Agglomeration–Flotation of Microplastics Using Kerosene as Bridging Liquid for Particle Size Enlargement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
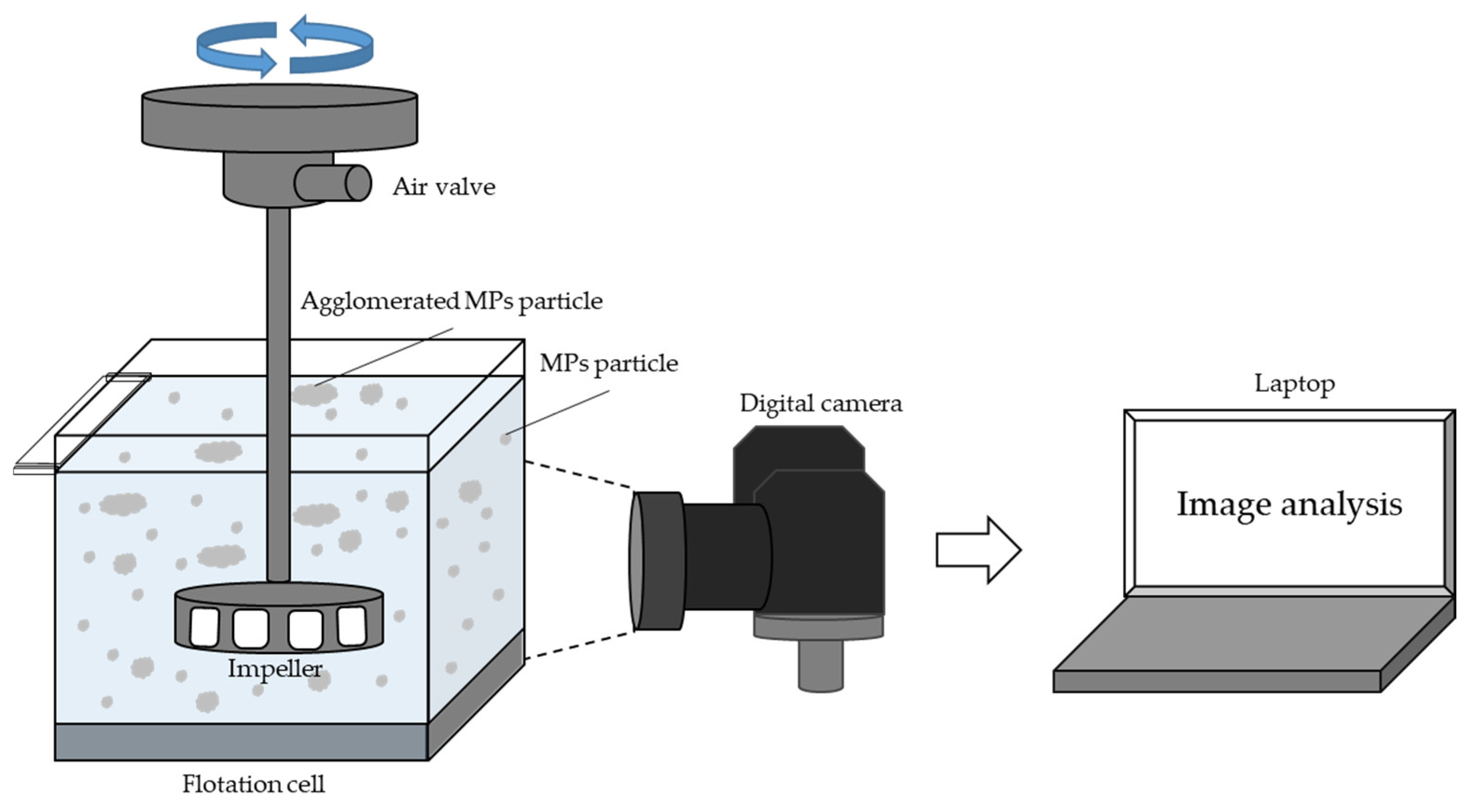
2.2. Microplastics Flotation Experiments
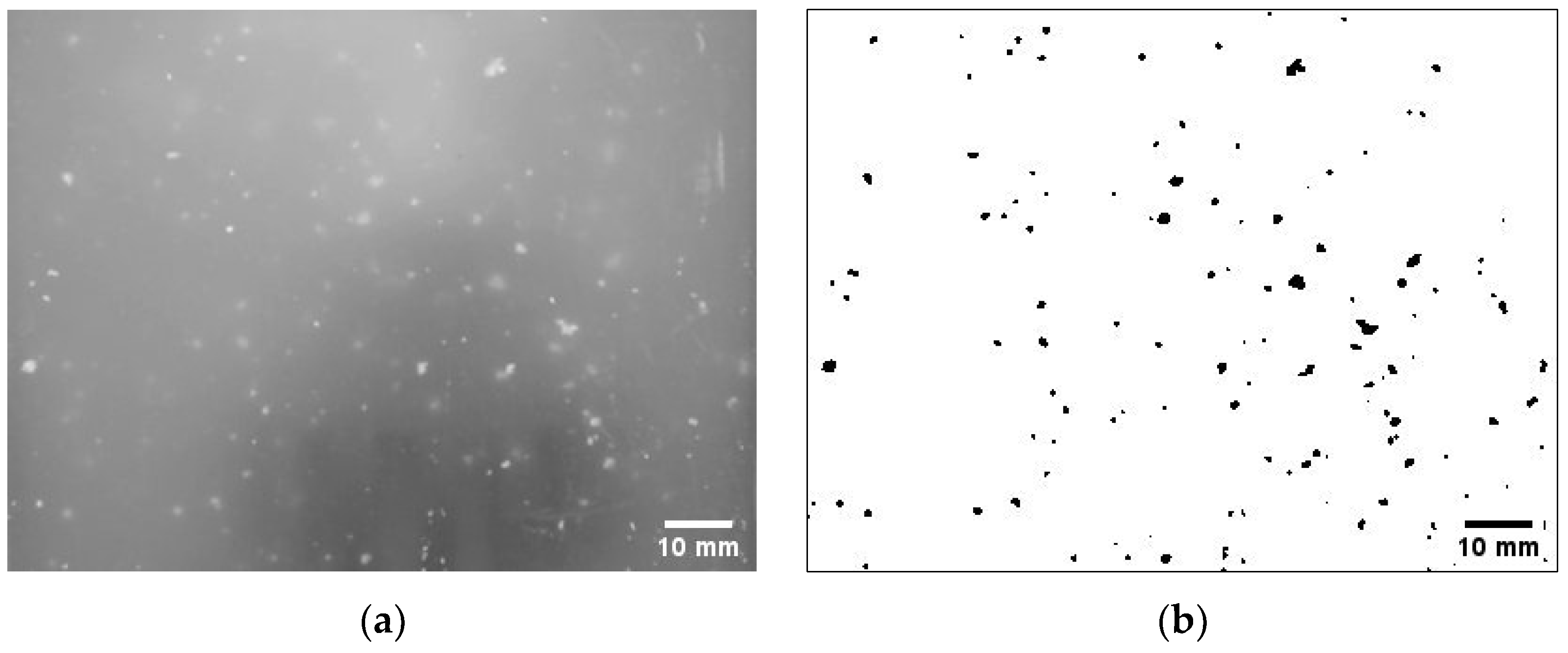
2.3. Particle Size Analysis of Microplastics
3. Results and Discussion
3.1. Effects of Plastic Types on the Floatability of Microplastics
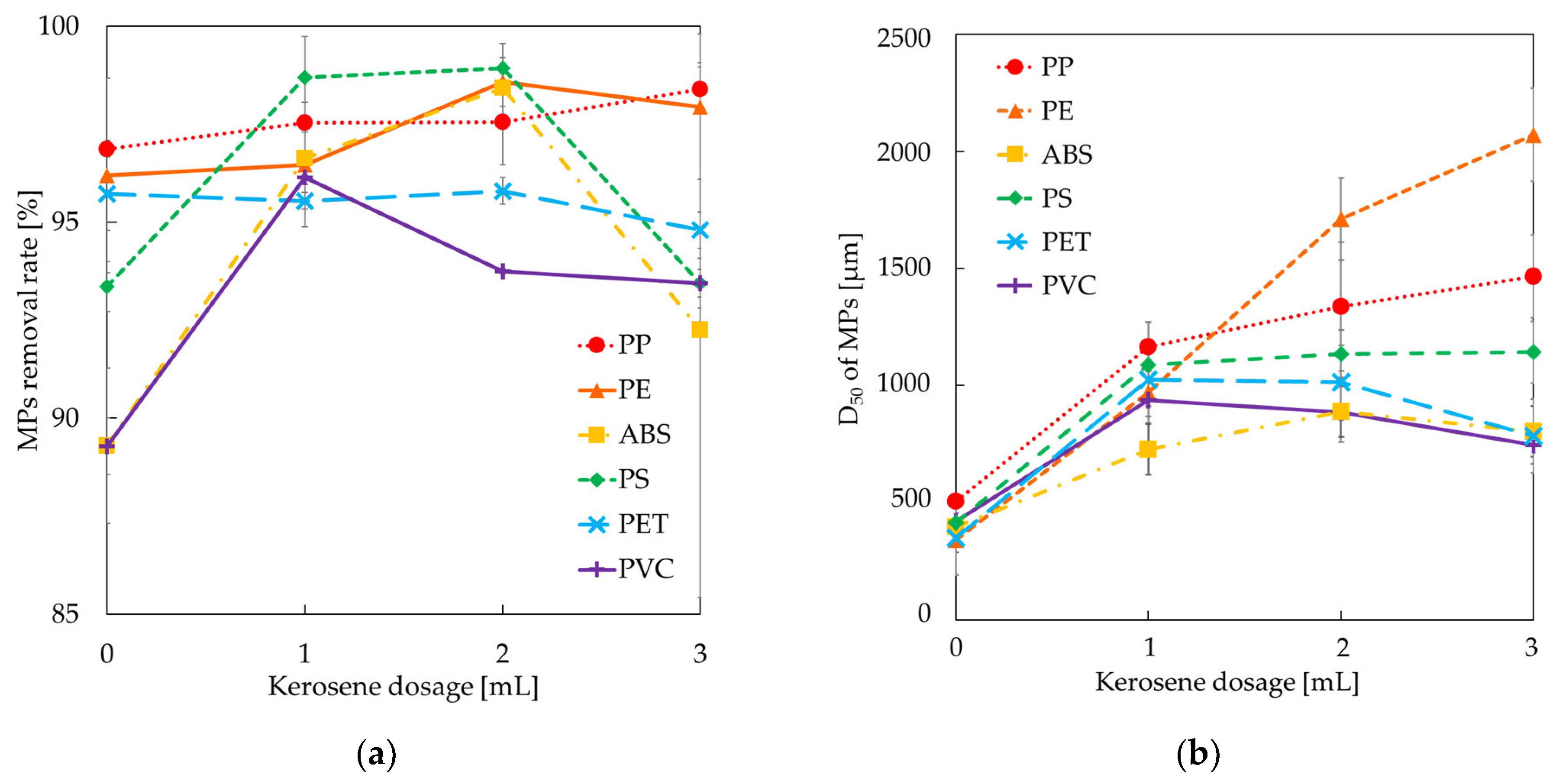
3.2. Effects of Kerosene Dosage on the Floatability of Microplastics
3.3. Effects of Kerosene Dosage on the Particle Size Enlargement of Microplastics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, M.; Saito, A.; Takeuchi, M.; Murase, N.; Phengsaart, T.; Tabelin, C.B.; Hiroyoshi, N. Development of the reverse hybrid jig: Separation of polyethylene and cross-linked polyethylene from eco-cable wire. Miner. Eng. 2021, 174, 107241. [Google Scholar] [CrossRef]
- Phengsaart, T.; Ito, M.; Kimura, S.; Azuma, A.; Hori, K.; Tanno, H.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Development of a restraining wall and screw-extractor discharge system for continuous jig separation of mixed plastics. Miner. Eng. 2021, 168, 106918. [Google Scholar] [CrossRef]
- Phengsaart, T.; Manositchaikul, C.; Srichonphaisarn, P.; Juntarasakul, O.; Maneeintr, K.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N.; Ito, M. Reverse Hybrid Jig Separation Efficiency Estimation of Floating Plastics Using Apparent Specific Gravity and Concentration Criterion. Front. Chem. 2022, 10, 1014441. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.U.; Fred-Ahmadu, O.H.; Bassey, D.E.; Atayero, A.A. COVID-19 pandemic and emerging plastic-based personal protective equipment waste pollution and management in Africa. J. Environ. Chem. Eng. 2021, 9, 105222. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Elder, P. End-of-Waste Criteria for Waste Plastic for Conversion; Technical Proposal, EUR 26843; Pubication Office of the European Union: Luxembourg, 2014; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC91637 (accessed on 13 June 2022).
