Climate Change Assessment of the Spatial Potential Aggregation Zones of Plectropomus pessuliferus marisrubri and Plectropomus areolatus along the Saudi Coast, Using RS and GIS
Abstract
1. Introduction
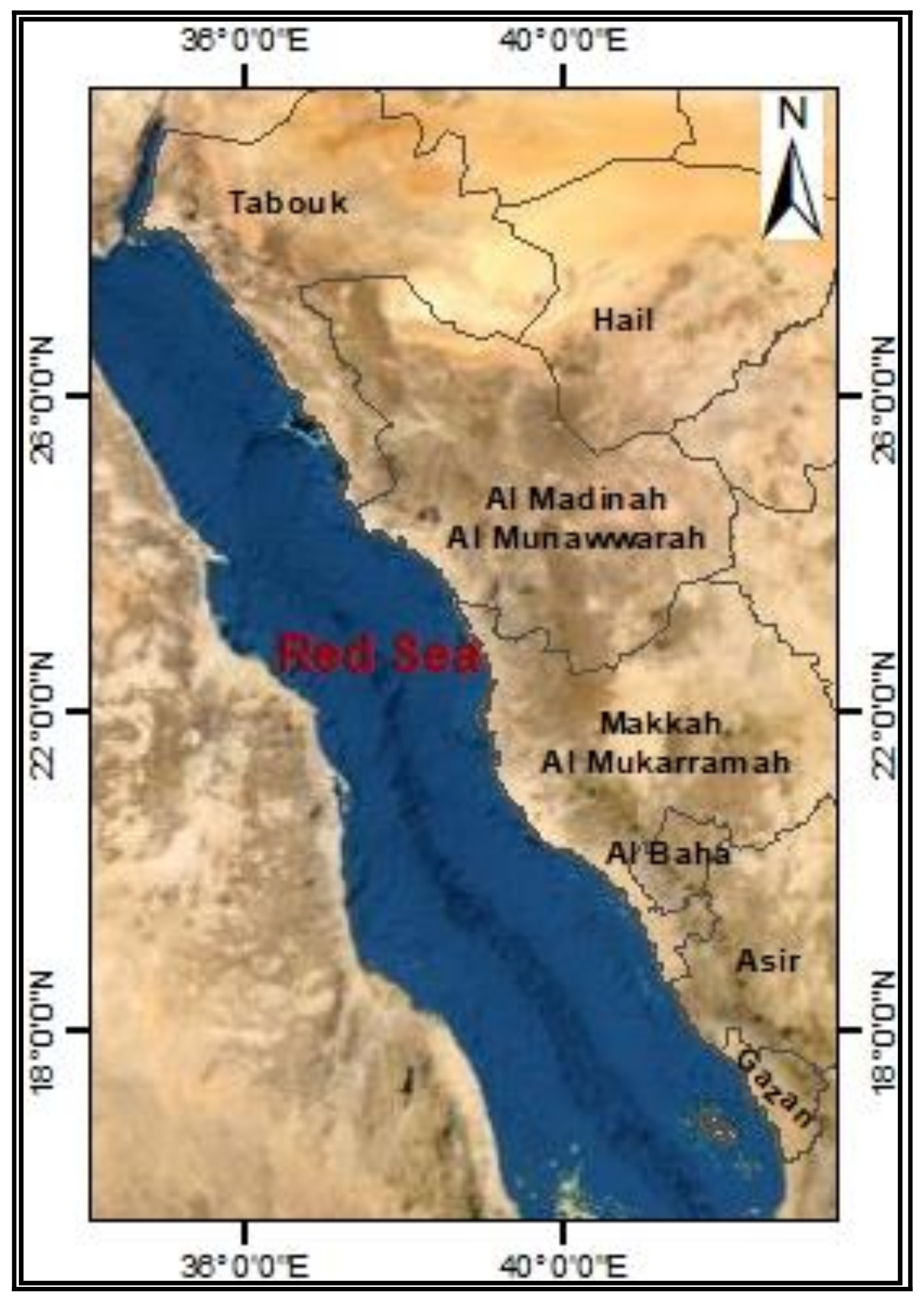
Study Area
2. Materials and Methods
2.1. Data
2.2. Data Determination of SST Changes
2.3. Suitability Map of Spatial Potential Fishing Zones
2.4. Risk Assessment of SST on Spatial Potential Aggregation Zones
3. Results and Discussion
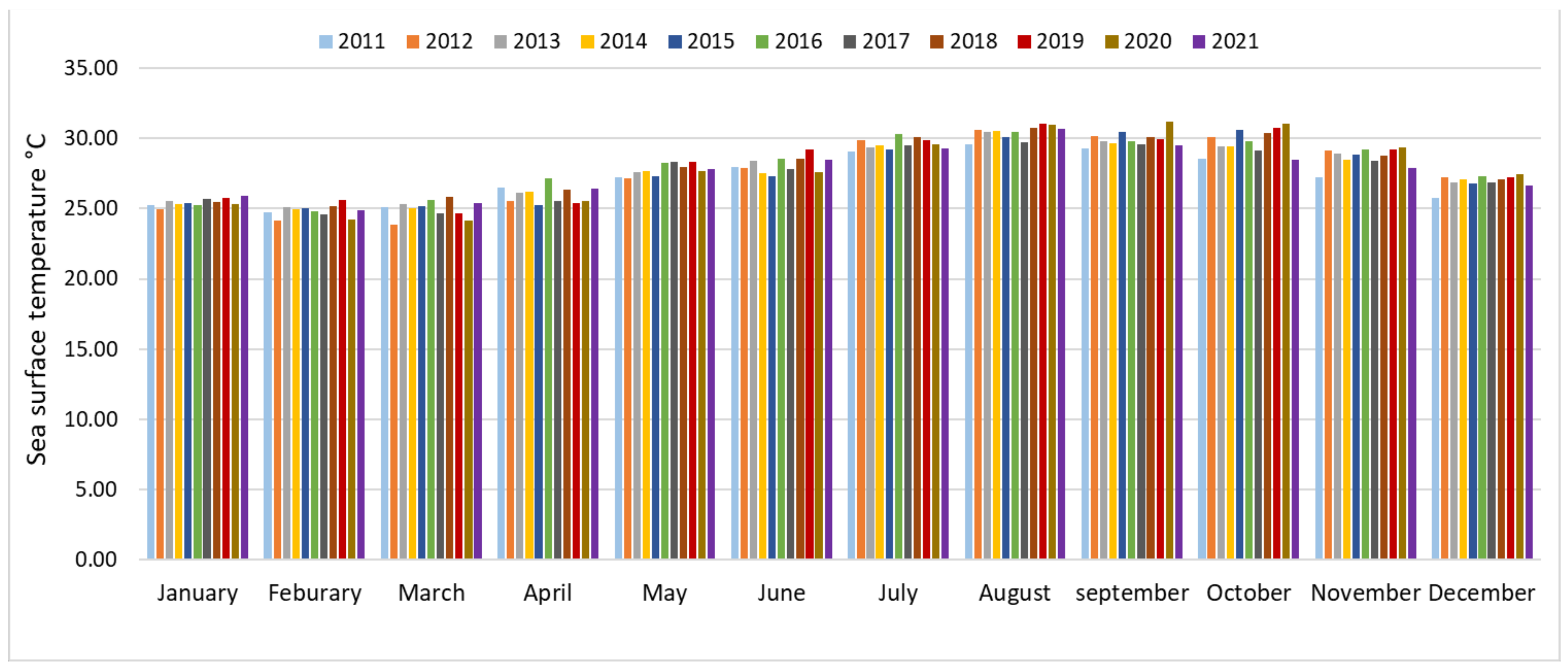
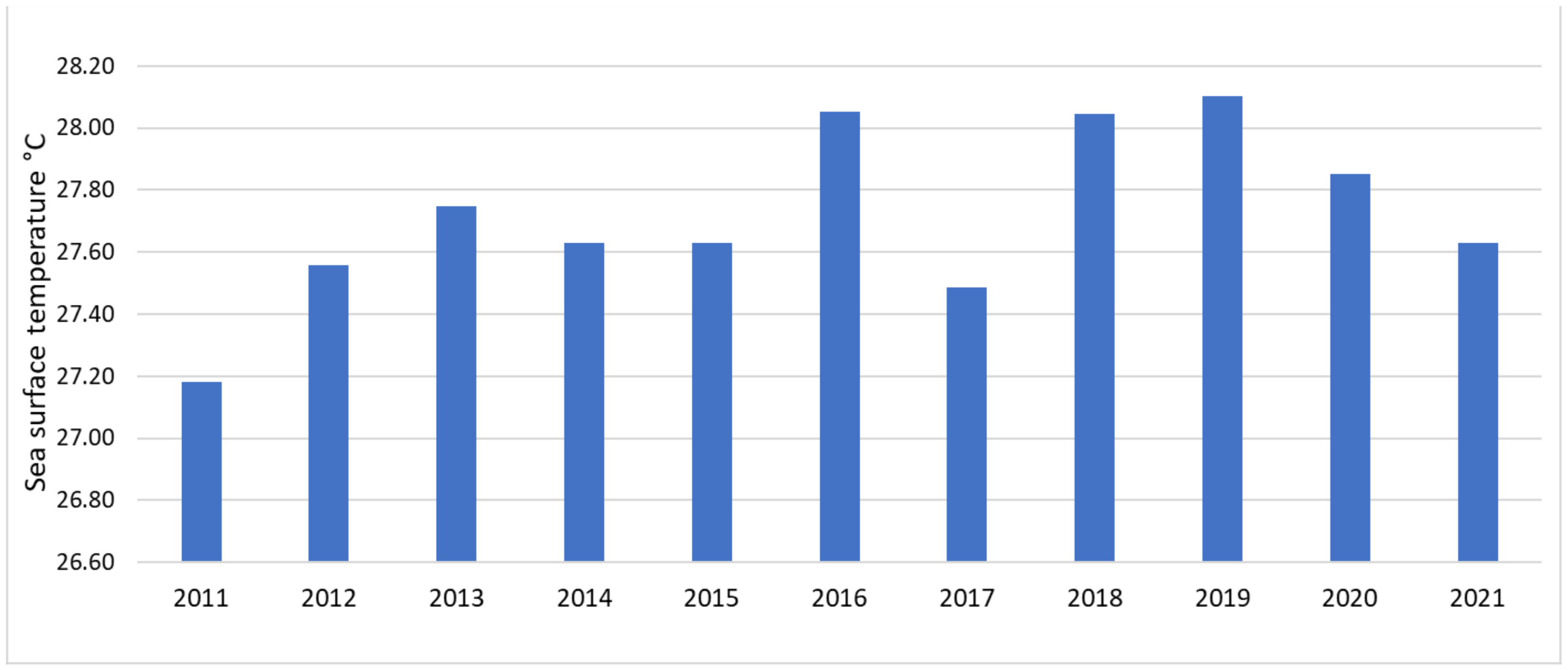
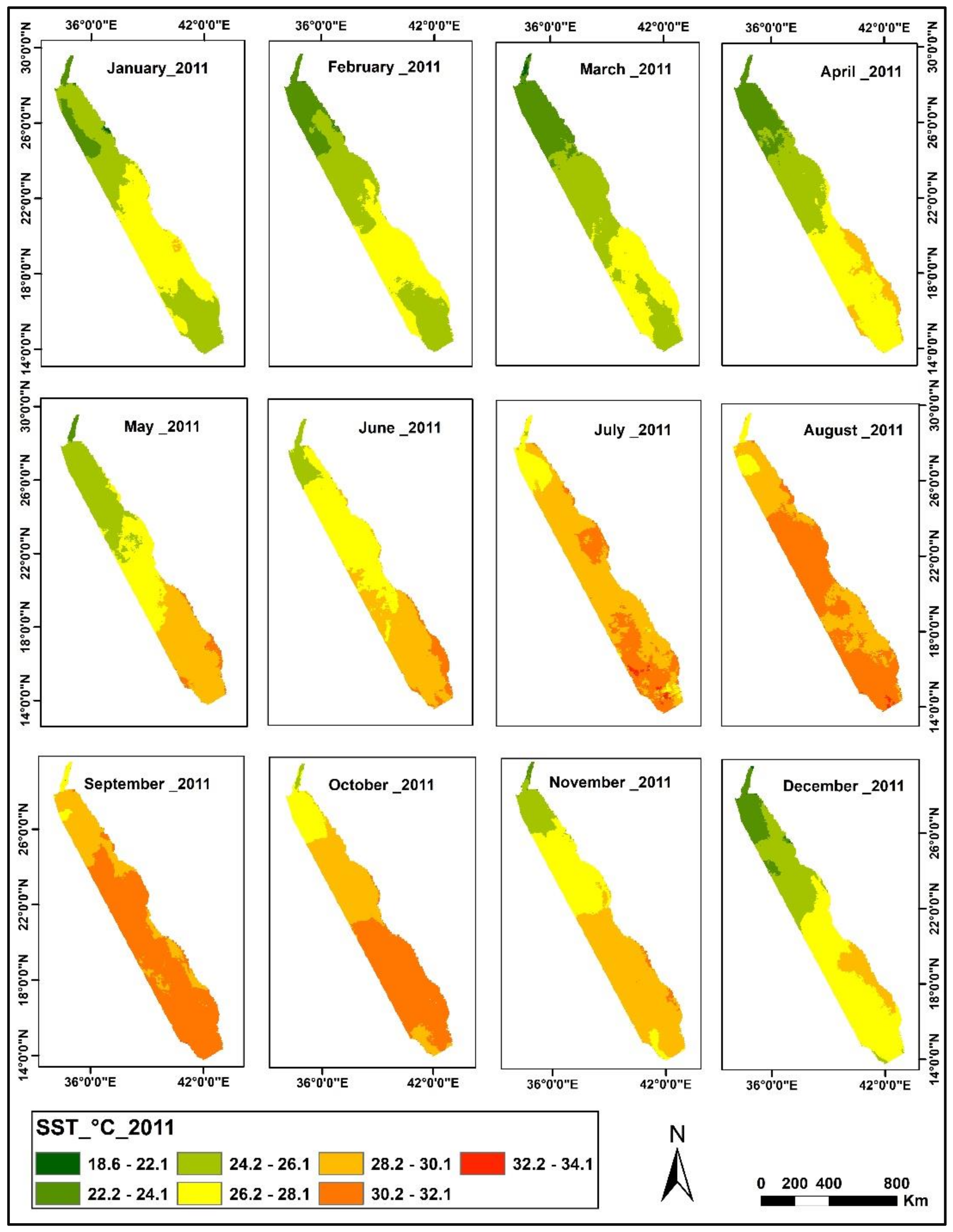
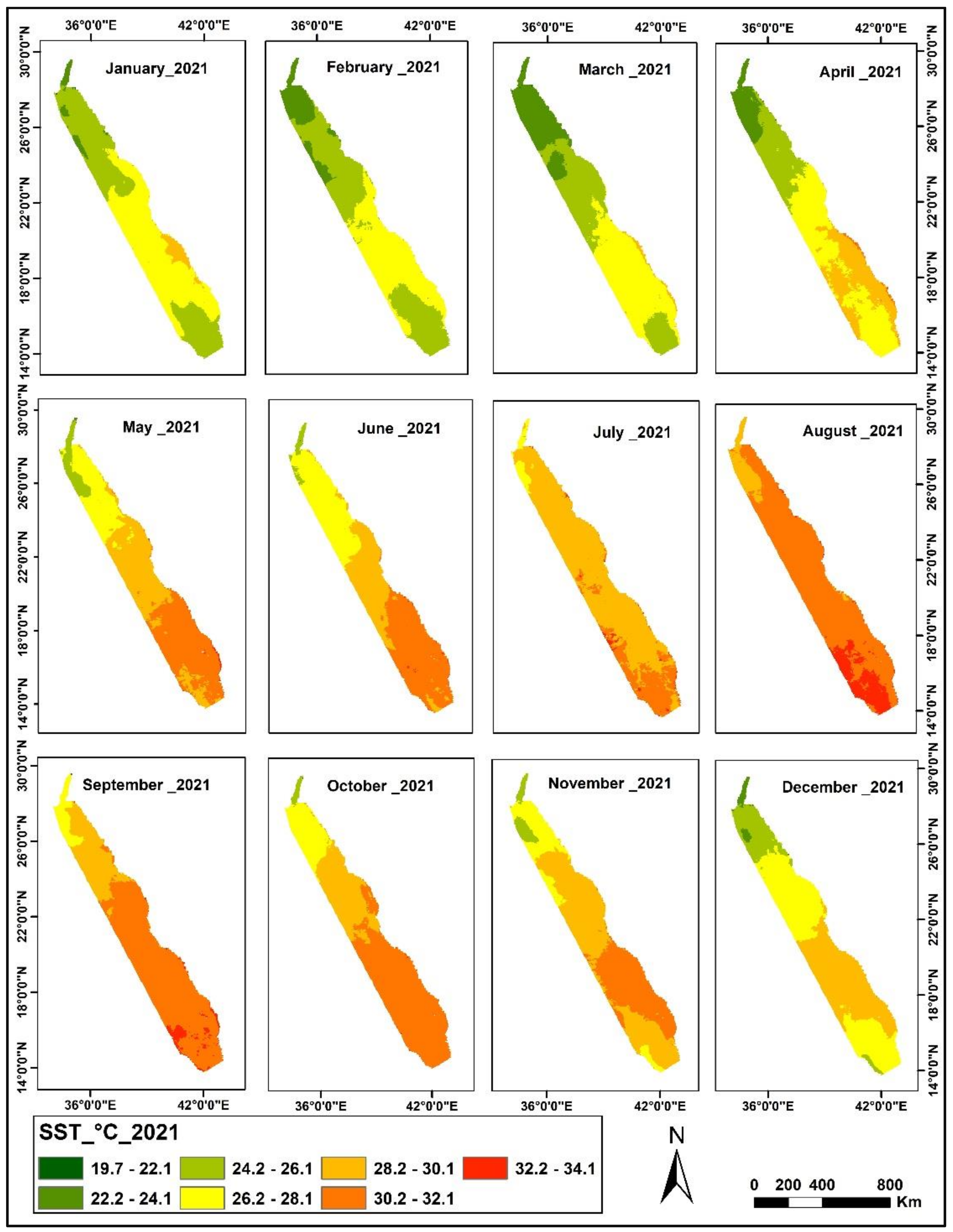
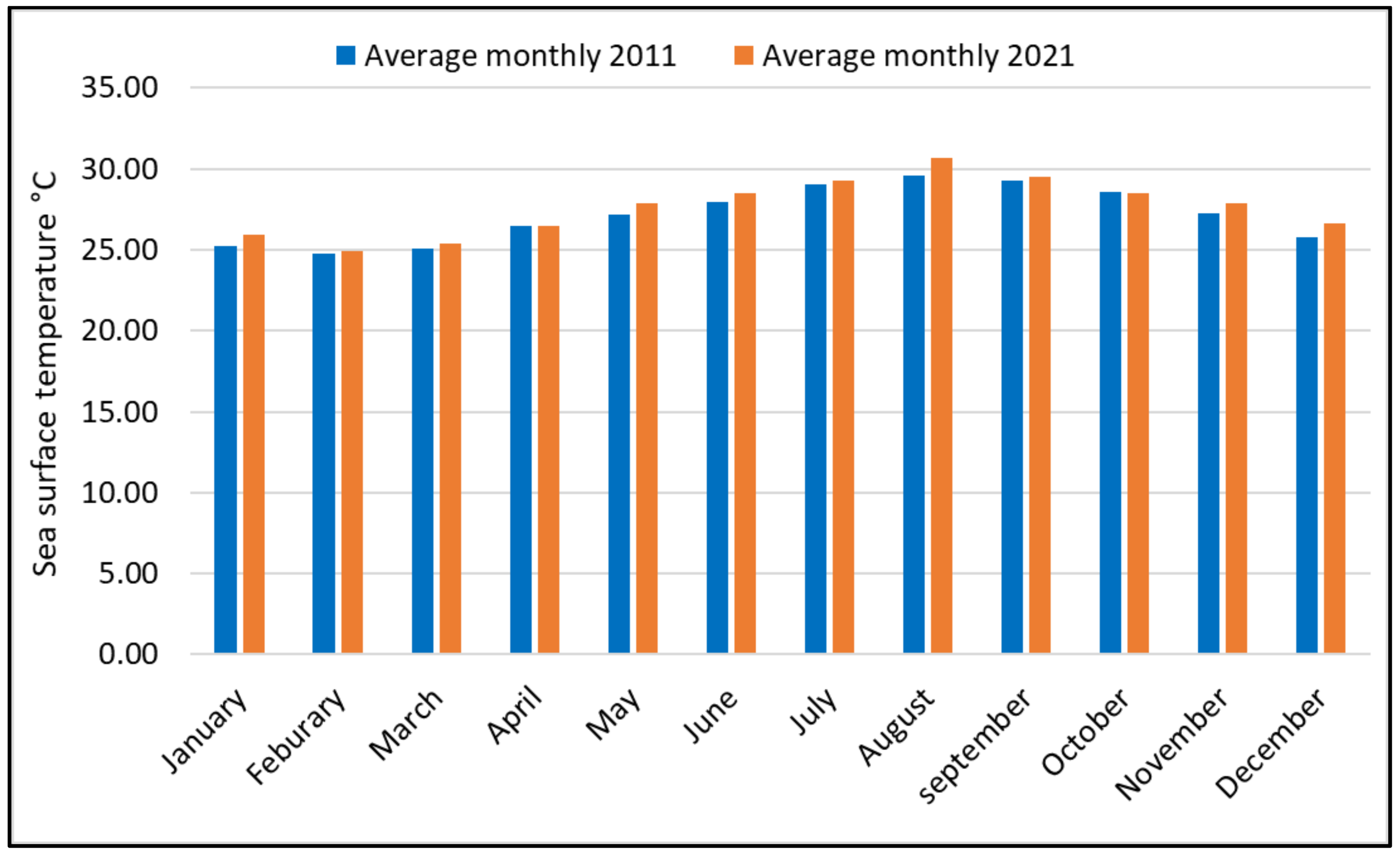
3.1. SST Change Detection
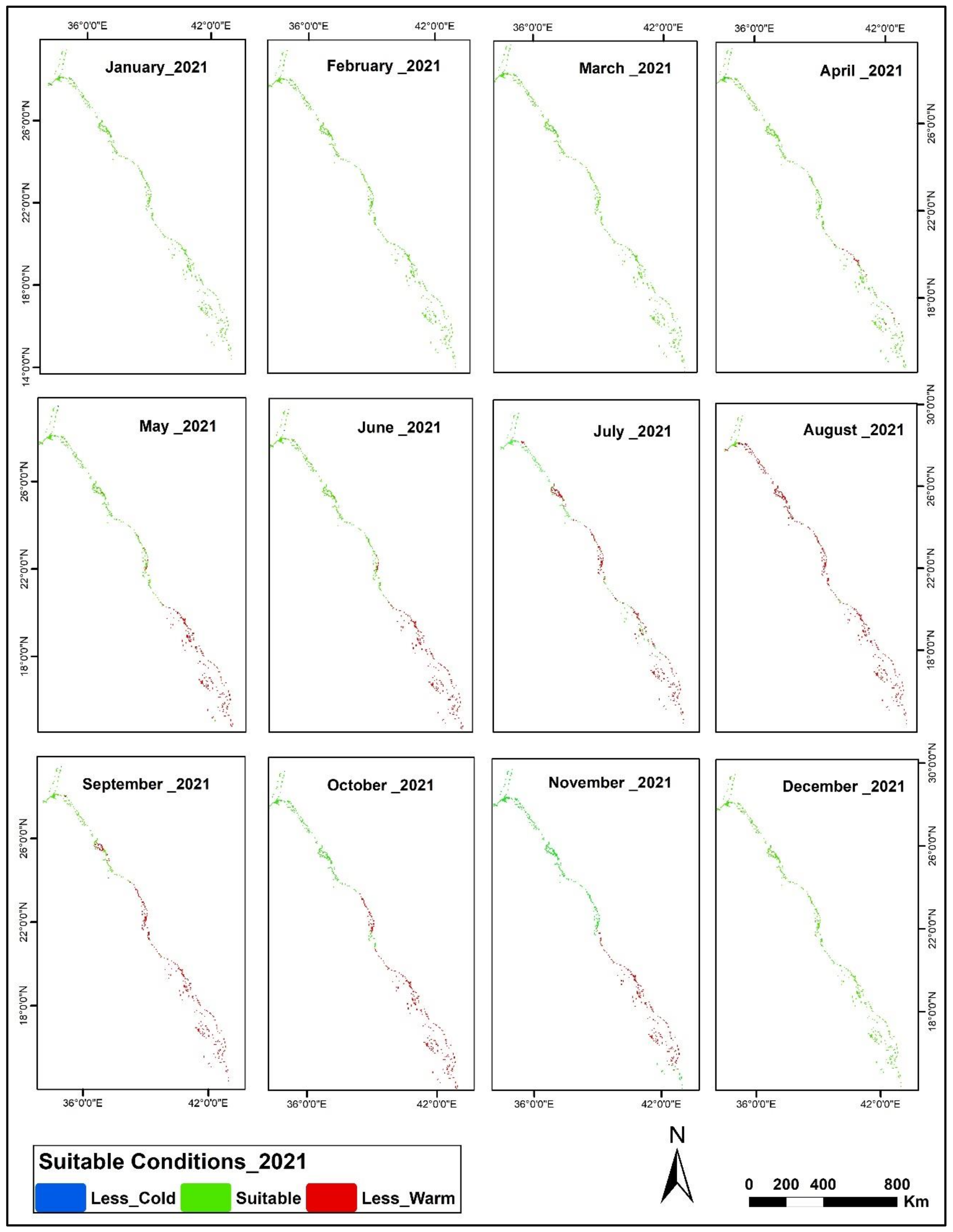
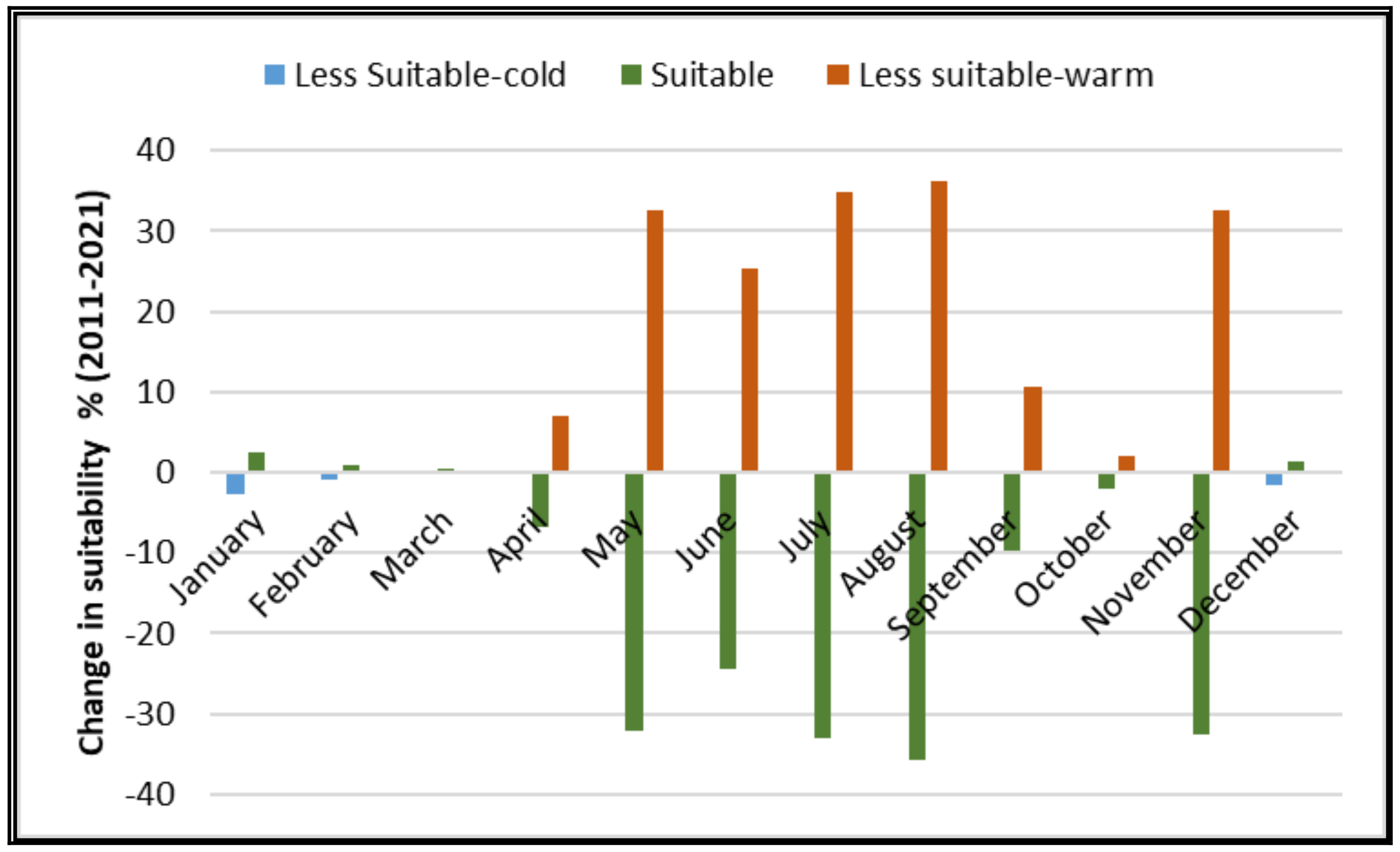
3.2. Suitability Map of Spatial Potential Aggregation Zones
3.3. Risk Map of Potential Aggregation Zones
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, J.D.; Kronen, M.; Vunisea, A.; Nash, W.J.; Keeble, G.; Demmke, A.; Pontifex, S.; Andréfouët, S. Planning the use of fish for food security in the Pacific. Mar. Policy 2009, 33, 64–76. [Google Scholar] [CrossRef]
- Gillett, R. Fisheries in the Economies of the Pacific Island Countries and Territories; Asian Development Bank: Mandaluyong City, Philippines, 2009. [Google Scholar]
