The Establishment of Rapid Propagation System of ‘RED SUN’ Phalaenopsis aphrodite
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Effect of Sampling, Sterilization Time, and Material Site on the Contamination Rate and Germination Rate of Explants
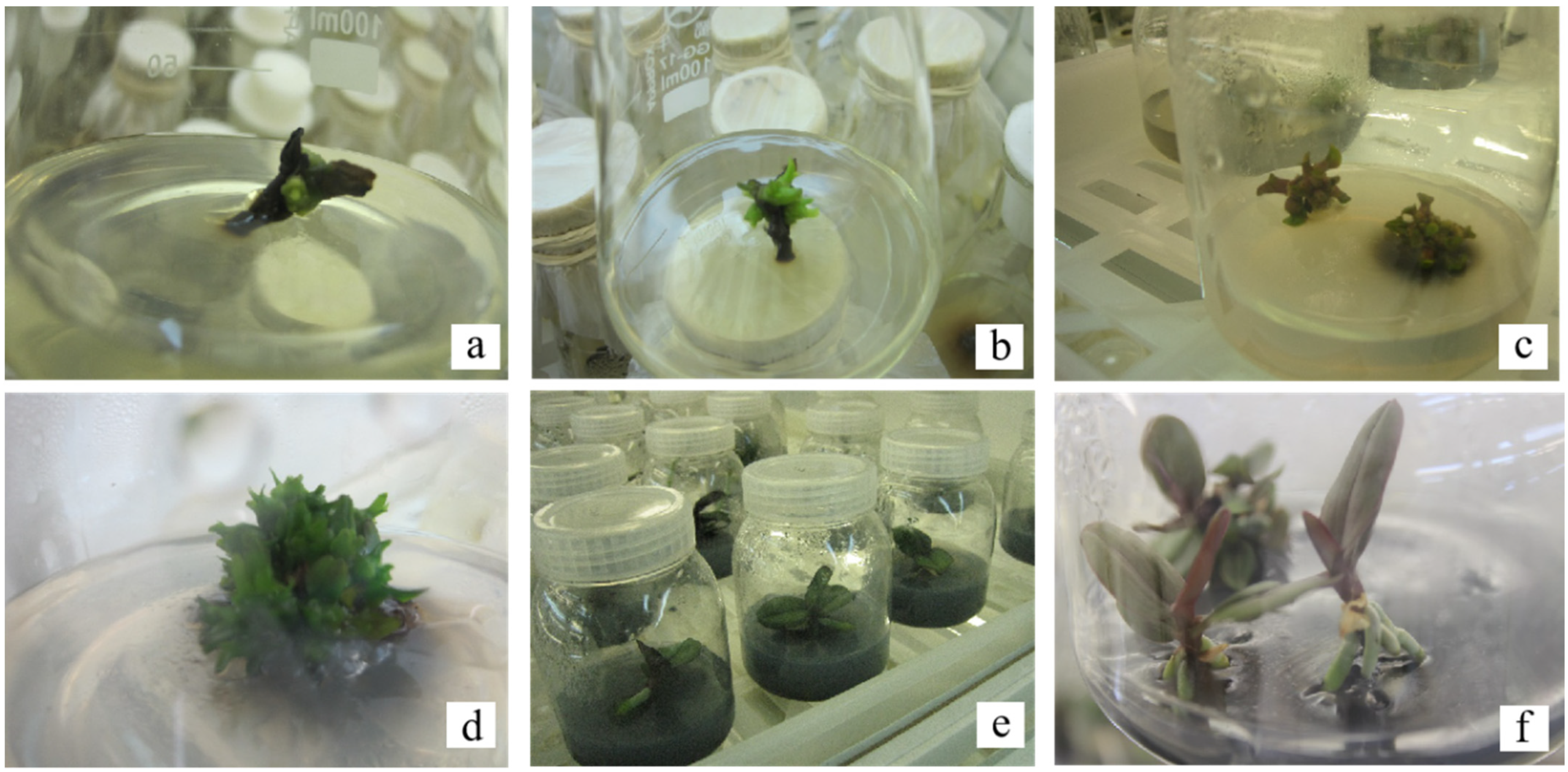
3.2. Induction of Adventitious Buds in Butterfly Pedicels
3.3. Proliferation Culture of Adventitious Buds Induced by Phalaenopsis Pedicels
3.4. Culture of Adventitious Buds and Strong Seedlings Induced by Phalaenopsis Pedicels
3.5. Effect of Medium, IBA, and Activated Carbon on Rooting of Sterile Seedlings
3.6. Grading and Maintenance of Sterile Seedling Transplants of Phalaenopsis
3.7. Preservation of Phalaenopsis Germplasm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, S.M.; Wei, B.; Li, X.C.; Luo, C.; Sun, F.F. The impacts of climate change on the potential distribution of Haloxylon ammodendron. Chin. J. Ecol. 2017, 36, 1243–1250. [Google Scholar] [CrossRef]
- Franklin, J.; Serra-Diaz, J.M.; Syphard, A.D.; Regan, H.M. Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Gao, S.; Liu, S.; Hong, M.; Zhu, Y.; Wu, Y.; Hu, D.; Zhang, L.; Lei, T. An aseptic rapid propagation system for obtaining plumbagin of ceratostigma willmottianum stapf. Plant Cell Tissue Organ Cult. 2019, 137, 369–377. [Google Scholar] [CrossRef]
- Gow, W.-P.; Chen, J.-T.; Chang, W.-C. Effects of genotype, light regime, explant position and orientation on direct somatic embryogenesis from leaf explants of phalaenopsis orchids. Acta Physiol. Plant. 2009, 31, 363–369. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chen, H.H.; Chen, W.H. Phalaenopsis. In Ornamental Crops; Springer: Cham, Switzerland, 2018; pp. 567–625. [Google Scholar] [CrossRef]
- Pramanik, D.; Shintiavira, H.; Winarto, B. Studi kualitas regeneran phalaenopsis hasil kultur in vitro dari eksplan tangkai infloresen, tunas pucuk, dan empulur (the quality study of phalaenopsis regenerants from in vitro propagation of inflorescence, shoot tip, and pith explants). J. Hortik. 2018, 28, 13–24. [Google Scholar] [CrossRef]
- Vir, R.; Kumar, V. Role of plant tissue culture in improvement of horticultural crops. Ann. Hortic. 2021, 14, 1–6. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Luan, C.; Yang, J.; Cheng, S.; Dai, Z.; Mei, P.; Huang, C. Somatic embryogenesis from mature zygotic embryos of distylium chinense (fr.) diels and assessment of genetic fidelity of regenerated plants by srap markers. Plant Growth Regul. 2014, 74, 11–21. [Google Scholar] [CrossRef]