- Ito, M.; Takeuchi, M.; Saito, A.; Murase, N.; Phengsaart, T.; Tabelin, C.B.; Hiroyoshi, N.; Tsunekawa, M. Improvement of hybrid jig separation efficiency using wetting agents for the recycling of mixed-plastic wastes. J. Mater. Cycles Waste Manag. 2019, 21, 1376–1383. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef] [Green Version]
- Fred-Ahmadu, O.H.; Tenebe, I.T.; Ayejuyo, O.O.; Benson, N.U. Microplastics and associated organic pollutants in beach sediments from the Gulf of Guinea (SE Atlantic) coastal ecosystems. Chemosphere 2022, 298, 134193. [Google Scholar] [CrossRef]
- Bissen, R.; Chawchai, S. Microplastics on beaches along the eastern Gulf of Thailand–A preliminary study. Mar. Pollut. Bull. 2020, 157, 1113445. [Google Scholar] [CrossRef]
- Thepwilai, S.; Wangritthikraikul, K.; Chawchai, S.; Bissen, R. Testing the factors controlling the numbers of microplastics on beaches along the western Gulf of Thailand. Mar. Pollut. Bull. 2021, 168, 112467. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef]
- Wang, R.; Ji, M.; Zhai, H.; Liu, Y. Occurrence of phthalate esters and microplastics in urban secondary effluents, receiving water bodies and reclaimed water treatment processes. Sci. Total Environ. 2020, 737, 140219. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Bian, K.; Wang, H.; Wang, C. Is froth flotation a potential scheme for microplastics removal? Analysis on flotation kinetics and surface characteristics. Sci. Total Environ. 2021, 792, 148345. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Ayejuyo, O.O.; Benson, N.U. Microplastics distribution and characterization in epipsammic sediments of tropical Atlantic Ocean, Nigeria. Reg. Stud. 2020, 38, 101365. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Ayejuyo, O.O.; Tenebe, I.T.; Benson, N.U. Occurrence and distribution of micro(meso)plastic-sorbed heavy metals and metalloids in sediments, Gulf of Guinea coast (SE Atlantic). Sci. Total Environ. 