- Bell, J.D.; Reid, C.; Batty, M.J.; Lehodey, P.; Rodwell, L.; Hobday, A.J.; Johnson, J.E.; Demmke, A. Effects of climate change on oceanic fisheries in the tropical Pacific: Implications for economic development and food security. Clim. Chang. 2013, 119, 199–212. [Google Scholar] [CrossRef]
- IPCC. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2007: Impacts, Adaptation and Vulnerability; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2017; 976p. [Google Scholar]
- IPCC. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. In 2018: Global Warming of 1.5 °C.; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; 616p. [Google Scholar] [CrossRef]
- Barros, A.F.; Campos, V.P.; da Silva, J.C.P.; Lopez, L.E.; da Silva, A.P.; Pozza, E.A.; Pedroso, L.A. Tempo de exposição de juvenis de segundo estádio a voláteis emitidos por macerados de nim e de mostarda e biofumigação contra Meloidogyne incognita. Nematropica 2014, 44, 190–199. [Google Scholar]
- Philippart, C.J.; Anadón, R.; Danovaro, R.; Dippner, J.W.; Drinkwater, K.F.; Hawkins, S.J.; Oguz, T.; O’Sullivan, G.; Reid, P.C. Impacts of climate change on European marine ecosystems: Observations, expectations and indicators. J. Exp. Mar. Biol. Ecol. 2011, 400, 52–69. [Google Scholar] [CrossRef]
- Belkin, I.M. Rapid warming of large marine ecosystems. Prog. Oceanography. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Riegl, B.M.; Bruckner, A.W.; Rowlands, G.P.; Purkis, S.J.; Renaud, P. Red Sea coral reef trajectories over 2 decades suggest increasing community homogenization and decline in coral size. PLoS ONE 2012, 7, e38396. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Hoteit, I.; Prihartato, P.K.; Chronis, T.; Triantafyllou, G.; Abualnaja, Y. Abrupt warming of the Red Sea. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T. (Ed.) Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Vulnerability of coral reef fisheries to a loss of structural complexity. Curr. Biol. 2014, 24, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.; Ferrel, A.; Seibel, B.; Pörtner, H.O.; Huey, R.B. Climate change tightens a metabolic constraint on marine habitats. Science 2015, 348, 1132–1135. [Google Scholar] [CrossRef]
- Portner, H.O.; Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D. Climatic vulnerability of the world’s freshwater and marine fishes. Nat. Clim. Change 2017, 7, 718–722. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Rogers, S.I.; Jennings, S.; Stelzenmüller, V.; Dye, S.R.; Skjoldal, H.R. Climate change and deepening of the North Sea fish assemblage: A biotic indicator of warming seas. J. Appl. Ecol. 2008, 45, 1029–1039. [Google Scholar] [CrossRef]
- Nye, J.A.; Link, J.S.; Hare, J.A.; Overholtz, W.J. Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United States continental shelf. Mar. Ecol. Prog. Ser. 2009, 393, 111–129. [Google Scholar] [CrossRef]
- Mueter, F.J.; Litzow, M.A. Sea ice retreat alters the biogeography of the Bering Sea continental shelf. Ecol. Appl. 2008, 18, 309–320. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; Gledhill, D.C.; Hobday, A.J.; Brown, R.; Edgar, G.J.; Pecl, G. Long-term shifts in abundance and distribution of a temperate fish fauna: A response to climate change and fishing practices. Glob. Ecol. Biogeogr. 2010, 20, 58–72. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Cheung, W.W.; Lam, V.W.; Pauly, D.; Herrick, S. Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Change 2011, 1, 449–456. [Google Scholar] [CrossRef]
- Heemstra, P.C.; Randall, J.E. Groupers of the World. FAO Fish. Synop. 1993, 16, 382. [Google Scholar]
- Russ, G.R.; Lou, D.C.; Higgs, J.B.; Ferreira, B.P. Mortality rate of a cohort of the coral trout, Plectropomus leopardus, in zones of the Great Barrier Reef Marine Park closed to fishing. Mar. Freshw. Res. 1998, 49, 507–511. [Google Scholar] [CrossRef]
- Choat, J.H.; Amorim, P.; Law, C.; Ma, K.; Myers, R.; Nair, R.; Pollard, D.A.; Rhodes, K.; Russell, B.; Samoilys, M.; et al. Plectropomus pessuliferus. IUCN Red List. Threat. Species 2018, e.T118359431A100469254. [Google Scholar] [CrossRef]
- DesRosiers, N. Growth and Maturation of Plectropomus spp. in the Saudi Arabian Red Sea. Ph.D. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2011. [Google Scholar]
- Burger, J.; Gochfeld, M.; Batang, Z.; Alikunhi, N.; Al-Jahdali, R.; Al-Jebreen, D.; Aziz, M.A.; Al-Suwailem, A. Fish consumption behavior and rates in native and non-native people in Saudi Arabia. Environ. Res. 2014, 133, 141–148. [Google Scholar] [CrossRef]
