaiyen, S.; Kudan, S. Increasing the Production of β-Glucan from Saccharomyces carlsbergensis RU01 by Using Tannic Acid. Appl. Biochem. Biotechnol. 2021, 193, 2591–2601. [Google Scholar] [CrossRef]
- Yagüe, S.; Terrón, M.C.; González, T.; Zapico, E.; Bocchini, P.; Galletti, G.C.; González, A.E. Biotreatment of tannin-rich beer-factory wastewater with white-rot basidiomycete Coriolopsis gallica monitored by pyrolysis/gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 905–910. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Liu, J.; Jiang, L.; Huang, W.; Huo, F.-W.; Tian, D. Colorimetric Assay for Heterogeneous-Catalyzed Lipase Activity: Enzyme-Regulated Gold Nanoparticle Aggregation. J. Agric. Food Chem. 2015, 63, 39–42. [Google Scholar] [CrossRef]
- Cantatore, V.; Filannino, P.; Gambacorta, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation to Re-cycle Apple By-Products for Wheat Bread Fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.-D.; Yoon, K.-L. Method for Production of a Wheat Bran Fermentation Product with Increased Water Soluble Mucilage through Mixed Fermentation and Method for Production of a High Fiber Bread with the Wheat Bran Fermentation Product. KR101475318B1, 22 December 2014. [Google Scholar]
- Costa, J.R.; Tonon, R.V.; Gottschalk, L.M.; Santiago, M.C.A.; Mellinger-Silva, C.; Pastrana, L.; Pintado, M.M.; Cabral, L.M. Enzymatic production of xylooligosaccharides from Brazilian Syrah grape pomace flour: A green alternative to conventional methods for adding value to agricultural by- products. J. Sci. Food Agric. 2019, 99, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Outeiriño, D.; Pérez Guerra, N.; Domínguez, J.M. Enzymatic hydrolysis of brewer’s spent grain to obtain fermentable sugars. Bioresour. Technol. 2019, 275, 402–409. [Google Scholar] [CrossRef]
- Amorim, T.L.; Duarte, L.M.; Chellini, P.R.; de Oliveira, M.A.L. A validated capillary electrophoresis method for fatty acid determination in encapsulated vegetable oils supplements. LWT 2019, 114, 108380. [Google Scholar] [CrossRef]
- Khummanee, N.; Rudeekulthamrong, P.; Kaulpiboon, J. Enzymatic Synthesis of Functional Xylose Glucoside and Its Application to Prebiotic. Appl. Biochem. Microbiol. 2021, 57, 212–218. [Google Scholar] [CrossRef]
- Bhanja, T.; Rout, S.; Banerjee, R.; Bhattacharyya, B.C. Comparative profiles of alpha-amylase production in conventional tray reactor and GROWTEK bioreactor. Bioprocess. Biosyst. Eng. 2007, 30, 369–376. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Cătoi, A.F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants. 2019, 8(9), 372. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Ma, T.; Bao, X.; Du, F.; Zhuang, G.; Qu, Y. Simultaneous saccharification and fermentation of acid-pretreated corncobs with a recombinant Saccharomyces cerevisiae expressing β-glucosidase. Bioresour. Technol. 2008, 99, 5099–5103. [Google Scholar] [CrossRef]
- Verduzco-Oliva, R.; Gutierrez-Uribe, J.A. Beyond Enzyme Production: Solid State Fermentation (SSF) as an Alternative Approach to Produce Antioxidant Polysaccharides. Sustainability 2020, 12, 495. [Google Scholar] [CrossRef]
- Liu, G.; Li, H.; Chu, C.; Yang, H. Method for Preparing Carotenoid-Enriched Yeast Single-Cell Protein by Using Bean Curd Yellow Water for Fermentation. CN103627645A, 12 March 2014. [Google Scholar]
- Yue, P. Method for Producing Grape Seed Vinegar by Using Solid Fermentation method. CN102154087B, 9 May 2012. [Google Scholar]
