The Effectiveness of Providing Shell Substrate for the Restoration of Adult Mussel Reefs
Abstract
:1. Introduction
2. Methods
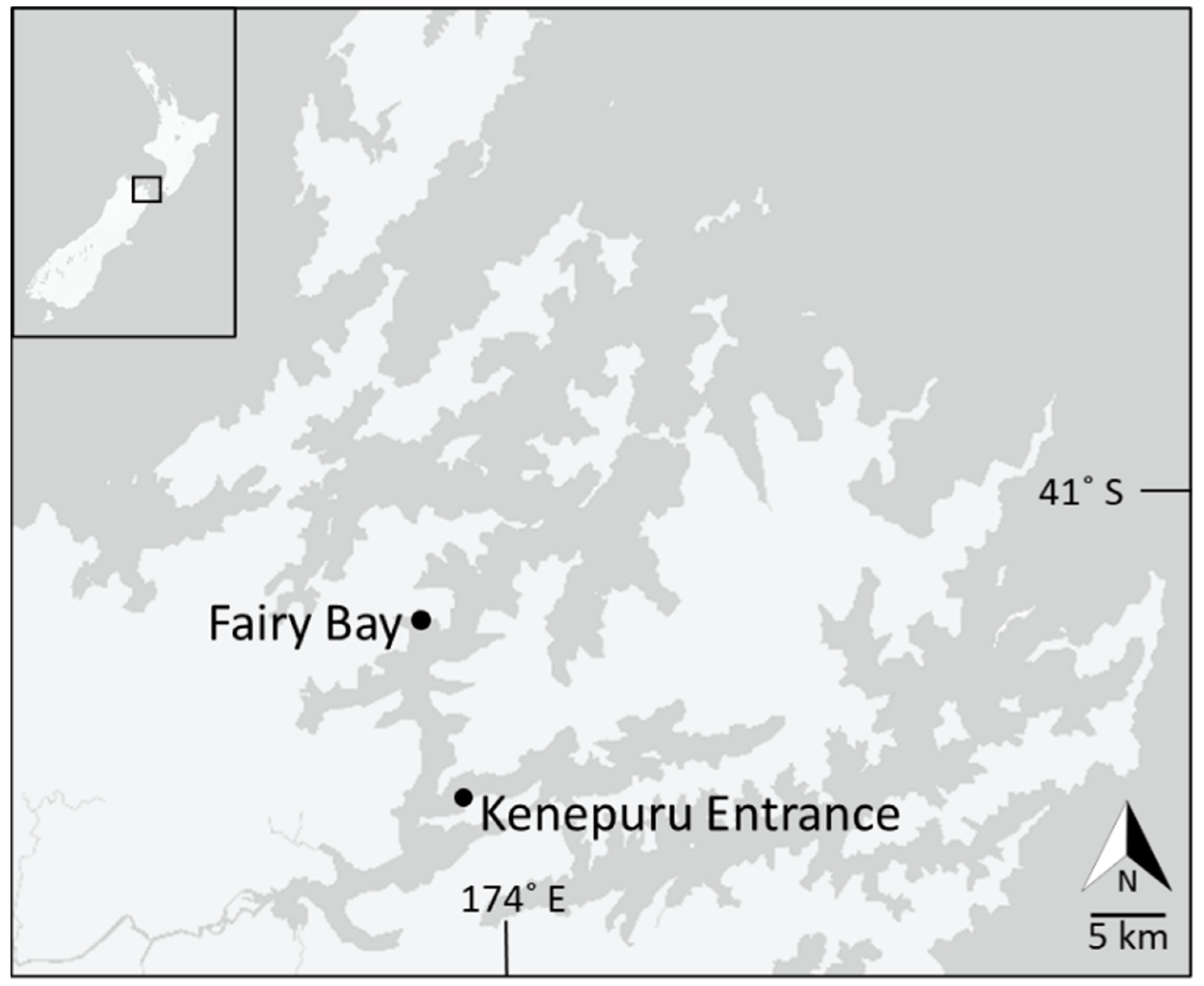
2.1. Study Area
2.2. Shell Substrate and Mussel Deployment
2.3. Sampling Design
2.4. Statistical Analyses
3. Results
3.1. Sediment Composition
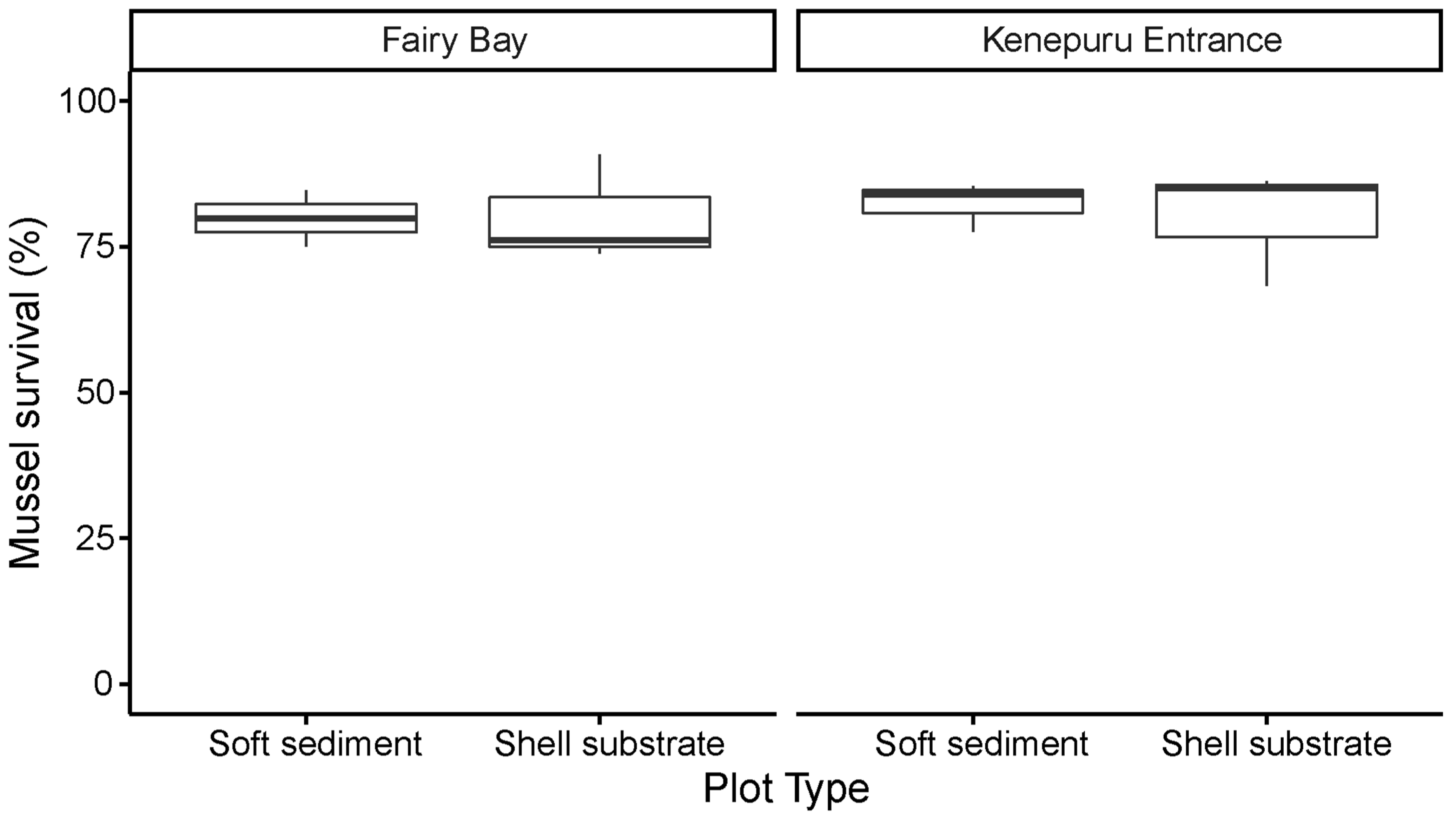
3.2. Mussel Survival
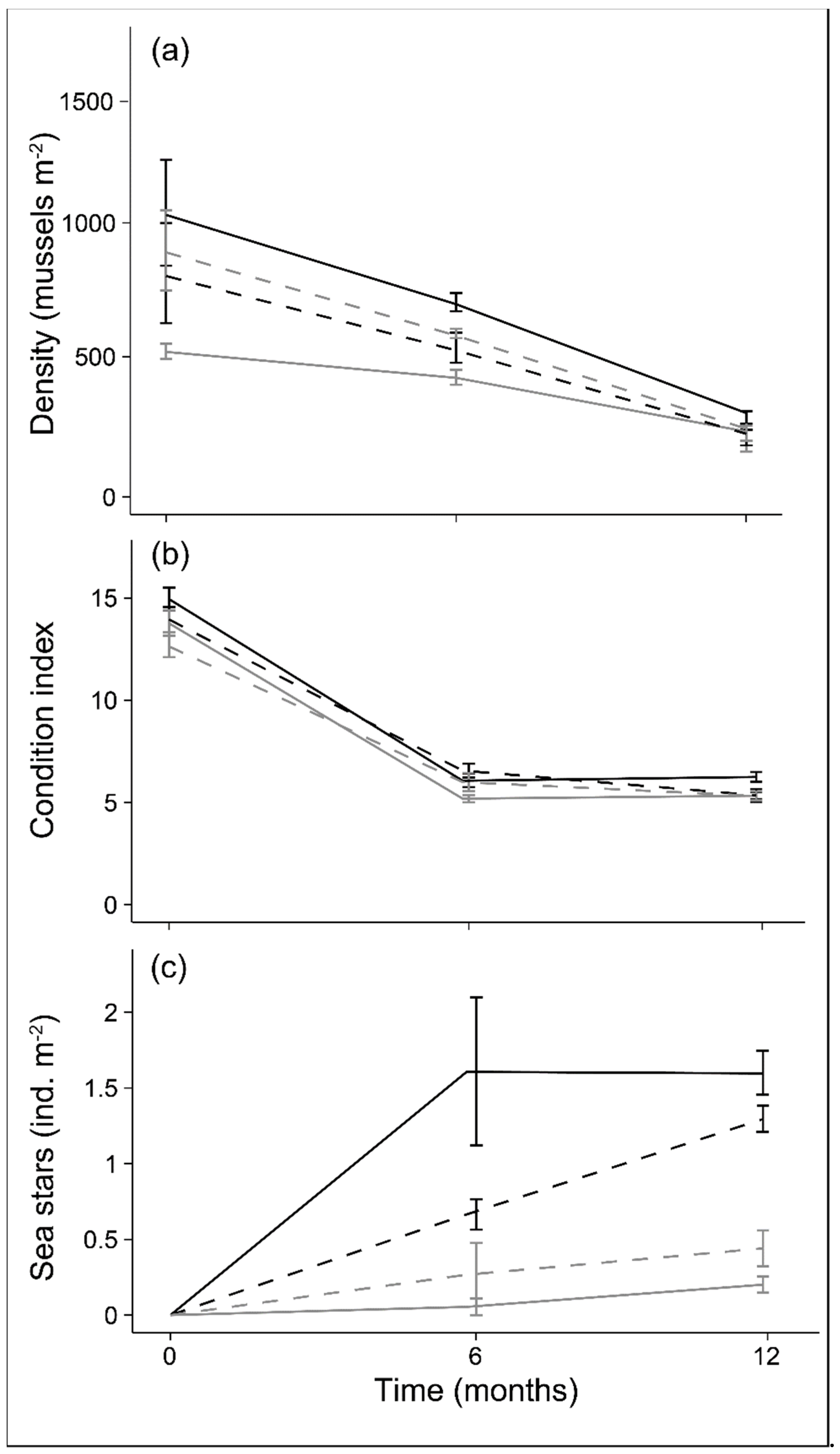
3.3. Mussel Density and Area