- Shellem, C.T.; Ellis, J.I.; Coker, D.J.; Berumen, M.L. Red Sea fish market assessments indicate high species diversity and potential overexploitation. Fish. Res. 2021, 239, 105922. [Google Scholar] [CrossRef]
- Johansen, J.L.; Messmer, V.; Coker, D.J.; Hoey, A.S.; Pratchett, M.S. Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob. Change Biol. 2014, 20, 1067–1074. [Google Scholar] [CrossRef]
- Johansen, J.L.; Pratchett, M.S.; Messmer, V.; Coker, D.J.; Tobin, A.J.; Hoey, A.S. Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci. Rep. 2015, 5, srep13830. [Google Scholar] [CrossRef] [PubMed]
- Prachett, M.S.; Messmer, V.; Reynolds, A.; Martin, J.; Clark, T.D.; Munday, P.L.; Tobin, A.J.; Hoey, A.S. Effects of Climate Change on Reproduction, Larval Development, and Adult Health of Coral Trout (Plectropomus spp.); Project Report; Fisheries Research and Development Corporation: Deakin West, Australia, 2013. [Google Scholar]
- Pratchett, M.S.; Cameron, D.S.; Donelson, J.; Evans, L.; Frisch, A.J.; Hobday, A.J.; Hoey, A.S.; Marshall, N.A.; Messmer, V.; Munday, P.L.; et al. Effects of climate change on coral grouper (Plectropomus spp.) and possible adaptation options. Rev. Fish Biol. Fish. 2017, 27, 297–316. [Google Scholar] [CrossRef]