- Jiang, L.; Luo, F.; Xia, B. Method for Processing Dry Chili Sauce by Using Chili Juice as Byproduct of Fermented Chili Processing Enterprise. CN106722835B, 3 April 2020. [Google Scholar]
- Xi, M.; Zhu, Y.; Li, J.; Li, X. Aspergillus Oryzae and Application Thereof. CN105695340A, 21 August 2020. [Google Scholar]
- Li, M.; You, X.; Zhang, Y.; Sun, J.; Li, Z.; Wei, P.; Wang, Y.; Zhou, K.; He, X. Preparation Method of Pearl Plum Flavor Fruitcake. CN106819352B, 8 January 2021. [Google Scholar]
- Gao, B.; Li, D.; Qi, Y.; Liu, Z.; Gao, Z. Preparation Method of Olive Paste Rich in Carotenoid. CN106858552B, 31 July 2020. [Google Scholar]
- Yoo, I.-H.; Lee, K.-J.; Kim, Y.-J.; Kim, H.-S. Process for Preparing Beverage Comprising Lactate Fermented Citron Pomace with Anti-Browing Properties. South. KR101922961B1, 29 November 2018. [Google Scholar]
- Yue, C. Preparation Method of Banana Peel Lactobacillus Fermented Beverage. CN105961586B, 11 August 2020. [Google Scholar]
- Liu, B.; Hu, R.; Huang, Z.; Jia, R.; Xiao, Z. Method for Comprehensively Developing and Utilizing Fruits and Vegetables. CN107259271B, 1 January 2021. [Google Scholar]
- Kwon, T.-S.; Seo, K.-S.; Kim, G.-J.; Jin, S.-W.; Go, Y.-W.; Im, S.-B.; Ha, N.L.; Jung, H.-K. Health Food Composition Using Pomegranate Fermented by Lactic Acid Bacteria and Manufacturing Method Thereof. South. KR102214532B1, 10 February 2021. [Google Scholar]
- Yuji, K.; Yuji, K.; Hiroki, A.; Hiroki, A. Method for Producing LPS-Rich Composition. JP6539400B1, 3 July 2019. [Google Scholar]
- Puupponen, P.R.N.L.; Virtanen, V. Process for Converting Berry and Fruit Materials into Fractions Containing Bioactive Compounds. FI127240B, 15 February 2018. [Google Scholar]
- Tao, M.; Pan, D.; Guo, Y. Flavored Yogurt Rich in Functional Factors and Preparation Method Thereof. CN107006606B, 16 June 2020. [Google Scholar]
- Nataliya Ivanovna, S. Oat Product of Functional Purpose (Versions). RU2734461C2, 16 October 2020. [Google Scholar]
- Browne, D.C.Y.; Dohnalek, M.; McDonagh, D.; Kleinbach-Sauter, H.; Shadix, K.; Tan, S.Y. Beverage and Food Production Using Greek Yogurt Acid Whey. U.S. Patent 20180116250A1, 5 December 2020. [Google Scholar]
- Malik, k.; Tokas, J.; Chand Anand, R. Characterization and Cytotoxicity Assay of Pigment Producing Microbes. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 370–376. [Google Scholar] [CrossRef]
- Dufossé, L. Chapter 4—Microbial Pigments From Bacteria, Yeasts, Fungi, and Microalgae for the Food and Feed Industries. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 113–132. [Google Scholar]
- Buzzini, P.; Martini, A. Production of carotenoids by strains of Rhodotorula glutinis cultured in raw materials of agro-industrial origin. Bioresour. Technol. 2000, 71, 41–44. [Google Scholar] [CrossRef]
- Chandi, G.K.; Gill, B.S. Production and Characterization of Microbial Carotenoids as an Alternative to Synthetic Colors: A Review. Int. J. Food Prop. 2011, 14, 503–513. [Google Scholar] [CrossRef]
- Mirzaei, M.; Shavandi, A.; Mirdamadi, S.; Soleymanzadeh, N.; Motahari, P.; Mirdamadi, N.; Moser, M.; Subra, G.; Alimoradi, H.; Goriely, S. Bioactive peptides from yeast: A comparative review on production methods, bioactivity, structure-function relationship, and stability. Trends Food Sci. Technol. 2021, 118, 297–315. [Google Scholar] [CrossRef]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta Mol. Cell 2020, 1865, 158699. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C.; Park, S. Advances in the valorization of spent brewer’s yeast. Innov. Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- Chafale, A.; Kapley, A. Biosurfactants as microbial bioactive compounds in microbial enhanced oil recovery. J. Biotechnol. 2022, 352, 1–15. [Google Scholar] [CrossRef]