- Li, X.Y.; Yin, T.P.; Niu, Y.H.; Cui, G.J.; Li, X.L. Research on Rapid Propagation and Cultivation Management of Phalaenopsis. Shandong Agric. Sci. 2000, 4, 13–14. [Google Scholar]
- Li, J.J.; Liao, J.J.; Ke, L.W.; Cai, P.L. Tissue Culture of the Root Segment of Phalaenopsis. Brief Commun. Plant Tissue Cult. 2000, 1, 37. [Google Scholar]
- Chai, X.H.; Li, J.; Zhu, B.Q.; Zeng, B.J.; Zhang, X.S.; Zhan, H.Y.; Wang, X.Y. Leaf Culture of Golden-edged Phalaenopsis. Chin. J. Trop. Agric. 2004, 3, 18–20. [Google Scholar] [CrossRef]
- Xu, H.; Lian, L.; Wang, F.; Jiang, J.; Lin, Q.; Xie, H.; Zhang, J. Brassinosteroid signaling may regulate the germination of axillary buds in ratoon rice. BMC Plant Biol. 2020, 20, 76. [Google Scholar] [CrossRef]
- Bu, C.Y.; Jiang, H.P.; Man, R.J. Study on the In Vitro Culture of Phalaenopsis Pedicels and Leaf Induction of Proto-bulbs. Jiangsu Agric. Sci. 2008, 3, 147–150. [Google Scholar]
- Cui, G.R.; Hou, X.L.; Zhang, Z.X.; Zhang, C.Y.; Hu, N.B.; Liu, Y.C. Efficient Somatic Embryogenesis from Leaf Explants of Phalaenopsis in Vitro Culture and Histological Observations. Acta Hortic. Sin. 2007, 34, 431–436. [Google Scholar]
- Liu, F.P.; Hong, L.P.; Zheng, M.Q. Effects of 6-BA,2,4-D on PLB Explants Induction from Phalaenopsis. Acta Agric. Jiangxi 2007, 8, 69–71. [Google Scholar] [CrossRef]
- Homma, Y.; Asahira, T. New means of phalaenopsis propagation with internodal sections of flower stalk. J. Jpn. Soc. Hortic. Sci. 2007, 54, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Jiu, L.J.; Wang, F.; Yang, F.M.; Yu, L. Study on Tissue Culture and Effect of Heat-shock Treatment on Explant Browing of Phalaenopsis spp. Acta Bot. Boreali-Occident. Sin. 2012, 32, 2352–2358. [Google Scholar]
- Cen, Z.; Su, J.; Tu, H.; Lin, S. Study on the establishment of a rapid propagation system for succulent plant haworthia heidelbergensis. J. Biobased Mater. Bioenergy 2020, 14, 632–638. [Google Scholar] [CrossRef]
- Fan, J.R. The optimizations of protocorm induction route and culture process in tissue culture of phalaenopsis amabilis. J. Hefei Univ. (Nat. Sci.) 2013, 23, 51–54. [Google Scholar]
- Ali, M.B.; Khatun, S.; Hahn, E.J.; Paek, K.Y. Enhancement of phenylpropanoidenzymes and lignin in Phalaenopsis orchid and their influence on plantacclimatization at different levels of photosynthetic photon flux. Plant Growth Regul. 2006, 49, 137–146. [Google Scholar] [CrossRef]
