Evaluation of Washing and Screening for Upgrading the Calcium Content of Oyster Shells Using a Simulated Wet-Type Trommel
Abstract
:1. Introduction
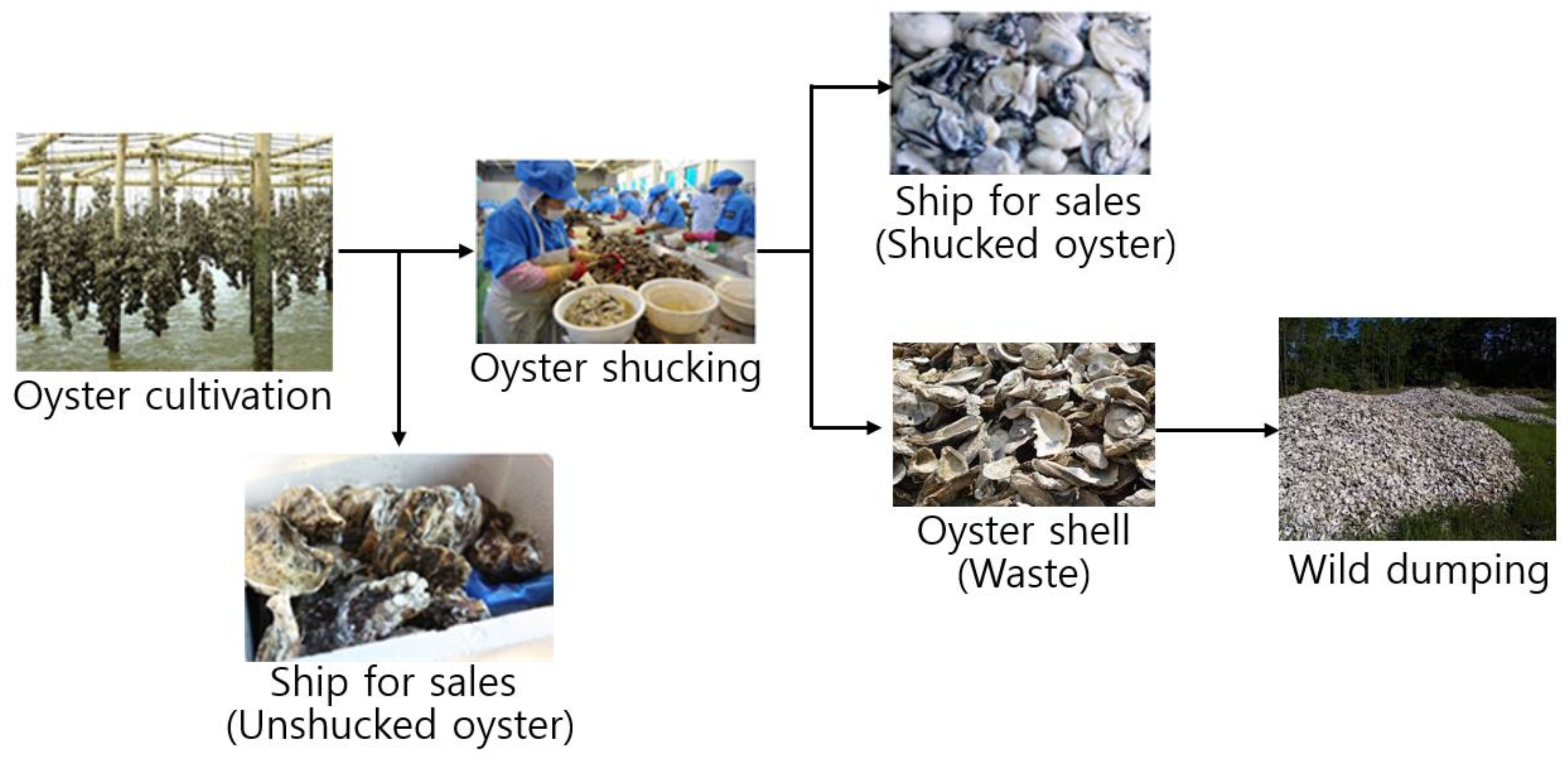
2. An Obstacle to Oyster Shell Recycling in Korea
3. Materials and Methods
3.1. Collection of Oyster Shell
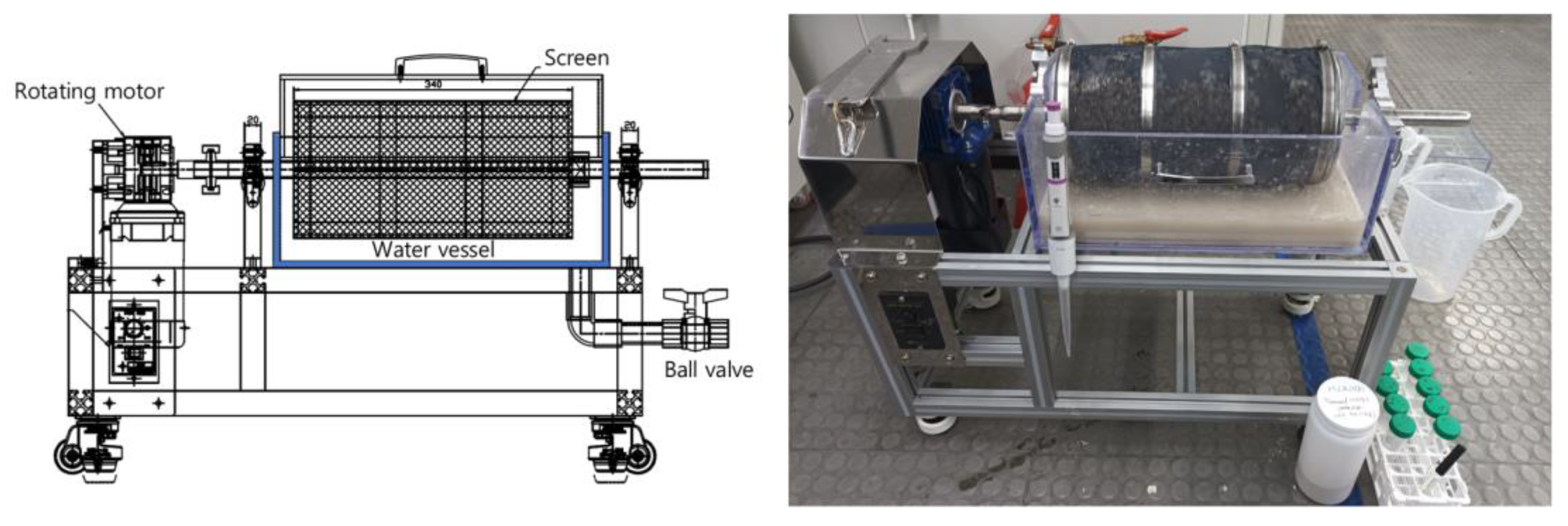
3.2. A Wet-Type Trommel System
3.3. Analytical Methods and Procedures
4. Results and Discussion
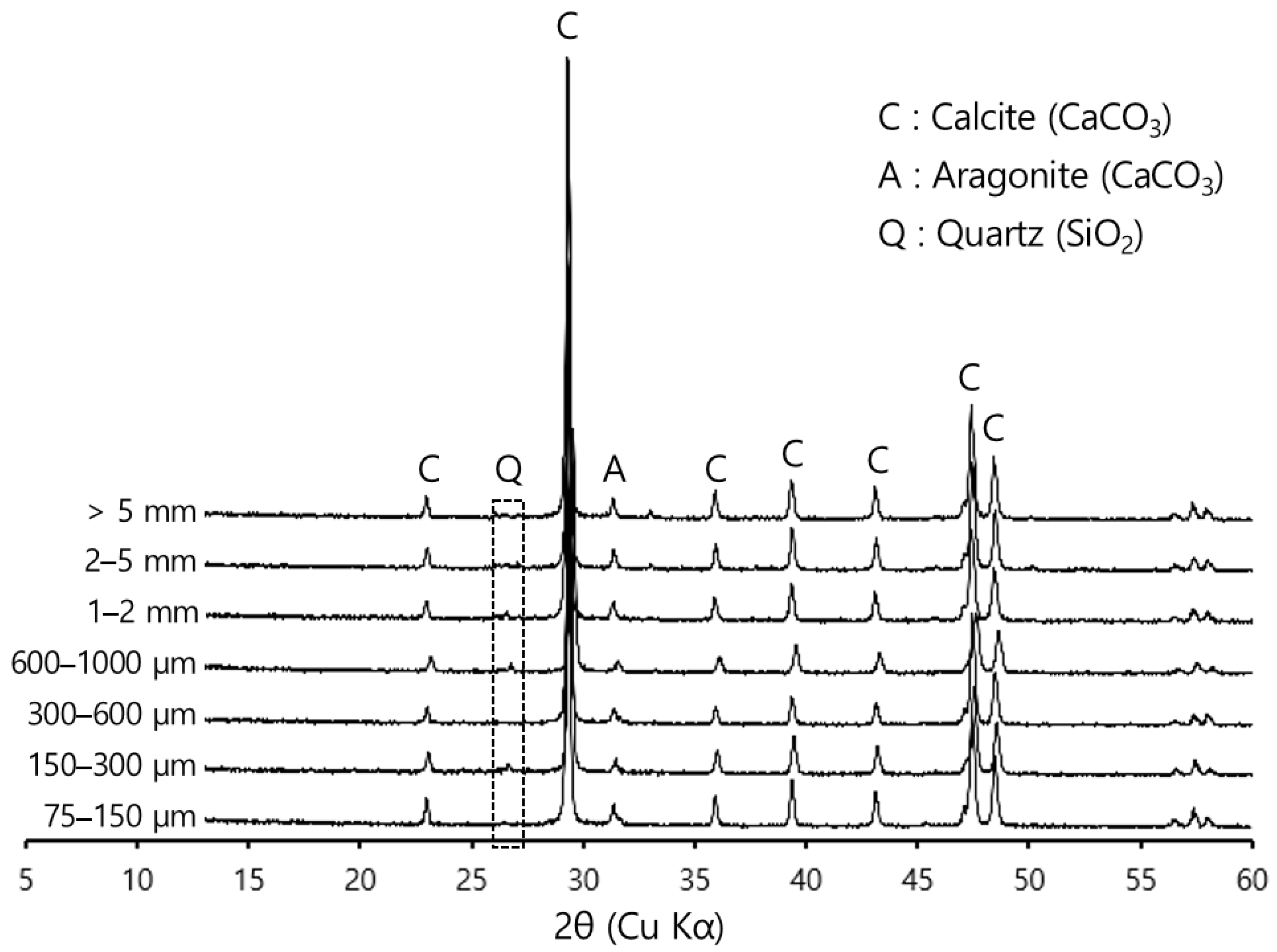
4.1. Particle Size and Chemical Composition
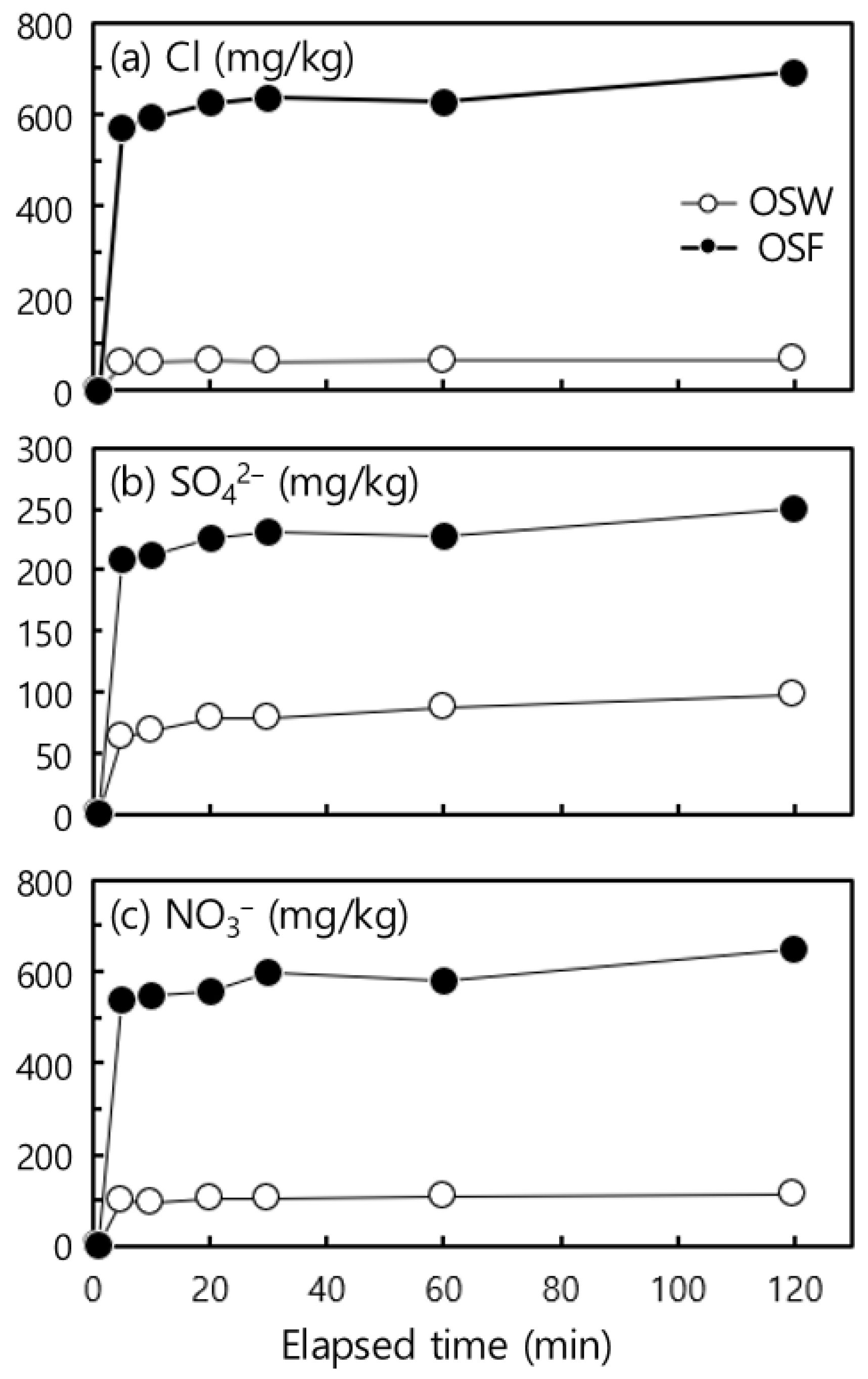
4.2. Washing Characteristics of Oyster Shell Applied to a Simulated Trommel
4.3. Comparisons with Pretreatment Methods on Impurities Removal Efficiency
5. Conclusions and Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barros, M.C.; Bello, P.M.; Bao, M.; Torrado, J.J. From waste to commodity: Transforming shells into high purity calcium carbonate. J. Clean. Prod. 2009, 17, 400–407. [Google Scholar] [CrossRef]