- Luna, J.M.; Santos Filho, A.; Rufino, R.D.; Sarubbo, L.A. Production of biosurfactant from Candida bombicola URM 3718 for environmental applications. Chem. Eng. Trans. 2016, 49, 583–588. [Google Scholar]
- Abd Razak, D.L.; Abd Rashid, N.Y.; Jamaluddin, A.; Sharifudin, S.A.; Abd Kahar, A.; Long, K. Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant. Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Sugiharto, S. A review of filamentous fungi in broiler production. Ann. Agric. Sci. 2019, 64, 1–8. [Google Scholar] [CrossRef]
- Cole, G.T. Basic Biology of Fungi. In Medical Microbiology.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Hameed, A.; Hussain, S.A.; Yang, J.; Ijaz, M.U.; Liu, Q.; Suleria, H.A.R.; Song, Y. Antioxidants Potential of the Filamentous Fungi (Mucor circinelloides). Nutrients 2017, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Kazda, M.; Langer, S.; Bengelsdorf, F.R. Fungi open new possibilities for anaerobic fermentation of organic residues. Energy Sustain. Soc. 2014, 4, 6. [Google Scholar] [CrossRef]
- FazeliNejad, S.; Ferreira, J.A.; Brandberg, T.; Lennartsson, P.R.; Taherzadeh, M.J. Fungal protein and ethanol from lignocelluloses using Rhizopus pellets under simultaneous saccharification, filtration and fermentation (SSFF). Biofuel Res. J. 2016, 3, 372–378. [Google Scholar] [CrossRef]
- Ibarruri, J.; Hernández, I. Rhizopus oryzae as fermentation agent in food derived sub-products. Waste Biomass Val. 2018, 9, 2107–2115. [Google Scholar] [CrossRef]
- Guimarães, L.H.S.; Peixoto-Nogueira, S.d.C.; Michelin, M.; Rizzatti, A.C.S.; Sandrim, V.C.; Zanoelo, F.F.; Aquino, A.C.M.d.S.; Junior, A.B.; Polizeli, M.d.L. Screening of filamentous fungi for production of enzymes of biotechnological interest. Braz. J. Microbiol. 2006, 37, 474–480. [Google Scholar] [CrossRef]
- Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. Fungal biotechnology in food and feed processing. Food Res. Int. 2009, 42, 577–587. [Google Scholar] [CrossRef]
- Hamada, S.; Suzuki, K.; Aoki, N.; Suzuki, Y. Improvements in the qualities of gluten-free bread after using a protease obtained from Aspergillus oryzae. J. Cereal Sci. 2013, 57, 91–97. [Google Scholar] [CrossRef]
- Bayitse, R.; Hou, X.; Laryea, G.; Bjerre, A.B. Protein enrichment of cassava residue using Trichoderma pseudokoningii (ATCC 26801). AMB Express 2015, 5, 80. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Oliveira, R.A.; Bon, E.P.S.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.F.; Tonon, R.V. Use of grape pomace for the production of hydrolytic enzymes by solid-state fermentation and recovery of its bioactive compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef]
- Uraz, T.; Özer, B.H. STARTER CULTURES | Molds Employed in Food Processing. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 522–528. [Google Scholar]
- Nitayavardhana, S.; Issarapayup, K.; Pavasant, P.; Khanal, S.K. Production of protein-rich fungal biomass in an airlift bioreactor using vinasse as substrate. Bioresour. Technol. 2013, 133, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.; Li, S.; Xu, Q.; Ying, H.; Huang, H.; Ouyang, P. Chitosan production from hemicellulose hydrolysate of corn straw: Impact of degradation products on Rhizopus oryzae growth and chitosan fermentation. Lett. Appl. Microbiol. 2010, 51, 278–284. [Google Scholar] [CrossRef] [PubMed]