3.4. Mussel Growth and Condition
3.5. Sea Star Abundance
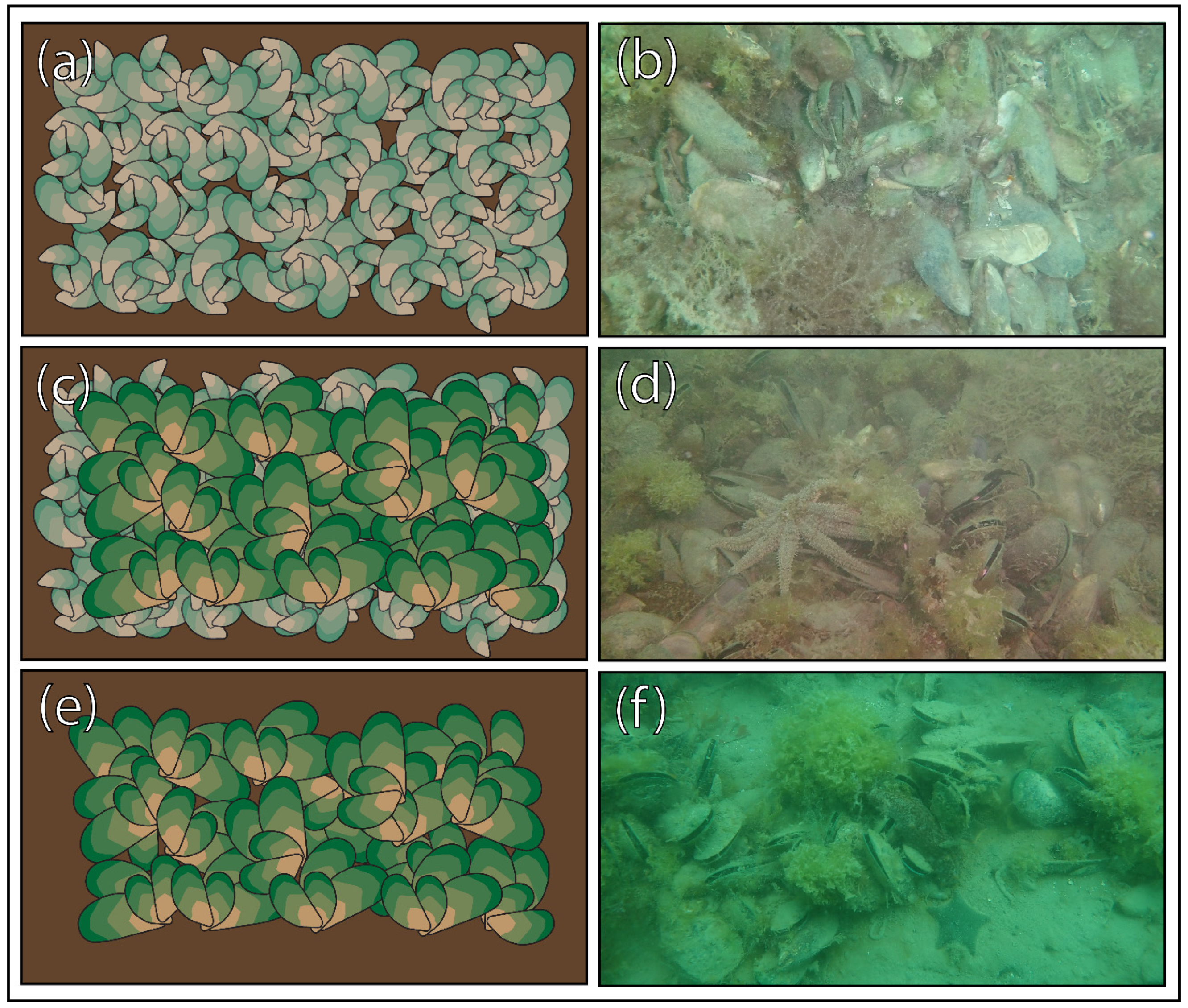
3.6. Visual Observations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piazza, B.P.; Banks, P.D.; La Peyre, M.K. The potential for created oyster shell reefs as a sustainable shoreline protection strategy in Louisiana. Restor. Ecol. 2005, 13, 499–506. [Google Scholar] [CrossRef]
- Commito, J.A.; Celano, E.A.; Celico, H.J.; Como, S.; Johnson, C.P. Mussels matter: Postlarval dispersal dynamics altered by a spatially complex ecosystem engineer. J. Exp. Mar. Bio. Ecol. 2005, 316, 133–147. [Google Scholar] [CrossRef]
- Newell, R. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. J. Shellfish Res. 2004, 23, 51–61. [Google Scholar]
- zu Ermgassen, P.S.E.; Grabowski, J.H.; Gair, J.R.; Powers, S.P. Quantifying fish and mobile invertebrate production from a threatened nursery habitat. J. Appl. Ecol. 2016, 53, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Knoche, S.; Ihde, T.F.; Samonte, G.; Townsend, H.M.; Lipton, D.; Lewis, K.A.; Steinback, S. Estimating Ecological Benefits and Socio-Economic Impacts from Oyster Reef Restoration in the Choptank River Complex, Chesapeake Bay; NOAA Technical Memorandum NMFS-OHC-6; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2020.