2022, 813, 152605. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Thavamani, P. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Khandaker, S.; Chowdhury, M.F.; Awual, R.; Islam, A.; Kuba, T. Efficient cesium encapsulation from contaminated water by cellulosic biomass based activated wood charcoal. Chemosphere 2021, 262, 127801. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, S.; Hasan, N.; Islam, A.; Hasan, M.; Awual, R. Utilizing an alternative composite material for effective copper (II) ion capturing from wastewater. J. Mol. Liq. 2021, 336, 116325. [Google Scholar] [CrossRef]
- Awual, R. A novel facial composite adsorbent for enhanced copper (II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Benson, N.U.; Fred-Ahmadu, O.H. Occurrence and distribution of microplastics-sorbed phthalic acid esters (PAEs) in coastal psammitic sediments of tropical Atlantic Ocean, Gulf of Guinea. Sci. Total Environ. 2020, 730, 139013. [Google Scholar] [CrossRef] [PubMed]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Sci. Total Environ. 2020, 706, 135978. [Google Scholar] [CrossRef] [PubMed]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Bilgin, M.; Meral, Y.; Fatih, K. Microplastic removal by aerated grit chambers versus settling tanks of a municipal wastewater treatment plant. J. Water Process Eng. 2020, 38, 101604. [Google Scholar] [CrossRef]
- Bayo, J.; Sonia, O.; Joaquín, L.C. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Bayo, J.; Joaquín, L.C.; Sonia, O. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Carmen, H.C.; Marcello, S.; Stijn, V.H.; Diederik, P.L.R. Horizontal subsurface flow constructed wetlands as tertiary treatment: Can they be an efficient barrier for microplastics pollution? Sci. Total Environ. 2020, 721, 137785. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Hidayaturrahman, H.; Peera, S.G.; Lee, T.G. Elimination of Microplastics at Different Stages in Wastewater Treatment Plants. Water 2022, 14, 2404. [Google Scholar] [CrossRef]
