Biodegradation of Cyanide by a New Isolated Aerococcus viridans and Optimization of Degradation Conditions by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Kits
2.2. Isolation and Screening of Cyanide-Degrading Bacteria
2.3. Determination of the Minimal Inhibitory Concentration of Cyanide
2.4. Identification of the Isolated Strain
2.5. Biodegradation of Cyanide by T1 Strain and Measurement of Concentration of Cyanide
2.6. Effects of Physical and Chemical Conditions on Biodegradation of Cyanide by T1 Strain
2.7. Optimization of Process Variables with Response Surface Methodology
2.7.1. Plackett–Burman Design
2.7.2. Central Composite Design
2.8. Statistical Analysis
3. Results
3.1. Isolation of Cyanide-Degrading Bacteria
3.2. Identification of Cyanide-Degrading Bacteria Strain T1
3.3. Cyanide-Degrading Experiment
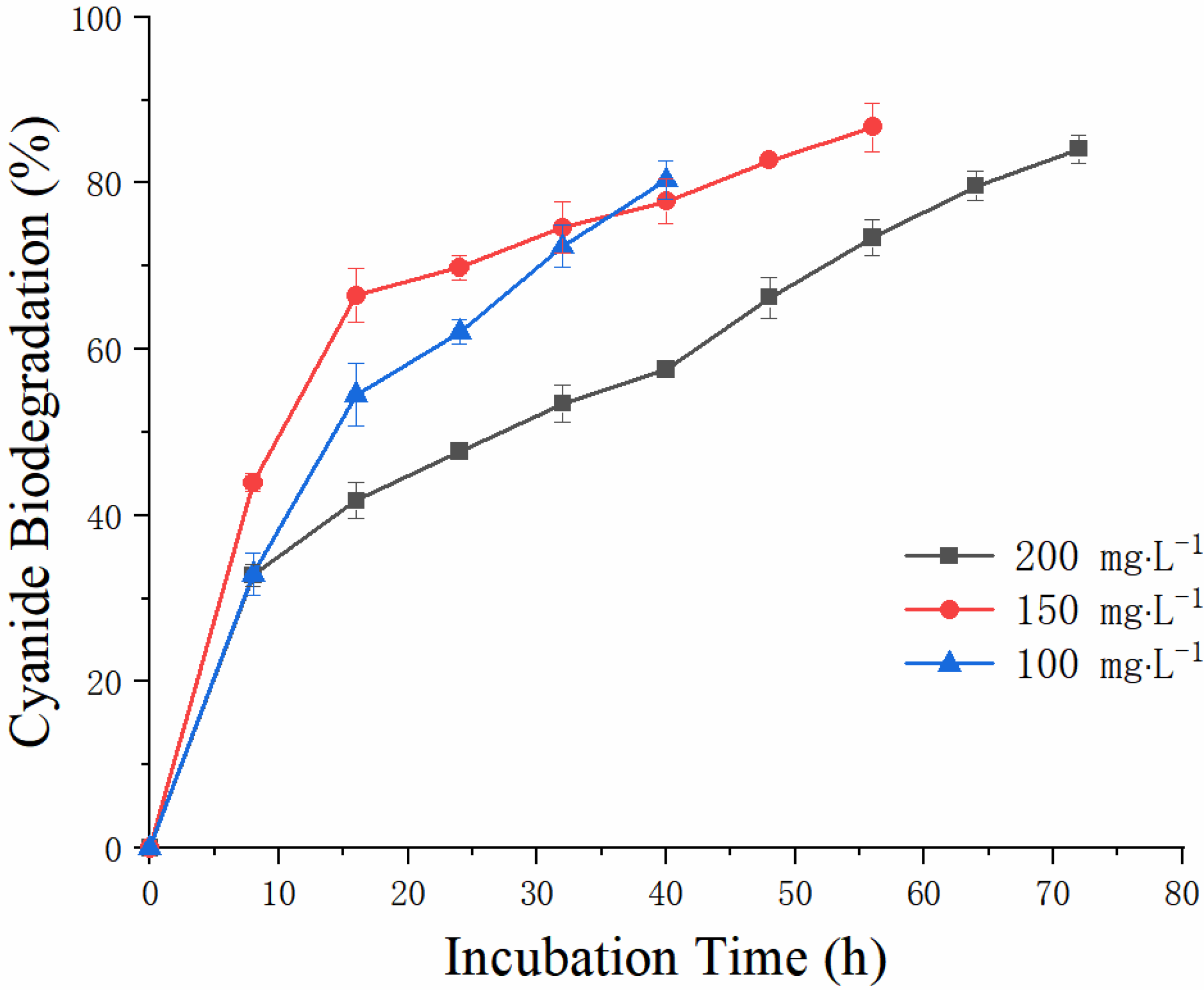
3.3.1. Cyanide Removal Efficiency of A. viridans T1
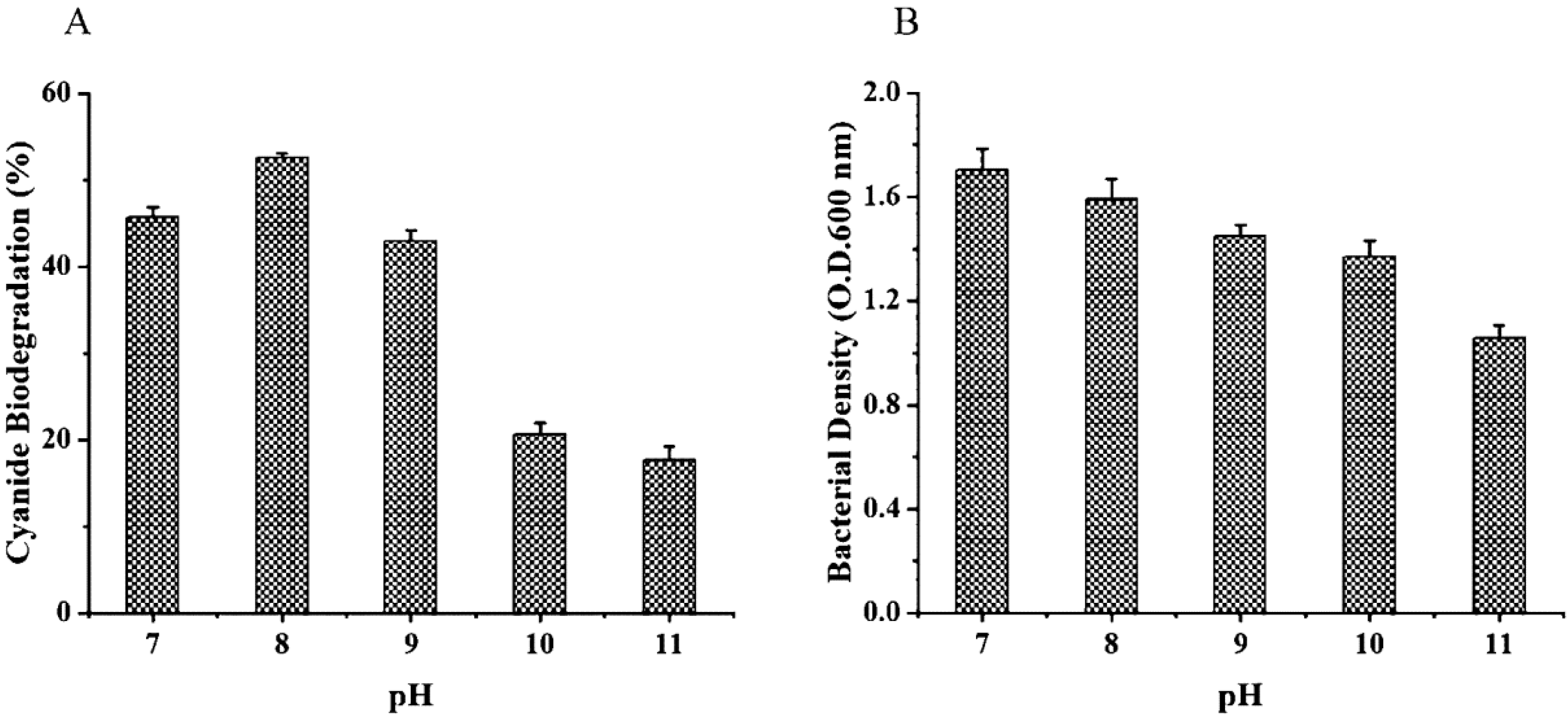
3.3.2. Effects of Temperature and pH on Cyanide Biodegradation and Growth of A. viridans T1
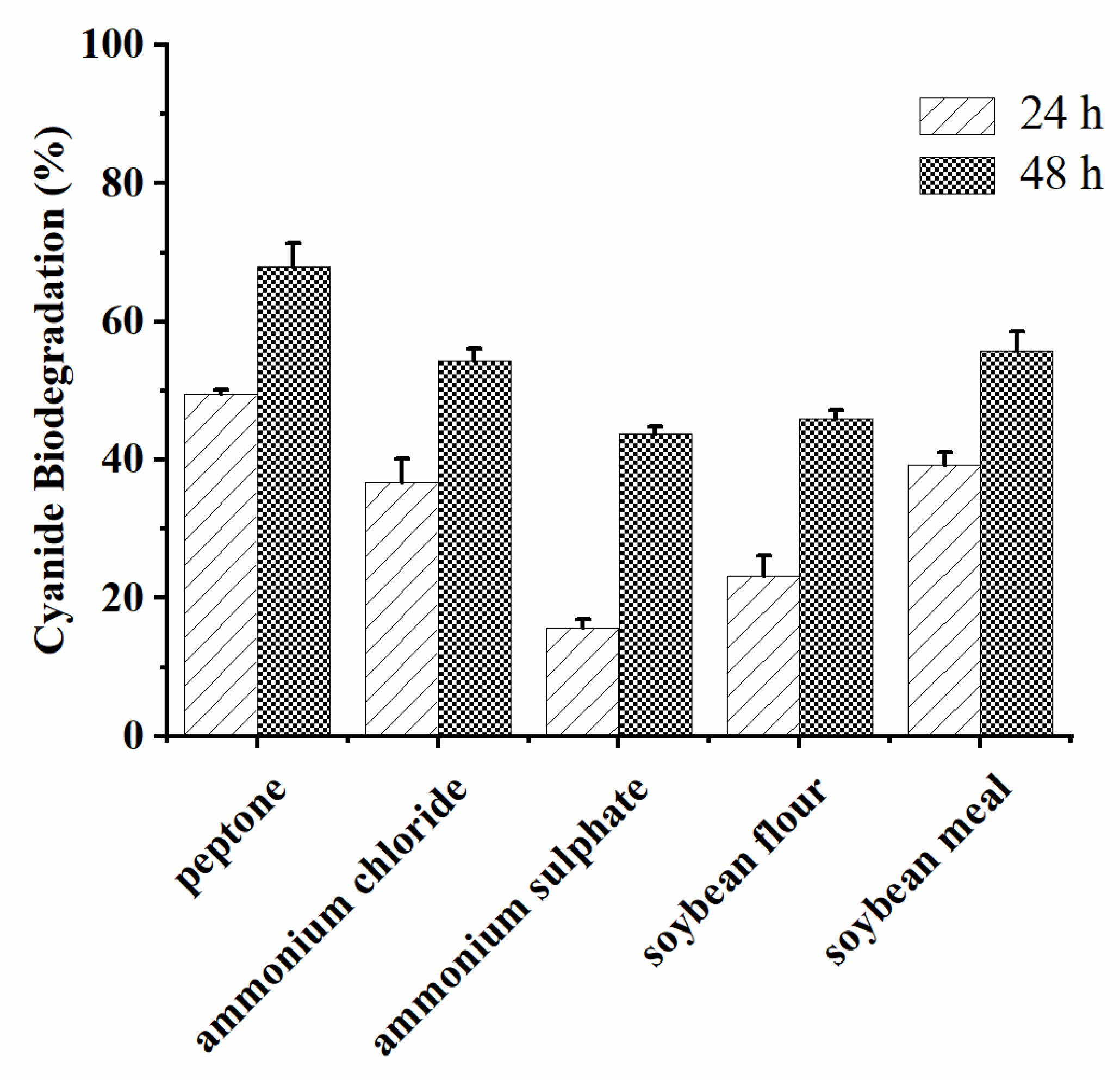
3.3.3. Effects of Carbon Source and Nitrogen Source on Cyanide Biodegradation by A. viridans
3.4. Significant Factors Identified by PBD
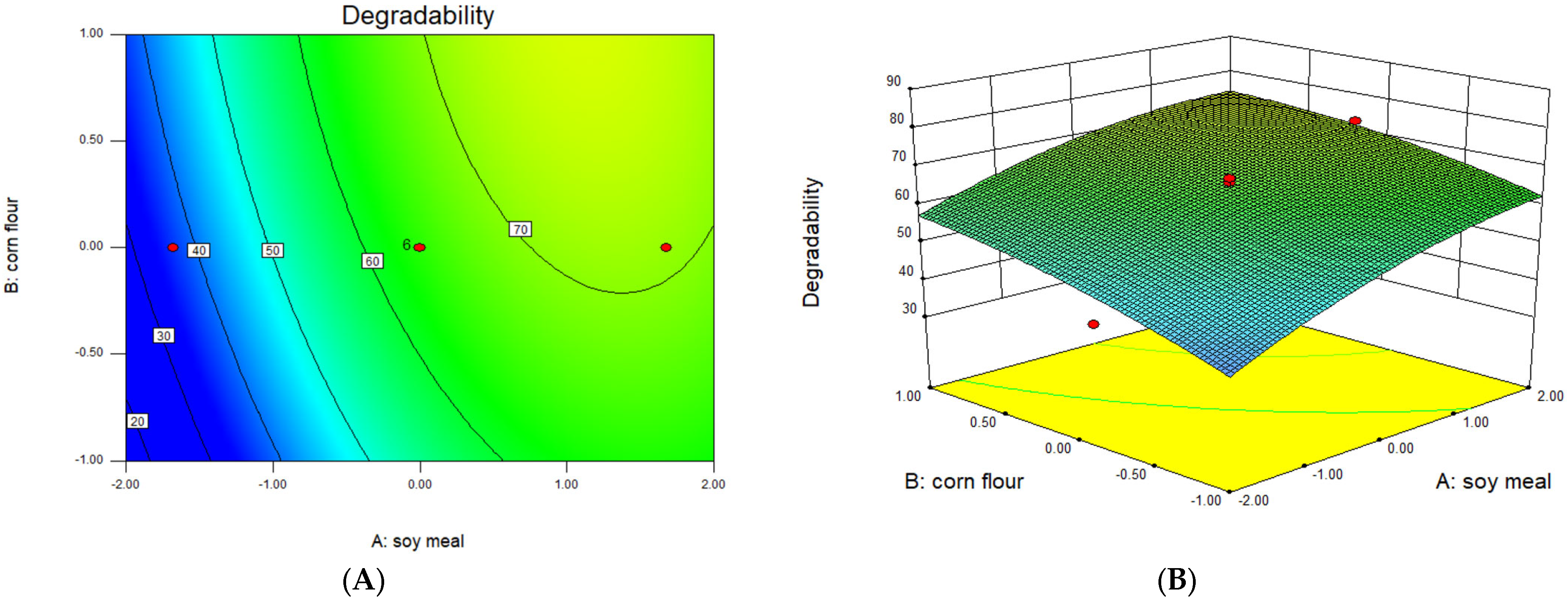
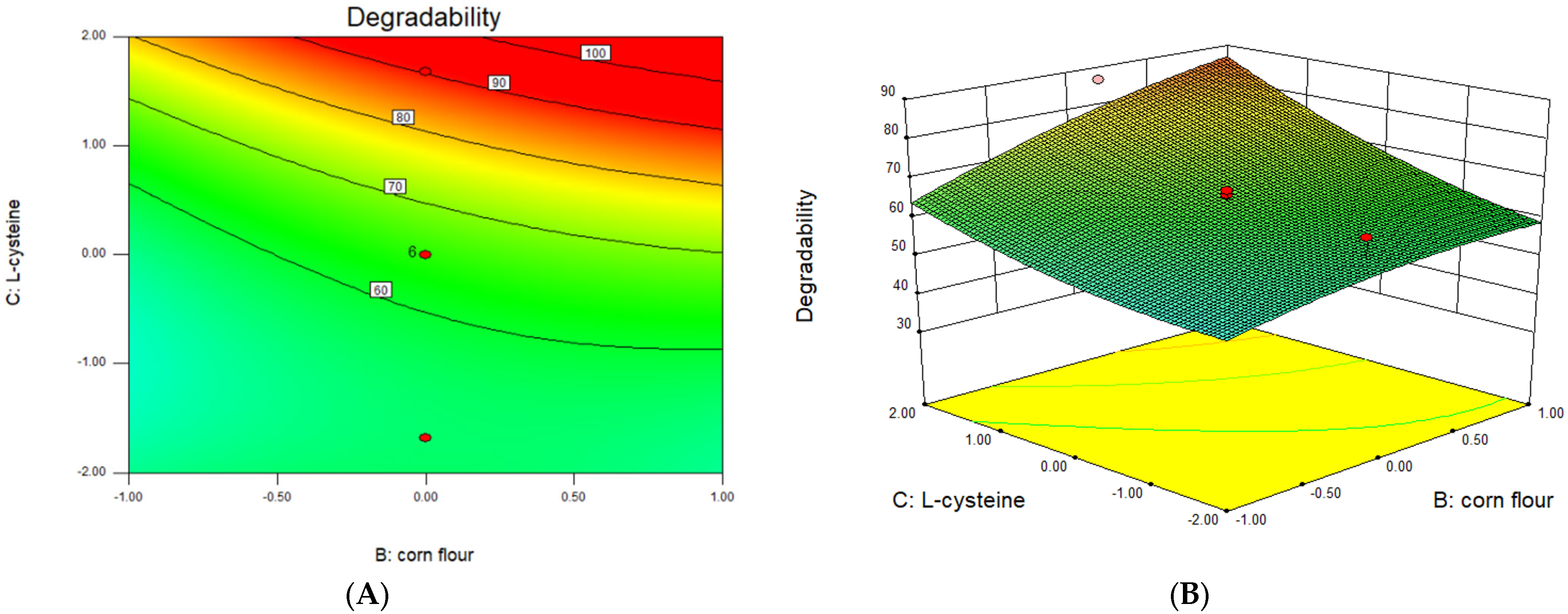