- Grabowski, J.H.; Hughes, A.R.; Kimbro, D.L.; Dolan, M.A. How habitat setting influences restored oyster reef communities. Ecology 2005, 86, 1926–1935. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; van der Heide, T.; Holthuijsen, S.J.; van der Reijden, K.J.; Borst, A.C.W.; Olff, H. Biodiversity and food web indicators of community recovery in intertidal shellfish reefs. Biol. Conserv. 2017, 213, 317–324. [Google Scholar] [CrossRef]
- Benjamin, E.D.; Handley, S.J.; Hale, R.; Toone, T.A.; Jeffs, A.; Hillman, J.R. Biodiversity associated with restored small-scale mussel habitats has restoration decision implications. Biodivers. Conserv. 2022, 31, 2833–2855. [Google Scholar] [CrossRef]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.; et al. Shellfish Reefs at Risk: A Global Analysis of Problems and Solutions; The Nature Conservancy: Arlington, VA, USA, 2009. [Google Scholar]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B.C. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Gillies, C.L.; McLeod, I.M.; Alleway, H.K.; Cook, P.; Crawford, C.; Creighton, C.; Diggles, B.; Ford, J.; Hamer, P.; Heller-Wagner, G.; et al. Australian shellfish ecosystems: Past distribution, current status and future direction. PLoS ONE 2018, 13, e0190914. [Google Scholar] [CrossRef] [Green Version]
- Paul, L.J. A history of the Firth of Thames dredge fishery for mussels: Use and abuse of a coastal resource. New Zeal. Aquat. Environ. Biodivers. Rep. 2012, 94, 1–27. [Google Scholar]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 2011, 61, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Bayraktarov, E.; Saunders, M.I.; Abdullah, S.; Mills, M.; Beher, J.; Possingham, H.P.; Mumby, P.J.; Lovelock, C.E. The cost and feasibility of marine coastal restoration. Ecol. Appl. 2016, 26, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Toone, T.A.; Hunter, R.; Benjamin, E.D.; Handley, S.; Jeffs, A.; Hillman, J.R. Conserving shellfish reefs-a systematic review reveals the need to broaden research efforts. Restor. Ecol. 2021, 29, e13375. [Google Scholar] [CrossRef]
- Brumbaugh, R.D.; Beck, M.W.; Coen, L.D.; Craig, L.; Hicks, P. A Practitioners’ Guide to the Design and Monitoring of Shellfish Restoration Projects; The Nature Conservancy: Arlington, VA, USA, 2006. [Google Scholar]
- Brown, S.; Handley, S.; Michael, K.; Schiel, D. Annual pattern of brooding and settlement in a population of the flat oyster Ostrea chilensis from central New Zealand. New Zeal. J. Mar. Freshw. Res. 2010, 44, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.B.; Brumbaugh, R.D.; Frederick, P.; Grizzle, R.; Luckenbach, M.W.; Peterson, C.H.; Angelini, C. Restoring the eastern oyster: How much progress has been made in 53 years? Front. Ecol. Environ. 2018, 16, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Kimbro, D.L.; Stallings, C.D.; White, J.W. Diminishing returns in habitat restoration by adding biogenic materials: A test using estuarine oysters and recycled oyster shell. Restor. Ecol. 2020, 28, 1633–1642. [Google Scholar] [CrossRef]
- Uddin, M.J.; Smith, K.J.; Hargis, C.W. Development of pervious oyster shell habitat (POSH) concrete for reef restoration and living shorelines. Constr. Build. Mater. 2021, 295, 123685. [Google Scholar] [CrossRef]