- Rotor, G. Methord of vegetative propation of phalaenopsis stem cuttings. Am. Orchid Soc. Bull. 1949, 18, 739–742. [Google Scholar]
- Narita, W.; Ikuma, M.; Takizawa, N.; Kano, A.; Takayama, S. Rapid clonal propagation of phalaenopsis plantlets by shake culture. J. Adv. Sci. 1994, 6, 14–16. [Google Scholar] [CrossRef]
- Cui, Q.H.; Sun, Y.Y.; Kun, L.I.; Zhou, Z.M.; Duan, M.L. Effects of different factors on proliferation coefficient of dendrobium devonianum and average seedling height. J. Cent. South Univ. For. Technol. 2012, 32, 200–203. [Google Scholar]
- Pan, X.F.; Wang, A.-S.; Li, H.Z. A review on the progress of phalaenopsis rapid propagation through tissue culture. Trop. For. 2005, 33, 45–47. [Google Scholar]
- Zhu, Z.G. Research on tissue culture and rapid propagation of phalaenopsis amabilis. J. Anhui Agric. Sci. 2009, 37, 14614–14615. [Google Scholar]
- Wang, D.Y.; Wang, J.Y.; Cai, Y.; Jiang, X.E. Optimization of Conditions for the Proliferation of Adventitious Shoots in Phalaenopsis Group Culture. J. Huazhong Agric. Univ. 2007, 12, 856–858. [Google Scholar]
- Xiao, Y.; Zhang, L.; Zhang, G.; Han, Y.; Zhao, A. Application of sucrose free tissue culture in gerbera jamesonii propagation. Acta Hortic. Sin. 1998, 25, 408–410. [Google Scholar]
- Tan, W. Study on Rooting of Tissure Culture Shoot of Phalaenopsis in the Bottle. For. By-Prod. Spec. China 2011, 4, 34–35. [Google Scholar] [CrossRef]
- Zou, J.H. Research on transplantation survival rate of phalaenopsis hybrimycin tissue culture seedlings. J. Anhui Agric. Sci. 2007, 35, 295–300. [Google Scholar] [CrossRef]
- Feng, J.-H.; Chen, J.-T. A NovelIn VitroProtocol for Inducing Direct Somatic Embryogenesis inPhalaenopsis aphroditewithout Taking Explants. Sci. World J. 2014, 2014, 263642. [Google Scholar] [CrossRef] [Green Version]
- Bu, C.Y.; Li, B.J. Economic Profit Analysis on Phalaenopsis Commercial Tissue Culture Micropropagation. J. Huazhong Agric. Univ. (Soc. Sci. Ed.) 2007, 1, 78–81. [Google Scholar]
- So Young, P.; Murthy, H.N.; Kee Yoeup, P. Mass multiplication ofprotocorm-like bodies using bioreactor system and subsequent plantregeneration in Phalaenopsis. Plant Cell Tissure Organ Cult. 2000, 63, 67–72. [Google Scholar] [CrossRef]
- Chen, H.M.; Lv, F.B.; Zuo, L.I.; Xiao, W.F. Classification of phalaenopsis germplasm based on phenotypic traits. Chin. Hortic. Abstr. 2017, 33, 1–7+26. [Google Scholar]