3.5. Optimization of Significant Variables by CCD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- Razanamahandry, L.C.; Andrianisa, H.A.; Karoui, H.; Kouakou, K.M.; Yacouba, H. Biodegradation of free cyanide by bacterial species isolated from cyanide-contaminated artisanal gold mining catchment area in Burkina Faso. Chemosphere 2016, 157, 71–78. [Google Scholar] [CrossRef]
- Ojaghi, A.; Shafaie Tonkaboni, S.Z.; Shariati, P.; Doulati Ardejani, F. Novel cyanide electro-biodegradation using Bacillus pumilus ATCC 7061 in aqueous solution. J. Environ. Health Sci. Eng. 2018, 16, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Karahan, A.G.; Ciftci, H.; Sagdic, O. Biological treatment of cyanide by natural isolated bacteria. Miner. Eng. 2003, 16, 643–649. [Google Scholar] [CrossRef]
- Ebbs, S. Biological degradation of cyanide compounds. Curr. Opin. Biotechnol. 2004, 15, 231–236. [Google Scholar] [CrossRef]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Enzymatic mechanism and biochemistry for cyanide degradation: A review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Merchán, F.; Blasco, R.; Igeño, M.I.; Martínez-Luque, M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D. Cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 involves a malate: Quinone oxidoreductase and an associated cyanide-insensitive electron transfer chain. Microbiology 2011, 157, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Avcioglu, N.H.; Bilkay, I.S. Biological Treatment of Cyanide by Using Klebsiella pneumoniae Species. Food. Technol. Biotechnol. 2016, 54, 450–454. [Google Scholar] [CrossRef]