- Xu, X.; Jian, Y.; Xue, Y.; Hou, Q.; Wang, L. Microplastics in the wastewater treatment plants (WWTPs): Occurrence and removal. Chemosphere 2019, 235, 1089–1096. [Google Scholar] [CrossRef]
- Rajala, K.; Gronfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef]
- Lapointe, M.; Jeffrey, M.F.; Laura, M.H.; Nathalie, T. Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef]
- Skaf, D.W.; Vito, L.P.; Javaz, T.R.; Kyle, A.K. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem. Eng. J. 2020, 386, 123807. [Google Scholar] [CrossRef]
- Shahi, N.K.; Maeng, M.; Kim, D.; Dockko, S. Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. PSEP 2020, 141, 9–17. [Google Scholar] [CrossRef]
- Wang, Z.; Majid, S.; Amanda, L.L. Filtration of microplastic spheres by biochar: Removal efficiency and immobilisation mechanisms. Water Res. 2020, 184, 116165. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.; Miao, C.; Wang, Y.-R.; Gao, G.-K.; Yang, R.-X.; Zhu, H.-J.; Wang, J.-H.; Li, S.-L.; Lan, Y.-Q. Metal–organic framework-based foams for efficient microplastics removal. J. Mater. Chem. 2020, 8, 14644–14652. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Cuartucci, M. Ultrafiltration, a cost-effective solution for treating surface water to potable standard. Water Pract. Technol. 2020, 15, 426–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Bian, K.; Wang, H.; Wang, C. Flotation separation of hazardous polyvinyl chloride towards source control of microplastics based on selective hydrophilization of plasticizer-doping surfaces. J. Hazard. Mater. 2022, 423, 127095. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, E.; Liu, X.; Miao, Z.; Jiang, X.; Han, Y. Flotation and separation of microplastics from the eye-glass polishing wastewater using sec-octyl alcohol and diesel oil. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Fu, J.; Liu, Y. Flotation separation of waste plastics for recycling—A review. Waste Manag. 