- Villalobos, V.M.; Engelman, F. Ex situ conservation of plant germplasm using biotechnology. World J. Microbiol. Biotechnol. 1995, 11, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Suo, S.; Xu, M.H. Effects of Different Disinfection Treatments on Seed Germination of Wild Leptodermis oblonga Bge. in Tissue Culture. Mol. Plant Breed. 2022, 20, 1325–1330. [Google Scholar]
- Chalupa, V. Effect of benzylaminopurine and thidiazuron on invitro shoot proliferation of Tilia cordata Mill., Sorbus aucuparia L. and Robinia pseudoacacia L. Biol. Plant. 1987, 29, 425–429. [Google Scholar] [CrossRef]
- Chevreau, E.; Skirvin, R.M.; Abu-Qaoud, H.A.; Sullivan, S. Adventitious shoot regeneration from leaf tissue of three pear (Pyrus sp.) cultivars in vitro. Plant Cell Rep. 1989, 7, 688–691. [Google Scholar] [CrossRef]
- Liu, L.; Yi, Z.L.; Jiang, J.X.; Chen, Z.Y.; Huang, L.F. A study on tissue culture of Phalaenopsis spp. Subtrop. Plant Sci. 2008, 36, 25–29. [Google Scholar]

| Sequence Number | Germplasm Types | Quantity |
|---|---|---|
| 1 | branch | ≥10 |
| 2 | root | ≥10 |
| 3 | bud | ≥50 |
| 4 | DNA | ≥6 |
| No. | NAA (ml/L) | TDZ (ml/L) | Inoculation Amount | Induction Rate (%) |
|---|---|---|---|---|
| 1 | 0.5 | 0.2 | 30 | 33.3 ± 3.3 G |
| 2 | 1.0 | 0.2 | 30 | 37.8 ± 1.9 FG |
| 3 | 1.5 | 0.2 | 30 | 46.7 ± 3.3 DE |
| 4 | 0.5 | 0.4 | 30 | 95.6 ± 1.9 A |
| 5 | 1.0 | 0.4 | 30 | 87.8 ± 1.9 B |
| 6 | 1.5 | 0.4 | 30 | 82.2 ± 1.9 B |
| 7 | 0.5 | 0.6 | 30 | 55.6 ± 1.9 C |
| 8 | 1.0 | 0.6 | 30 | 43.3 ± 3.3 EF |
| 9 | 1.5 | 0.6 | 30 | 50.0 ± 3.3 CD |
| No. | TDZ (mg/L) | NAA (mg/L) | Inoculation Amount | Proliferation Rates (%) | Proliferation Fold |
|---|---|---|---|---|---|
| 1 | 0.1 | 0.2 | 30 | 28.9 ± 1.9 Cd | 3.6 ± 0.1 Cd |
| 2 | 0.1 | 0.4 | 30 | 64.4 ± 5.1 Bb | 5.0 ± 0.1 Bb |
| 3 | 0.1 | 0.6 | 30 | 36.7 ± 3.3 Cc | 3.8 ± 0.3 Ccd |
| 4 | 0.2 | 0.2 | 30 | 30.0 ± 3.3 Ccd | 4.2 ± 0.2 Cc |
| 5 | 0.2 | 0.4 | 30 | 87.8 ± 5.1 Aa | 8.1 ± 0.3 Aa |
| 6 | 0.2 | 0.6 | 30 | 32.2 ± 3.8 Ccd | 4.2 ± 0.5 Cc |
| 7 | 0.3 | 0.2 | 30 | 33.3 ± 3.3 Ccd | 3.5 ± 0.2 Cd |
| 8 | 0.3 | 0.4 | 30 | 66.7 ± 3.3 Bb | 5.0 ± 0.0 Bb |
| 9 | 0.3 | 0.6 | 30 | 36.7 ± 3.3 Cc | 3.8 ± 0.2 Ccd |
| No. | Banana Powder (g) | Tryptone (g) | Inoculation Amount | Leaf Number | Plant Height (cm) | Stem Diameter (cm) | Height/ Diameter |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 1 | 30 | 2.0 ± 0.0 Cd | 0.6 ± 0.10 F e | 0.5 ± 0.1 Fe | 1.20 |
| 2 | 10 | 2 | 30 | 2.7 ± 0.6 BCcd | 0.8 ± 0.1 EF de | 0.9 ± 0.1 CDbc | 0.96 |
| 3 | 10 | 3 | 30 | 2.3 ± 0.6 BCcd | 1.3 ± 0.2 CD c | 0.6 ± 0.1 EFde | 2.11 |