- FEFAC, European Feed Manufacturer’s Federation. Environment Report, 2nd ed.; FEFAC: Brussels, Belgium, 2012. [Google Scholar]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Application of Fermentation to Recover High-Added Value Compounds from Food By-Products. In Fermentation Processes; BoD–Books on Demand: Norderstedt, Germany, 2021; pp. 195–219. [Google Scholar]
- Smith, H.; Doyle, S.; Murphy, R. Filamentous fungi as a source of natural antioxidants. Food Chem 2015, 185, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Klempová, T.; Slaný, O.; Šišmiš, M.; Marcinčák, S.; Čertík, M. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. J. Biotechnol. 2020, 311, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. A fermentative approach towards optimizing directed biosynthesis of fumaric acid by Rhizopus oryzae 1526 utilizing apple industry waste biomass. Fungal. Biol. 2015, 119, 1279–1290. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Potential use of pulp and paper solid waste for the bio-production of fumaric acid through submerged and solid state fermentation. J. Clean. Prod. 2016, 112, 4435–4444. [Google Scholar] [CrossRef]
- Lun, O.K.; Wai, T.; Ling, L.S. Pineapple cannery waste as a potential substrate for microbial biotranformation to produce vanillic acid and vanillin. Int. Food Res. J. 2014, 21, 953–958. [Google Scholar]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules 2018, 23, 10. [Google Scholar] [CrossRef]
- Cai, S.; Wang, O.; Wu, W.; Zhu, S.; Zhou, F.; Ji, B.; Gao, F.; Zhang, D.; Liu, J.; Cheng, Q. Comparative study of the effects of solid-state fermentation with three filamentous fungi on the total phenolics content (TPC), flavonoids, and antioxidant activities of subfractions from oats (Avena sativa L.). J. Agric. Food Chem. 2012, 60, 507–513. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, Y.-D.; Lee, S.-R. Method of Using Beta-Glucan from Schizophyllum Commune. U.S. Patent 200,900,236,81A1, 22 January 2009. [Google Scholar]
- Filannino, P.; Di Cagno, R.; Trani, A.; Cantatore, V.; Gambacorta, G.; Gobbetti, M. Lactic acid fermentation enriches the profile of biogenic compounds and enhances the functional features of common purslane (Portulaca oleracea L.). J. Funct. Foods 2017, 39, 175–185. [Google Scholar] [CrossRef]
- Ebah, E.E.; Wusuum, B.; Akande, T.; Emmanuel, O.O.; Ikala, R.O.; Ode, T.A. Effect of lactic-acid fermentation on the shelf life of vegetables. Am. J. Innova. Res. App. Sci. 2019, 90, 328–334. [Google Scholar]
- Filannino, P.; Di Cagno, R.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Fructose-rich niches traced the evolution of lactic acid bacteria toward fructophilic species. Crit. Rev. Microbiol. 2019, 45, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Reverón, I.; Plaza-Vinuesa, L.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Transcriptional Reprogramming at Genome-Scale of Lactobacillus plantarum WCFS1 in Response to Olive Oil Challenge. Front. Microbiol. 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Crecchio, C.; De Virgilio, C.; De Angelis, M.; Gobbetti, M. Transcriptional reprogramming and phenotypic switching associated with the adaptation of Lactobacillus plantarum C2 to plant niches. Sci. Rep. 2016, 6, 27392. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Shah, N.P.; Wang, M.-F.; Lui, W.-Y.; Corke, H. Fermentation alters antioxidant capacity and polyphenol distribution in selected edible legumes. Int. J. Food Sci. Technol. 2016, 51, 875–884. [Google Scholar] [CrossRef]