- Huertas, M.J.; Sáez, L.P.; Roldán, M.D.; Luque-Almagro, V.M.; Martínez-Luque, M.; Blasco, R.; Castillo, F.; Moreno-Vivián, C.; García-García, I. Alkaline cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 in a batch reactor. Influence of pH. J. Hazard. Mater. 2010, 179, 72–78. [Google Scholar] [CrossRef]
- Singh, U.; Arora, N.K.; Sachan, P. Simultaneous biodegradation of phenol and cyanide present in coke-oven effluent using immobilized Pseudomonas putida and Pseudomonas stutzeri. Braz. J. Microbiol. 2018, 49, 38–44. [Google Scholar] [CrossRef]
- Meyers, P.R.; Gokool, P.; Rawlings, D.E.; Woods, D.R. An efficient cyanide-degrading Bacillus pumilus strain. J. Gen. Microbiol. 1991, 137, 1397–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamba, K.I.; Ahmad, S.A.; Zulkharnain, A.; Yasid, N.A.; Ibrahim, S.; Shukor, M.Y. Batch growth kinetic studies of locally isolated cyanide-degrading Serratia marcescens strain AQ07. 3 Biotech 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Mekuto, L.; Alegbeleye, O.O.; Ntwampe, S.K.; Ngongang, M.M.; Mudumbi, J.B.; Akinpelu, E.A. Co-metabolism of thiocyanate and free cyanide by Exiguobacterium acetylicum and Bacillus marisflavi under alkaline conditions. 3 Biotech 2016, 6, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, M.M.; Ciminelli, V.S.; de Andrade, M.C.; Linardi, V.R. Cyanide degradation by an Escherichia coli strain. Can. J. Microbiol. 1996, 42, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, F.; Ciftci, H.; Akcil, A. Biodegradation of cyanide containing effluents by Scenedesmus obliquus. J. Hazard. Mater. 