- Tang, D.; Kawamura, H.; Lee, M.A.; Van Dien, T. Seasonal and spatial distribution of chlorophyll-a concentrations and water conditions in the Gulf of Tonkin, South China Sea. Remote Sens. Environ. 2003, 85, 475–483. [Google Scholar] [CrossRef]
- Shattri, M.; Tan, C.K.; Ibrahim, H.M.; Rashid, M.S.A. Satellite fish forecasting in south china sea. In Proceedings of the 22nd Asian Conference on Remote Sensing, Singapore, 5–9 November 2001; pp. 22–25. [Google Scholar]
- Dan Teknik, M.S.; Nurdin, S.; Mustapha, M.A.; Lihan, T.; Abd Ghaffar, M.A. Determination of potential fishing grounds of Rastrelliger kanagurta using satellite remote sensing and GIS technique. Sains Malays. 2015, 44, 225–232. [Google Scholar]
- Kassi, J.B.; Racault, M.F.; Mobio, B.A.; Platt, T.; Sathyendranath, S.; Raitsos, D.E.; Affian, K. Remotely sensing the biophysical drivers of Sardinella aurita variability in Ivorian waters. Remote Sens. 2018, 10, 785. [Google Scholar] [CrossRef]
- Balaguru, B.; Ramakrishnan, S.S.; Vidhya, R.; Thanabalan, P. A comparative study on utilization of multi-sensor satellite data to detect potential fishing zone (PFZ). In International Archives of the Photogrammetry, Remote Sensing & Spatial Information Sciences, XL-8, ISPRS Technical Commission VIII Symposium, Hyderabad, India, 9–12 December 2014; ISPRS: Hyderabad, India, 2014. [Google Scholar]
- Sun, Z.; Xia, S.; Feng, S.; Zhang, Z.; Rahman, M.M.; Rajkumar, M.; Jiang, S. Effects of water temperature on survival, growth, digestive enzyme activities, and body composition of the leopard coral grouper Plectropomus leopardus. Fish. Sci. 2015, 81, 107–112. [Google Scholar] [CrossRef]