- Gobbetti, M.; Di Cagno, R.; Calasso, M.; Neviani, E.; Fox, P.F.; De Angelis, M. Drivers that establish and assembly the lactic acid bacteria biota in cheeses. Trends Food Sci. Technol. 2018, 78, 244–254. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Da Ros, A.; Filannino, P.; Vincentini, O.; Gobbetti, M.; Di Cagno, R. Biotechnological re-cycling of apple by-products: A reservoir model to produce a dietary supplement fortified with biogenic phenolic compounds. Food Chem. 2021, 336, 127616. [Google Scholar] [CrossRef]
- Durante, M.; Bleve, G.; Selvaggini, R.; Veneziani, G.; Servili, M.; Mita, G. Bioactive Compounds and Stability of a Typical Italian Bakery Products “Taralli” Enriched with Fermented Olive Paste. Molecules 2019, 24, 18. [Google Scholar] [CrossRef]
- Arte, E.; Rizzello, C.G.; Verni, M.; Nordlund, E.; Katina, K.; Coda, R. Impact of Enzymatic and Microbial Bioprocessing on Protein Modification and Nutritional Properties of Wheat Bran. J. Agric. Food Chem. 2015, 63, 8685–8693. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef]
- Mao, M.; Wang, P.; Shi, K.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lv, F.-x. Effect of solid state fermentation by Enterococcus faecalis M2 on antioxidant and nutritional properties of wheat bran. J. Cereal Sci. 2020, 94, 102997. [Google Scholar] [CrossRef]
- Tamang, J.P.; Tamang, B.; Schillinger, U.; Guigas, C.; Holzapfel, W.H. Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. Int. J. Food Microbiol. 2009, 135, 28–33. [Google Scholar] [CrossRef]
- Bergamo, P.; Luongo, D.; Miyamoto, J.; Cocca, E.; Kishino, S.; Ogawa, J.; Tanabe, S.; Rossi, M. Immunomodulatory activity of a gut microbial metabolite of dietary linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, associated with improved antioxidant/detoxifying defences. J. Funct. Foods 2014, 11, 192–202. [Google Scholar] [CrossRef]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Filannino, P.; Cavoski, I.; Lanera, A.; Mamdouh, B.M.; Gobbetti, M. Bioprocessing technology to exploit organic palm date (Phoenix dactylifera L. cultivar Siwi) fruit as a functional dietary supplement. J. Funct. Foods 2017, 31, 9–19. [Google Scholar] [CrossRef]
- Monedero, V.; Pérez-Martínez, G.; Yebra, M.J. Perspectives of engineering lactic acid bacteria for biotechnological polyol production. Appl. Microbiol. Biotechnol. 2010, 86, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Rodríguez, L.G.; Aller, K.; Bru, E.; De Vuyst, L.; Hébert, E.M.; Mozzi, F. Enhanced mannitol biosynthesis by the fruit origin strain Fructobacillus tropaeoli CRL 2034. Appl. Microbiol. Biotechnol. 2017, 101, 6165–6177. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.A.; Kopit, L.; Doyle, C.; Yu, A.O.; Hugenholtz, J.; Marco, M.L. Polyol production during heterofermentative growth of the plant isolate Lactobacillus florum 2F. J. Appl. Microbiol. 2016, 120, 1336–1345. [Google Scholar] [CrossRef]
- El-Bialy, H.A.A.; Abd El-Khalek, H.H. A comparative study on astaxanthin recovery from shrimp wastes using lactic fermentation and green solvents:an applied model on minced Tilapia. J. Radiat Res. Appl. Sci. 2020, 13, 594–605. [Google Scholar] [CrossRef]
- Barbosa, J.R.; de Carvalho Junior, R.N. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Baharudin, M.M.A.; Ngalimat, M.S.; Mohd Shariff, F.; Balia Yusof, Z.N.; Karim, M.; Baharum, S.N.; Sabri, S. Antimicrobial activities of Bacillus velezensis strains isolated from stingless bee products against methicillin-resistant Staphylococcus aureus. PLoS ONE 2021, 16, e0251514. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; James, K. Economic Growth Potential of More Circular Economies; Waste and Resources Action Programme (WRAP): Banbury, UK, 2015. [Google Scholar]