2015, 41, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Forssberg, E.; Pugh, R. Selective flotation separation of plastics by particle control. Resour. Conserv. Recycl. 2001, 33, 37–50. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite and Quartz: Effects of Agitation Strength during Agglomeration Using Emulsified Oil on Chalcopyrite. Minerals 2020, 10, 380. [Google Scholar] [CrossRef]
- Wang, L.; Li, C. A brief review of pulp and froth rheology in mineral flotation. J. Chem. 2020, 16, 3894542. [Google Scholar] [CrossRef]
- Sajjad, M.; Otsuki, A. Correlation between flotation and rheology of fine particle suspensions. Metals 2022, 12, 270. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals 2020, 10, 912. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Yamazawa, R.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Kinetic Analysis for Agglomeration-Flotation of Finely Ground Chalcopyrite: Comparison of First Order Kinetic Model and Experimental Results. Mater. Trans. 2020, 61, 1940–1948. [Google Scholar] [CrossRef]
- Hornn, V.; Park, I.; Ito, M.; Shimada, H.; Suto, T.; Tabelin, C.B.; Jeon, S.; Hiroyoshi, N. Agglomeration-flotation of finely ground chalcopyrite using surfactant-stabilized oil emulsions: Effects of co-existing minerals and ions. Miner. Eng. 2021, 171, 107076. [Google Scholar] [CrossRef]
- Tavares, R.; Ramos, A.; Rouboa, A. Microplastics thermal treatment by polyethylene terephthalate-biomass gasification. Energy Convers. Manag. 2018, 162, 118–131. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, T.; Geng, M.; Cui, K.; Peng, J.; Luo, G.; Núñez Delgado, A.; Zhou, Y.; Liu, J.; Fei, J. Occurrence of Microplastics from Plastic Fragments in Cultivated Soil of Sichuan Province: The Key Controls. Water 2022, 14, 1417. [Google Scholar] [CrossRef]
- Mazahernasab, R.; Ahamadi, R.; Ravanasa, E. Direct Bubble Size Measurement in a Mechanical Flotation Cell by Image Analysis and Laser Diffraction Technique—A Comparative Study. IJCCE 2021, 40, 1653–1664. [Google Scholar]