2009, 162, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, R.; Bs, S.; Thandeeswaran, M.; kG, K.; Vijayasarathy, M.; Angayarkanni, J.; Muthusamy, G. Microbial (Enzymatic) degradation of cyanide to produce pterins as cofactors. Curr. Microbiol. 2020, 77, 578–587. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Bhalla, T.C. In vitro cyanide degradation by Serretia marcescens RL2b. Int. J. Environ. Sci. 2013, 3, 1969–1979. [Google Scholar]
- Maniyam, M.N.; Sjahrir, F.; Ibrahim, A.; Cass, A.G. Biodegradation of cyanide by Rhodococcus UKMP-5 M. Biologia 2013, 68, 177–185. [Google Scholar] [CrossRef]
- Mirizadeh, S.; Yaghmaei, S.; Ghobadi Nejad, Z. Biodegradation of cyanide by a new isolated strain under alkaline conditions and optimization by response surface methodology. J. Environ. Health Sci. Eng. 2014, 12, 85. [Google Scholar] [CrossRef] [Green Version]
- Nasution, F.; Theanhom, A.A.; Sukartini; Bhuyar, P.; Chumpookam, J. Genetic diversity evaluation in wild Muntingia calabura L. based on Random Amplified Polymorphic DNA (RAPD) markers. Gene Rep. 2021, 25, 101335. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.O.; Kena, M.; Golela, M.T.; Amodu, O.S. Free cyanide and thiocyanate biodegradation by Pseudomonas aeruginosa STK 03 capable of heterotrophic nitrification under alkaline conditions. 