- Galanakis, C.M.; Schieber, A. Editorial. Food Res. Int. 2014, 65, 299–300. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, A.; López de Cerain, A.; Sanz-Serrano, J.; Gil, A.G.; Azqueta, A. European Regulatory Framework and Safety Assessment of Food-Related Bioactive Compounds. Nutrients 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Bansal, U.; Bhardwaj, A.; Singh, S.N.; Khubber, S.; Sharma, N.; Bansal, V. Effect of incorporating plant-based quercetin on physicochemical properties, consumer acceptability and sensory profiling of nutrition bars. Funct. Foods Health Dis. Online 2022, 22, 2378–7007. [Google Scholar] [CrossRef]
- Roni, R.A.; Sani, M.N.H.; Munira, S.; Wazed, M.A.; Siddiquee, S. Nutritional Composition and Sensory Evaluation of Cake Fortified with Moringa oleifera Leaf Powder and Ripe Banana Flour. Appl. Sci 2021, 11, 8474. [Google Scholar] [CrossRef]
- Sucheta; Singla, G.; Chaturvedi, K.; Sandhu, P.P. 2—Status and recent trends in fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 17–49. [Google Scholar]
- Zepeda-Ruiz, G.C.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Robles-Sánchez, M.; Salazar-López, N.J.; López-Díaz, J.A.; González-Aguilar, G.A. Supplementing corn chips with mango cv. “Ataulfo” peel improves their sensory acceptability and phenolic profile, and decreases in vitro dialyzed glucose. J. Food Process. Preserv. 2020, 44, e14954. [Google Scholar] [CrossRef]
- Bothma, C.; Cronjé, N.; Koen, M.; Hugo, A. Product development and consumer acceptability of soup made from Clarias gariepinus. CyTA J. Food 2020, 18, 572–579. [Google Scholar] [CrossRef]
| Food Waste/by-Product | Microorganisms | Metabolic Pathway/Enzymes | Effects | Ref. |
|---|---|---|---|---|
| Apple by-product | Lactiplantibacillus plantarum, Lactiplantibacillus fabifermentans, Saccharomyces cerevisiae | Phenolic compound metabolism (phenolic acid carboxylase and reductase) | Phenolic acids ↑, DPPH scavenging activity ↑, Antiradical and anti-inflammatory features in Caco-2 cell ↑ | [18] |
| Apple pomace | Phanerochaete chrysosporium | Hydrolytic enzymes | Phenolic compound production ↑ | [64] |
| Coconut water | Rhodotorula rubra Xanthophyllomyces dendrorhous | Levels of carotenoids ↑ | [65,66] | |
| Cocoa pod husk, cassava peel and palm kernel cake | Rhizopus stolonifer | Ligno-cellulolytic enzymes | Fiber and cyanide contents ↓, protein content ↑, radical scavenging activities | [67] |
| Papaya fruit waste | Yarrowia lipolytica | Lipases and proteases | Recombinant therapeutic proteins ↑, enzymes ↑ and antibodies | [68] |
| Olive Mill Wastewater (OMW) | Magnusiomyces capitatus Yarrowia lipolytica | Extracellular lipolytic activity Phenols degradation | Lipase activity ↑, olive oil concentration ↑ Citric and oleic acid production | [69,70] |
| Plum by-products | Aspergillus niger Rhizopus oligosporus | Cholesterol and fatty acid metabolism | Sterol esters and polar lipids ↑, Stimulator antioxidants ↑ | [71] |
| Cellulose substrate | Lactiplantibacillus plantarum | Proteolysis | GABA production ↑ | [72] |
| Maize by-products | Lactiplantibacillus plantarum, Weissella confusa | Cellular process/ Lipase activity | Phytic acid ↓, radical scavenging activity ↑, stability ↑, oxidative processes ↓ | [73] |