- Das, S.K.; Xiang, T.W.; Noor, N.M.; De, M.; Mazumder, S.K.; Goutham-Bharathi, M.P. Temperature physiology in grouper (Epinephelinae: Serranidae) aquaculture: A brief review. Aquac. Rep. 2021, 20, 100682. [Google Scholar] [CrossRef]
- Martínez-Palacios, C.A.; Tovar, E.B.; Taylor, J.F.; Durán, G.R.; Ross, L.G. Effect of temperature on growth and survival of Chirostoma estor estor, Jordan 1879, monitored using a simple video technique for remote measurement of length and mass of larval and juvenile fishes. Aquaculture 2002, 209, 369–377. [Google Scholar] [CrossRef]
- Cheng, L.; Abraham, J.; Zhu, J.; Trenberth, K.E.; Fasullo, J.; Boyer, T.; Locarnini, R.; Zhang, B.; Yu, F.; Wan, L.; et al. Record-Setting Ocean Warmth Continued in 2019. Adv. Atmos. Sci. 2020, 37, 137–142. [Google Scholar] [CrossRef]
- Dasari, H.P.; Langodan, S.; Viswanadhapalli, Y.; Vadlamudi, B.R.; Papadopoulos, V.P.; Hoteit, I. ENSO influence on the interannual variability of the Red Sea convergence zone and associated rainfall. Int. J. Climatol. 2018, 38, 761–775. [Google Scholar] [CrossRef]
- Donegan, B. El Niño Conditions Strengthen, Could Last Through Summer. Weather. Co. 2019. Available online: https://weather.com/news/weather/news/2019-03-14-el-nino-conditions-strengthen-could-last-through-summer (accessed on 29 September 2022).