3 Biotech 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Xu, X.M.; Zhu, Q.; Deng, M.C.; Feng, L.; Peng, J.; Yuan, J.P.; Wang, J.H. An effective method for the detoxification of cyanide-rich wastewater by Bacillus sp. CN-22. Appl. Microbiol. Biotechnol. 2014, 98, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.; Hirch, A.; Cowan, S.T. Aerococcus, a new bacterial genus. J. Gen. Microbiol. 1953, 8, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradkhani, M.; Yaghmaei, S.; Nejad, Z.G. Biodegradation of cyanide under alkaline conditions by a srain of Pseudomonas putida isolated from gold mine soil and optimization of process variables through response surface methodology (RSM). Period Polytech-Chem. 2018, 63, 265–273. [Google Scholar]
- Jandhyala, D.M.; Willson, R.C.; Sewell, B.T.; Benedik, M.J. Comparison of cyanide-degrading nitrilases. Appl. Microbiol. Biotechnol. 2005, 68, 327–335. [Google Scholar] [CrossRef]
- Adjei, M.D.; Ohta, Y. Factors affecting the biodegradation of cyanide by Burkholderia cepacia strain C-3. J. Biosci. Bioeng. 2000, 89, 274–277. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Adweson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley and Sons, Inc.: New York, NY, USA, 2008. [Google Scholar]



| Characteristics | Properties | Characteristics | Properties |
|---|---|---|---|
| Glucosesemi-solidagar | + | Melibiose | − |
| Ornithinedecarboxylase | − | Raffinose | − |
| Lysinedecarboxylase | − | Glucose | + |
| Ureaenzyme | + | Sucrose | + |
| Hydrogensulfide | − | Xylose | + |
| V-Ptest | + | Escin | + |
| Phenylalanine | − | Rhamnose | + |