| Barley by-products | Pediococcus acidilactici | Fatty acid metabolism (Hydratase) | Oleic, arachidic, eicosadienoic, behenic, and lignoceric fatty acids ↑, health-promoting features ↑ | [74] |
| Wheat bran and cornmeal | Mucor spp. | Biosynthesis of secondary metabolites | γ-linolenic acid and β-carotene ↑ | [75,76] |
| Wheat bran | Saccharomyces cerevisiae | Xylanase activity | Folate content | [77] |
| Wheat bran | Kazachstania exigua | Phenolic compound metabolism and hydrolytic activity | Level of folates ↑, free phenolic acids ↑, and soluble arabinoxylans ↑ | [78] |
| Rice bran | Issatchenkia orientalis | Glycolysis | ↑ Free phenolic content | [79] |
| Rice pasta | Monascus purpureus | Cellular process | Natural pigments ↑, Monacolin K ↑, citrinin ↓ | [80] |
| Black rice bran | Aspergillus awamori | Glycoside hydrolase, polysaccharides degrading enzymes and esterase | Phenolic compound production ↑ | [81] |
| Cheese whey | Kluyveromyces marxianus, Kluyveromyces fragilis, Candida pseudotropicalis, Candida versatilis | Lipolytic activity | Microbial mass production ↑ | [82] |
| Whey | Lactiplantibacillus plantarum | Cellular processes | Bacteriocins ↑, shelf life ↑, growth of Penicillium expansum and Penicillium brevicompactum ↓ | [83] |
| Yogurt whey | Lacticaseibacillus casei | Embden–Meyerhof–Parnas pathway for glycolysis | Lactic acid production ↑ | [84] |
| Whey | Leuconostoc mesenteroides, Lactobacillus jensenii, Lactobacillus acidophilus | Proteolysis | Bioactive peptides ↑, ABTS+ antioxidant activity ↑ | [85] |
| Dairy waste water | Candida bombicola | Cellular process | Level of sophorolipids ↑ | [86] |
| Dairy whey | Kluyveromyces fragilis Candida bombicola | Acidogenic fermentation | Bioenergy, organic acids, bioactive peptides Surfactants | [87] |
| Dairy wastewater | C. tropicalis, C. rugosa, C. lipolytica, Y. lipolytica | Fats and esters utilization | Microbial mass production ↑ | [88] |
| Chicken egg shell membrane | Lactiplantibacillus plantarum | Proteolysis | Bioactive peptides ↑, Radical scavenging activity ↑ | [89] |
| Fish head | Pediococcus acidilactici and Enterococcus faecium | Proteolysis | Antimicrobial peptides ↑ | [90] |
| Slaughterhouse wastewater | Yarrowia lipolytica | Lipolytic activity | Level of lipids ↑ (palmitic, stearic and oleic acids), biodiesel recovery | [91,92] |
| Brewers’ spent grain (BSG) | Pichia stipitis Kluyveromyces marxianus | Fermentation pathways | Phenol compounds | [93] |
| Patent ID | Food/Plant by-Product | Microorganisms | Effect/Outcome | Application | Ref. |
|---|---|---|---|---|---|
| CN-103627645-A | Bean curd yellow water | Yeasts | Carotenoid production | Carotenoid-enriched protein—food supplement | [122] |
| CN-107937234-A | Grape seed | Yeasts | Polyphenols | Procyanidin- and polyphenol-enriched grape vinegar | [123] |
| KR-101990582-B1 | Wheat bran | Saccharomyces cerevisiae SPC-SNU 70-1, Latilactobacillus curvatus SPC-SNU 70-3, Lactiplantibacillus plantarum SPC-SNU 72-1, Levilactobacillus brevis SPC-SNU 70-2 Fructilactobacillus sanfranciscensis SPC-SNU 70-4 | High fiber content, improvement of taste and reduced firmness | High dietary fiber bread | [113] |
| CN-106722835-B | By-product chili juice | Saccharomyces cerevisiae | Taste improvement | Novel flavor pepper sauce | [124] |
| CN-105695340-B | Orange peel | Aspergillus oryzae | Health promoting effects, production of naringinase | Food supplement | [125] |
| CN-106819352-B | Orange peel | Bifidobacterium longum Trichoderma viride Aspergillus niger | Appetite improvement and beauty preservation | Fruitcake | [126] |