- Crisp, D.J. Chemical factors inducing settlement in Crassostrea virginica. J. Anim. Ecol. 1967, 36, 329–335. [Google Scholar] [CrossRef]
- Poirier, L.A.; Clements, J.C.; Davidson, J.D.P.; Miron, G.; Davidson, J.; Comeau, L.A. Sink before you settle: Settlement behaviour of Eastern oyster (Crassostrea virginica) larvae on artificial spat collectors and natural substrate. Aquac. Rep. 2019, 13, 100181. [Google Scholar] [CrossRef]
- Sedanza, M.G.; Kim, H.J.; Seposo, X.; Yoshida, A.; Yamaguchi, K.; Satuito, C.G. Regulatory role of sugars on the settlement inducing activity of a conspecific cue in Pacific oyster Crassostrea gigas. Int. J. Mol. Sci. 2021, 22, 3273. [Google Scholar] [CrossRef]
- Branigan, S.; Fitzsimons, J.; Gillies, C.L. Modern middens: Shell recycling for restoring an endangered marine ecosystem in Victoria, Australia. Ecol. Manag. Restor. 2020, 21, 198–204. [Google Scholar] [CrossRef]
- Schulte, D.M.; Burke, R.P.; Lipcius, R.N. Unprecedented restoration of a native oyster metapopulation. Science 2009, 325, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Dinnel, P.A.; Peabody, B.; Peter-contesse, T. Rebuilding Olympia oysters, Ostrea lurida Carpenter 1864, in Fidalgo Bay, Washington. J. Shellfish Res. 2009, 28, 79–85. [Google Scholar] [CrossRef]
- Brazee, S.L.; Carrington, E. Interspecific comparison of the mechanical properties of mussel byssus. Biol. Bull. 2006, 211, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, G.A. The effect of sediment type upon the position and depth at which byssal attachment occurs in Mytilus edulis. J. Mar. Biol. Assoc. United Kingd. 1983, 63, 641–651. [Google Scholar] [CrossRef]
- Fey, F.; Dankers, N.; Steenbergen, J.; Goudswaard, K. Development and distribution of the non-indigenous Pacific oyster (Crassostrea gigas) in the Dutch Wadden Sea. Aquac. Int. 2010, 18, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Commito, J.A.; Commito, A.E.; Platt, R.V.; Grupe, B.M.; Dow Piniak, W.E.; Gownaris, N.J.; Reeves, K.A.; Vissichelli, A.M. Recruitment facilitation and spatial pattern formation in soft-bottom mussel beds. Ecosphere 2014, 5, art160. [Google Scholar] [CrossRef]
- Wilcox, M.A. Population Dynamics of Restored Green-Lipped Mussel (Perna canaliculus) beds in the Hauraki Gulf, New Zealand; University of Auckland: Auckland, New Zealand, 2017. [Google Scholar]
- Hunt, H.L.; Scheibling, R.E. Movement and wave dislodgment of mussels on a wave-exposed rocky shore. Veliger 2002, 45, 273–277. [Google Scholar]
- Dawber, C. Lines in the Water: A History of Greenshell Mussel farming in New Zealand; River Press for NZ Marine Farming Association: Picton, New Zealand, 2004. [Google Scholar]
- Wilcox, M.; Jeffs, A. Impacts of sea star predation on mussel bed restoration. Restor. Ecol. 2019, 27, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Alder, A.; Jeffs, A.; Hillman, J.R. Considering the use of subadult and juvenile mussels for mussel reef restoration. Restor. Ecol. 2021, 29, 1–10. [Google Scholar] [CrossRef]
- Benjamin, E.D.; Handley, S.J.; Jeffs, A.; Olsen, L.; Toone, T.A.; Hillman, J.R. Testing habitat suitability for shellfish restoration with small-scale pilot experiments. Conserv. Sci. Pract. 2022; In review. [Google Scholar]