| Mannitol | + | Argininedihydrolysis | − |
| Sorbose | + | Nitratereduction | − |
| Sorbitol | + | Gramstaining | + |
| Run | Variables | Degradability (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||
| 1 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | 95.7 |
| 2 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | 59 |
| 3 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | 80.8 |
| 4 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | 72.1 |
| 5 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | 87.7 |
| 6 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | 63.5 |
| 7 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | 50.6 |
| 8 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | 57.5 |
| 9 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | 43.3 |
| 10 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | 67 |
| 11 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | 96.2 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 37.1 |
| Variables | Coefficient | F Value | p Value | Ranking |
|---|---|---|---|---|
| pH | 3.14 | 2.93 | 0.1856 | 6 |
| Inoculum amount | −1.77 | 0.93 | 0.4050 | 7 |
| L-methionine | 4.79 | 6.81 | 0.0797 | 5 |
| L-cysteine | 11.68 | 40.43 | 0.0079 | 1 ** |
| Corn flour | 9.34 | 25.88 | 0.0147 | 2 * |
| Soy meal | 6.49 | 12.50 | 0.0385 | 3 * |
| Soy flour | −5.99 | 10.65 | 0.0470 | 4 * |
| Glucose | 1.74 | 0.90 | 0.4128 | 8 |
| Factor | Variables | Range Examined | Levels | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |||