| CN-106858552-B | Olive pomace | Aspergillus niger Rhodotorula glutinis CICC 31229 | Improved fragrance and digestibility | Food applications | [127] |
| KR-101955775-B1 | Grape or cereal by-products | Bacillus subtilis Bacillus thuringiensis Lactic acid bacteria Photosynthetic bacteria Yeast Bacteria Gram-positive actinomycetes Fungi | Antioxidant and immunostimulatory effect | Functional foods or ingredients | [104] |
| KR-101922961-B1 | Citron residues | Lactiplantibacillus plantarum GAVOL-07 | Production of polysaccharides with antioxidant and anti-browning activity | Novel beverage | [128] |
| CN-105961586-B | Banana peel | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | Increased content of dietary fibers and trace elements, improved flavor | Fermented beverage | [129] |
| CN-107259271-B | Fruit and vegetable residues | Lactiplantibacillus plantarum Lactobacillus acidophilus Lacticaseibacillus rhamnosus | Free radical scavenging activity, alpha-glucosidase inhibition | Fermented fruit and vegetable juice | [130] |
| KR-102214532-B1 | Garcinia cambogia fruit peel | Lactobacillus acidophilus Lactiplantibacillus plantarum Limosilactobacillus fermentum | Anti-obesity and anti-inflammatory effect | Food ingredient | [131] |
| JP-6539400-B1 | Grape pomace | Bacteria and yeasts | Production of LPS | Functional ingredient | [132] |
| FI-127240-B | Berry by-products | Lactococcus sp. Lactobacillus sp. Pediococcus sp. Oenococcus sp. | Production of antimicrobials | Cosmetics, hygiene products, food supplements, food products, feeds, pharmaceutical products | [133] |
| CN-107006606-B | Red bean dregs | Lactobacillus delbrueckii subsp. bulgaricus Streptococcus thermophilus Lactiplantibacillus plantarum Levilactobacillus brevis | Antioxidant, antifatigue and immunoregulatory effects | Functional yogurt | [134] |
| RU-2734461-C2 | Cereal bran or crushed cereal grains | Lactobacillus acidophilus LA-5 Bifidobacterium animalis subsp. lactis BB-12 Streptococcus thermophilus Lactobacillus delbrueckii subsp. bulgaricus | Antidiabetic effect | Antidiabetic yogurt | [135] |
| US-10645954-B2 | Yogurt whey | Lactobacillus sp. Streptococcus thermophilus | Increased content of calcium, potassium, phosphorous and fatty acids | Beverage/food ingredient | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minervini, F.; Comitini, F.; De Boni, A.; Fiorino, G.M.; Rodrigues, F.; Tlais, A.Z.A.; Carafa, I.; De Angelis, M. Sustainable and Health-Protecting Food Ingredients from Bioprocessed Food by-Products and Wastes. Sustainability 2022, 14, 15283. https://doi.org/10.3390/su142215283
Minervini F, Comitini F, De Boni A, Fiorino GM, Rodrigues F, Tlais AZA, Carafa I, De Angelis M. Sustainable and Health-Protecting Food Ingredients from Bioprocessed Food by-Products and Wastes. Sustainability. 2022; 14(22):15283. https://doi.org/10.3390/su142215283
Chicago/Turabian StyleMinervini, Fabio, Francesca Comitini, Annalisa De Boni, Giuseppina Maria Fiorino, Francisca Rodrigues, Ali Zein Alabiden Tlais, Ilaria Carafa, and Maria De Angelis. 2022. "Sustainable and Health-Protecting Food Ingredients from Bioprocessed Food by-Products and Wastes" Sustainability 14, no. 22: 15283. https://doi.org/10.3390/su142215283
APA StyleMinervini, F., Comitini, F., De Boni, A., Fiorino, G. M., Rodrigues, F., Tlais, A. Z. A., Carafa, I., & De Angelis, M. (2022). Sustainable and Health-Protecting Food Ingredients from Bioprocessed Food by-Products and Wastes. Sustainability, 14(22), 15283. https://doi.org/10.3390/su142215283