| 4 | 20 | 1 | 30 | 3.7 ± 0.6 ABab | 3.2 ± 0.1 B b | 1.2 ± 0.1 ABa | 2.67 |
| 5 | 20 | 2 | 30 | 4.3 ± 0.6 Aa | 5.2 ± 0.2 A a | 1.3 ± 0.2 Aa | 4.13 |
| 6 | 20 | 3 | 30 | 3.7 ± 0.6 ABab | 3.4 ± 0.10 B b | 1.2 ± 0.1 Aa | 2.76 |
| 7 | 30 | 1 | 30 | 2.3 ± 0.6 BCcd | 0.8 ± 0.2 EF e | 0.7 ± 0.1 DEcd | 1.05 |
| 8 | 30 | 2 | 30 | 3.0 ± 0.0 BCcd | 1.4 ± 0.2 C c | 1.0 ± 0.1 BCb | 1.43 |
| 9 | 30 | 3 | 30 | 2.3 ± 0.6 BCcd | 1.1 ± 0.2 DE d | 1.2 ± 0.1 ABa | 0.89 |
| Medium | IBA | Activated Carbon | Explant Number | Roots Number | Rooting Rate |
|---|---|---|---|---|---|
| MS | 0.5 | 1 | 30 | 39.7 ± 5.7 BCd | 41.8 ± 2.9 BCbc |
| MS | 1.0 | 2 | 30 | 36.7 ± 3.5 BCd | 40.5 ± 3.0 BCcd |
| MS | 1.5 | 3 | 30 | 15.3 ± 3.1 Ee | 30.3 ± 3.4 Ef |
| 1/2 MS | 0.5 | 2 | 30 | 111.0 ± 3.6 Aa | 76.6 ± 2.6 Aa |
| 1/2 MS | 1.0 | 3 | 30 | 35.3 ± 3.1 BCb | 50.2 ± 3.0 BCcd |
| 1/2 MS | 1.5 | 1 | 30 | 30.0 ± 4.4 CDcd | 43.7 ± 4.0 CDd |
| 1/4 MS | 0.5 | 3 | 30 | 34.7 ± 3.5 Cd | 38.6 ± 3.0 Ccd |
| 1/4 MS | 1.0 | 1 | 30 | 23.0 ± 2.6 DEd | 37.9 ± 3.0 Dee |
| 1/4 MS | 1.5 | 2 | 30 | 45.3 ± 5.5 Bbc | 47.6 ± 2.9 Bb |
| No. | Substrate | Environment | Leaf Size | Plant Height | Survival Rate | Growth Potential |
|---|---|---|---|---|---|---|
| 1 | moss | Glasshouse | 5.3 ± 1.0 Aa | 1.7 ± 0.0 Aa | 1.0 ± 0.1 Aa | rapid and strong |
| 2 | moss | Greenhouse | 3.5 ± 0.1 Bb | 0.9 ± 0.1 Cc | 0.5 ± 0.1 Bb | slow |
| 3 | peat | Glasshouse | 4.1 ± 0.1 ABb | 1.2 ± 0.1 Bb | 0.9 ± 0.1 Aa | slow and yellow |
| 4 | peat | Greenhouse | 3.2 ± 0.0 Bb | 0.7 ± 0.0 Dd | 0.5 ± 0.1 Bb | slow |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; He, D.; Li, X.; Dun, B.; Wu, D.; Huang, G. The Establishment of Rapid Propagation System of ‘RED SUN’ Phalaenopsis aphrodite. Sustainability 2022, 14, 15305. https://doi.org/10.3390/su142215305
Zhang H, He D, Li X, Dun B, Wu D, Huang G. The Establishment of Rapid Propagation System of ‘RED SUN’ Phalaenopsis aphrodite. Sustainability. 2022; 14(22):15305. https://doi.org/10.3390/su142215305
Chicago/Turabian StyleZhang, Haibo, Di He, Xiaoling Li, Bicheng Dun, Di Wu, and Guiyun Huang. 2022. "The Establishment of Rapid Propagation System of ‘RED SUN’ Phalaenopsis aphrodite" Sustainability 14, no. 22: 15305. https://doi.org/10.3390/su142215305
APA StyleZhang, H., He, D., Li, X., Dun, B., Wu, D., & Huang, G. (2022). The Establishment of Rapid Propagation System of ‘RED SUN’ Phalaenopsis aphrodite. Sustainability, 14(22), 15305. https://doi.org/10.3390/su142215305