- Capelle, J.J.; Leuchter, L.; de Wit, M.; Hartog, E.; Bouma, T.J. Creating a window of opportunity for establishing ecosystem engineers by adding substratum: A case study on mussels. Ecosphere 2019, 10, e02688. [Google Scholar] [CrossRef] [Green Version]
- Schotanus, J.; Capelle, J.J.; Paree, E.; Fivash, G.S.; van de Koppel, J.; Bouma, T.J. Restoring mussel beds in highly dynamic environments by lowering environmental stressors. Restor. Ecol. 2020, 28, 1124–1134. [Google Scholar] [CrossRef]
- Fariñas-Franco, J.M.; Allcock, L.; Smyth, D.; Roberts, D. Community convergence and recruitment of keystone species as performance indicators of artificial reefs. J. Sea Res. 2013, 78, 59–74. [Google Scholar] [CrossRef]
- Bertolini, C.; Geraldi, N.R.; Montgomery, W.I.; O’Connor, N.E. Substratum type and conspecific density as drivers of mussel patch formation. J. Sea Res. 2017, 121, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Handley, S.; Gibbs, M.; Swales, A.; Olsen, G.; Ovenden, R.; Bradley, A. A 1000 Year History of Seabed Change in Pelorus Sound/Te Hoiere; National Institute of Water and Atmospheric Research Client Report Prepared for Marlborough District Council: Nelson, NZ, USA, 2017.
- Stead, D.H. Fisheries Technical Report No. 62. Pelorus Sound: Mussel Survey December 1969; Fisheries Division, Marine Department: Wellington, NZ, USA, 1969.
- Flaws, D.E. Aspects of the Biology of Mussels in the Cook Strait Area. Ph.D. Thesis, Victoria University, Wellington, NZ, USA, 1975. [Google Scholar]
- Parker, J.G. A comparison of methods used for the measurement of organic matter in marine sediment. Chem. Ecol. 1983, 1, 201–209. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Lucas, A.; Beninger, P.G. The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 1985, 44, 187–200. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fivash, G.S.; Stüben, D.; Bachmann, M.; Walles, B.; van Belzen, J.; Didderen, K.; Temmink, R.J.M.; Lengkeek, W.; van der Heide, T.; Bouma, T.J. Can we enhance ecosystem-based coastal defense by connecting oysters to marsh edges? Analyzing the limits of oyster reef establishment. Ecol. Eng. 2021, 165, 106221. [Google Scholar] [CrossRef]
- Commito, J.A.; Gownaris, N.J.; Haulsee, D.E.; Coleman, S.E.; Beal, B.F. Separation anxiety: Mussels self-organize into similar power-law clusters regardless of predation threat cues. Mar. Ecol. Prog. Ser. 2016, 547, 107–119. [Google Scholar] [CrossRef]
- Bertolini, C.; Montgomery, W.I.; O’Connor, N.E. Habitat with small inter-structural spaces promotes mussel survival and reef generation. Mar. Biol. 2018, 165, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro, A.C.; Copp, B.R.; Appleton, D.R.; Kelly, S.; Jeffs, A.G. Chemical cues promote settlement in larvae of the green-lipped mussel, Perna canaliculus. Aquac. Int. 2006, 14, 405–412. [Google Scholar] [CrossRef]
- Atalah, J.; Forrest, B.M. Forecasting mussel settlement using historical data and boosted regression trees. Aquac. Environ. Interact. 2019, 11, 625–638. [Google Scholar] [CrossRef]
- Toone, T.A.; Benjamin, E.D.; Handley, S.; Jeffs, A.; Hillman, J. Expansion of shellfish aquaculture has no impact on settlement rates. Aquac. Environ. Interact. 2022, 14, 135–145. [Google Scholar] [CrossRef]