| X1 | Soy meal (%) | 0.5–1.5 | 0.5 | 0.75 | 1 | 1.25 | 1.5 |
| X2 | Corn flour (%) | 0.5–1.5 | 0.5 | 0.75 | 1 | 1.25 | 1.5 |
| X3 | L-cysteine (%) | 0.4–1.2 | 0.4 | 0.6 | 0.8 | 1.0 | 1.2 |
| Run | Soy Meal (%) | Corn Flour (%) | L-Cysteine (%) | Degradability (%) |
|---|---|---|---|---|
| 1 | 0.75 | 0.75 | 0.6 | 33.7 |
| 2 | 1.25 | 0.75 | 0.6 | 51.9 |
| 3 | 0.75 | 1.25 | 0.6 | 43.4 |
| 4 | 1.25 | 1.25 | 0.6 | 61.2 |
| 5 | 0.75 | 0.75 | 1.0 | 45.9 |
| 6 | 1.25 | 0.75 | 1.0 | 72.3 |
| 7 | 0.75 | 1.25 | 1.0 | 77 |
| 8 | 1.25 | 1.25 | 1.0 | 91 |
| 9 | 0.5 | 1 | 0.8 | 37.7 |
| 10 | 1.5 | 1 | 0.8 | 75 |
| 11 | 1 | 0.5 | 0.8 | 50.1 |
| 12 | 1 | 1.5 | 0.8 | 70.9 |
| 13 | 1 | 1 | 0.4 | 63.4 |
| 14 | 1 | 1 | 1.2 | 88.7 |
| 15 | 1 | 1 | 0.8 | 63 |
| 16 | 1 | 1 | 0.8 | 67 |
| 17 | 1 | 1 | 0.8 | 63 |
| 18 | 1 | 1 | 0.8 | 60 |
| 19 | 1 | 1 | 0.8 | 66 |
| 20 | 1 | 1 | 0.8 | 67 |
| Term Model | Sum of Squares | DF | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 4221.61 | 9 | 469.07 | 21.04 | <0.0001 |
| X1 | 1417.41 | 1 | 1417.41 | 63.57 | <0.0001 |
| X2 | 788.66 | 1 | 788.66 | 35.37 | 0.0001 |
| X3 | 1405.59 | 1 | 1405.59 | 63.04 | <0.0001 |
| X12 | 210.79 | 1 | 210.79 | 9.45 | 0.0117 |
| X22 | 80.08 | 1 | 80.08 | 3.59 | 0.0873 |
| X32 | 142.14 | 1 | 142.14 | 6.37 | 0.0301 |
| X1X2 | 20.48 | 1 | 20.48 | 0.92 | 0.3605 |
| X1X3 | 2.42 | 1 | 2.42 | 0.11 | 0.7486 |
| X2X3 | 118.58 | 1 | 118.58 | 5.32 | 0.0438 |
| Residual | 222.98 | 10 | 22.30 | ||