- Nagano, A.; Wakita, M.; Fujiki, T.; Uchida, H. El Niño-Related Vertical Mixing Enhancement Under the Winter Mixed Layer at Western Subarctic North Pacific Station K2. J. Geophys. Res. Ocean. 2021, 126, e2020JC016913. [Google Scholar] [CrossRef]
- Wang, S.; Mu, L.; Liu, D. A hybrid approach for El Niño prediction based on Empirical Mode Decomposition and convolutional LSTM Encoder-Decoder. Comput. Geosci. 2021, 149, 104695. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, Y.; Hendon, H.H.; McPhaden, M.J.; Marshall, A.G. Niño 4 West (Niño-4W) Sea Surface Temperature Variability. J. Geophys. Res. Ocean. 2021, 126, e2021JC017591. [Google Scholar] [CrossRef]
- Liang, X.S.; Xu, F.; Rong, Y.; Zhang, R.; Tang, X.; Zhang, F. El Niño Modoki can be mostly predicted more than 10 years ahead of time. Sci. Rep. 2021, 11, 17860. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, R.S. Dynamics of Reef Fish and Decapod Crustacean Spawning Aggregations: Underlying Mechanisms, Habitat Linkages, and Trophic Interactions. In Ecological Connectivity among Tropical Coastal Ecosystems; Nagelkerken, I., Ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Shoji, J.; Toshito, S.I.; Mizuno, K.I.; Kamimura, Y.; Hori, M.; Hirakawa, K. Possible effects of global warming on fish recruitment: Shifts in spawning season and latitudinal distribution can alter growth of fish early life stages through changes in daylength. ICES J. Mar. Sci. 2011, 68, 1165–1169. [Google Scholar] [CrossRef]
- Tsuji, M.; Abe, H.; Hanyuu, K.; Kuriyama, I.; Tsuchihashi, Y.; Tsumoto, K.; Nigou, T.; Kasuya, T.; Katou, T.; Kawamura, T.; et al. Effect of temperature on survival, growth and malformation of cultured larvae and juveniles of the seven-band grouper Epinephelus septemfasciatus. Fish. Sci. 2014, 80, 69–81. [Google Scholar] [CrossRef]
- Abram, N.; Gattuso, J.-P.; Prakash, A.; Cheng, L.; Chidichimo, M.P.; Crate, S.; Enomoto, H.; Garschagen, M.; Gruber, N.; Harper, S.; et al. Framing and Context of the Report. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 73–129. [Google Scholar] [CrossRef]
- Bindoff, N.L.; Cheung, W.W.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.J.M.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; pp. 477–587. [Google Scholar]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairy, N.; Ramadan, R.H.; Alogayell, H.M.; Alkadi, I.I.; Ismail, I.Y.; Ramadan, M.S. Climate Change Assessment of the Spatial Potential Aggregation Zones of Plectropomus pessuliferus marisrubri and Plectropomus areolatus along the Saudi Coast, Using RS and GIS. Sustainability 2022, 14, 15825. https://doi.org/10.3390/su142315825
Khairy N, Ramadan RH, Alogayell HM, Alkadi II, Ismail IY, Ramadan MS. Climate Change Assessment of the Spatial Potential Aggregation Zones of Plectropomus pessuliferus marisrubri and Plectropomus areolatus along the Saudi Coast, Using RS and GIS. Sustainability. 2022; 14(23):15825. https://doi.org/10.3390/su142315825
Chicago/Turabian StyleKhairy, Nesren, Rasha H. Ramadan, Haya M. Alogayell, Ibtesam I. Alkadi, Ismail Y. Ismail, and Mona S. Ramadan. 2022. "Climate Change Assessment of the Spatial Potential Aggregation Zones of Plectropomus pessuliferus marisrubri and Plectropomus areolatus along the Saudi Coast, Using RS and GIS" Sustainability 14, no. 23: 15825. https://doi.org/10.3390/su142315825
APA StyleKhairy, N., Ramadan, R. H., Alogayell, H. M., Alkadi, I. I., Ismail, I. Y., & Ramadan, M. S. (2022). Climate Change Assessment of the Spatial Potential Aggregation Zones of Plectropomus pessuliferus marisrubri and Plectropomus areolatus along the Saudi Coast, Using RS and GIS. Sustainability, 14(23), 15825. https://doi.org/10.3390/su142315825