- Shent, H.; Pugh, R.; Forssberg, E. A review of plastics waste recycling and the flotation of plastics. Resour. Conserv. Recycl. 1999, 25, 85–109. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Zhang, N. Efficiency and mechanism analysis of the flotation of anthracite coal using soybean oil as an alternative sustainable collector. Energy Sources Part A 2021, 43, 2210–2217. [Google Scholar] [CrossRef]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Tanaka, S.; Kitajima, N.; Saito, A.; Park, I.; Hiroyoshi, N. A physical separation scheme to improve ammonium thiosulfate leaching of gold by separation of base metals in crushed mobile phones. Miner. Eng. 2019, 138, 168–177. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julapong, P.; Ekasin, J.; Katethol, P.; Srichonphaisarn, P.; Juntarasakul, O.; Numprasanthai, A.; Tabelin, C.B.; Phengsaart, T. Agglomeration–Flotation of Microplastics Using Kerosene as Bridging Liquid for Particle Size Enlargement. Sustainability 2022, 14, 15584. https://doi.org/10.3390/su142315584
Julapong P, Ekasin J, Katethol P, Srichonphaisarn P, Juntarasakul O, Numprasanthai A, Tabelin CB, Phengsaart T. Agglomeration–Flotation of Microplastics Using Kerosene as Bridging Liquid for Particle Size Enlargement. Sustainability. 2022; 14(23):15584. https://doi.org/10.3390/su142315584
Chicago/Turabian StyleJulapong, Pongsiri, Jiraphon Ekasin, Pattaranun Katethol, Palot Srichonphaisarn, Onchanok Juntarasakul, Apisit Numprasanthai, Carlito Baltazar Tabelin, and Theerayut Phengsaart. 2022. "Agglomeration–Flotation of Microplastics Using Kerosene as Bridging Liquid for Particle Size Enlargement" Sustainability 14, no. 23: 15584. https://doi.org/10.3390/su142315584
APA StyleJulapong, P., Ekasin, J., Katethol, P., Srichonphaisarn, P., Juntarasakul, O., Numprasanthai, A., Tabelin, C. B., & Phengsaart, T. (2022). Agglomeration–Flotation of Microplastics Using Kerosene as Bridging Liquid for Particle Size Enlargement. Sustainability, 14(23), 15584. https://doi.org/10.3390/su142315584