| Lack of fit | 183.65 | 5 | 36.73 | 4.67 | 0.580 |
| Pure error | 39.33 | 5 | 7.87 | ||
| Cor. total | 4444.60 | 19 |
| Soybean Meal (%) | Corn Flour (%) | L-Cysteine (%) | CN Degradation (%) |
|---|---|---|---|
| 1.11 | 1.5 | 1.2 | 97.3 ± 2.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Lu, Y.; Feng, Z.; Yu, H.; Ma, P.; Zhu, J.; Wang, Y.; Sun, J. Biodegradation of Cyanide by a New Isolated Aerococcus viridans and Optimization of Degradation Conditions by Response Surface Methodology. Sustainability 2022, 14, 15560. https://doi.org/10.3390/su142315560
Jiang W, Lu Y, Feng Z, Yu H, Ma P, Zhu J, Wang Y, Sun J. Biodegradation of Cyanide by a New Isolated Aerococcus viridans and Optimization of Degradation Conditions by Response Surface Methodology. Sustainability. 2022; 14(23):15560. https://doi.org/10.3390/su142315560
Chicago/Turabian StyleJiang, Wenjin, Yang Lu, Zezhong Feng, Haixiao Yu, Ping Ma, Jinqi Zhu, Yingnan Wang, and Jinfu Sun. 2022. "Biodegradation of Cyanide by a New Isolated Aerococcus viridans and Optimization of Degradation Conditions by Response Surface Methodology" Sustainability 14, no. 23: 15560. https://doi.org/10.3390/su142315560
APA StyleJiang, W., Lu, Y., Feng, Z., Yu, H., Ma, P., Zhu, J., Wang, Y., & Sun, J. (2022). Biodegradation of Cyanide by a New Isolated Aerococcus viridans and Optimization of Degradation Conditions by Response Surface Methodology. Sustainability, 14(23), 15560. https://doi.org/10.3390/su142315560