- Paul-Burke, K.; Burke, J. Monitoring Assessment of Kūtai (Perna Canaliculus) Green-Lipped Mussel and Pātangaroa (Coscinasterias Muricata) Seastar Populations in the Western Side of Ōhiwa Harbour 2013: Technical Report; Te Rūnanga o Ngāti Awa: Whakatāne, New Zealand, 2013. [Google Scholar]
- McLeod, I.M.; Parsons, D.M.; Morrison, M.A.; Le Port, A.; Taylor, R.B. Factors affecting the recovery of soft-sediment mussel reefs in the Firth of Thames, New Zealand. Mar. Freshw. Res. 2012, 63, 78–83. [Google Scholar] [CrossRef]
- ten Brinke, W.B.M.; Augustinus, P.G.E.F.; Berger, G.W. Fine-grained sediment deposition on mussel beds in the Oosterschelde (The Netherlands), determined from echosoundings, radio-isotopes and biodeposition field experiments. Estuar. Coast. Shelf Sci. 1995, 40, 195–217. [Google Scholar] [CrossRef]
- Widdows, J.; Brinsley, M. Impact of biotic and abiotic processes on sediment dynamics and the consequences to the structure and functioning of the intertidal zone. J. Sea Res. 2002, 48, 143–156. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- van Leeuwen, B.; Augustijn, D.C.M.; van Wesenbeeck, B.K.; Hulscher, S.J.M.H.; de Vries, M.B. Modeling the influence of a young mussel bed on fine sediment dynamics on an intertidal flat in the Wadden Sea. Ecol. Eng. 2010, 36, 145–153. [Google Scholar] [CrossRef]
- Whitman, E.R.; Reidenbach, M.A. Benthic flow environments affect recruitment of Crassostrea virginica larvae to an intertidal oyster reef. Mar. Ecol. Prog. Ser. 2012, 463, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Colden, A.M.; Latour, R.J.; Lipcius, R.N. Reef height drives threshold dynamics of restored oyster reefs. Mar. Ecol. Prog. Ser. 2017, 582, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.P.; Peterson, C.H.; Grabowski, J.H.; Lenihan, H.S. Success of constructed oyster reefs in no-harvest sanctuaries: Implications for restoration. Mar. Ecol. Prog. Ser. 2009, 389, 159–170. [Google Scholar] [CrossRef] [Green Version]
- zu Ermgassen, P.S.E.; Thurstan, R.H.; Corrales, J.; Alleway, H.; Carranza, A.; Dankers, N.; De Angelis, B.; Hancock, B.; Kent, F.; McLeod, I.; et al. The benefits of bivalve reef restoration: A global synthesis of underrepresented species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 2050–2065. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjamin, E.D.; Hillman, J.R.; Handley, S.J.; Toone, T.A.; Jeffs, A. The Effectiveness of Providing Shell Substrate for the Restoration of Adult Mussel Reefs. Sustainability 2022, 14, 15746. https://doi.org/10.3390/su142315746
Benjamin ED, Hillman JR, Handley SJ, Toone TA, Jeffs A. The Effectiveness of Providing Shell Substrate for the Restoration of Adult Mussel Reefs. Sustainability. 2022; 14(23):15746. https://doi.org/10.3390/su142315746
Chicago/Turabian StyleBenjamin, Emilee D., Jenny R. Hillman, Sean J. Handley, Trevyn A. Toone, and Andrew Jeffs. 2022. "The Effectiveness of Providing Shell Substrate for the Restoration of Adult Mussel Reefs" Sustainability 14, no. 23: 15746. https://doi.org/10.3390/su142315746
APA StyleBenjamin, E. D., Hillman, J. R., Handley, S. J., Toone, T. A., & Jeffs, A. (2022). The Effectiveness of Providing Shell Substrate for the Restoration of Adult Mussel Reefs. Sustainability, 14(23), 15746. https://doi.org/10.3390/su142315746