- Directive, E.C. Directive 2006/12/EC of the European Parliament and of the Council of 5 April 2006 on waste. Off. J. Eur. Union 2006, 114, 9–14. [Google Scholar]
- Baek, E.Y. Oyster shell recycling and marine ecosystems: A comparative analysis in the Republic of Korea and Japan. J. Coast. Res. 2021, 114, 350–354. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Scottish Environment Protection Agency. Aquaculture Waste Minimisation Guide; Scottish Environment Protection Agency: Motherwell, UK, 2005.
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.; Fredel, M. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.T.; Wang, H.Y.; Shu, C.Y.; Su, D.S. Engineering properties of controlled low-strength materials containing waste oyster shells. Constr. Build. Mater. 2013, 46, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Mo, K.H.; Algngaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, S.M.; Yang, B.H.; Jang, J.G. Calcined oyster shell powder as an expansive additive in cement mortar. Material 2019, 12, 1322. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.B.; Lee, C.W.; Jun, B.S.; Yun, J.D.; Weon, S.Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Recour. Conserv. Recycl. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sakoda, A.; Suzuki, M. Removal of phosphate by adsorption onto oyster shell powder-kinetic studies. J. Chem. Technol. Biothechnol. 2005, 80, 356–358. [Google Scholar] [CrossRef]
- Jung, Y.J.; Koh, H.W.; Shin, W.T.; Sung, N.C. A novel approach to an advanced tertiary wastewater treatment: Combination of a membrane bioreactor and an oyster-zeolite column. Desalination 2006, 190, 243–255. [Google Scholar] [CrossRef]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, N.; Takamori, H.; Takeda, K.; Sakugawa, H. Transesterification of soybean oil using combusted oyster shell waste as catalyst. Bioresour. Technol. 2009, 100, 1510–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.H.; Chun, B.C.; Chung, Y.C.; Cho, B.G. Fire-retardant plastic material from Oyster shell hell powder and recycled polyethylene. J. Appl. Polym. Sci. 2006, 99, 1583–1589. [Google Scholar] [CrossRef]
- Funabashi, M.; Ninomiya, F.; Flores, E.D.; Kunioka, M. Biomass carbon ratio of polymer composites measured by accelerator mass spectrometry. J. Polym. Environ. 2010, 18, 85–93. [Google Scholar] [CrossRef]
- Gigante, V.; Cinelli, P.; Righetti, M.C.; Sandroni, M.; Tognotti, L.; Seggiani, M.; Lazzeri, A. Evaluation of mussel shells powder as reinforced for PLA-based biocomposites. Int. J. Mol. Sci. 2020, 21, 5364. [Google Scholar] [CrossRef]
- Tsou, C.H.; Wu, C.S.; Hung, W.S.; De Guzman, M.R.; Gao, C.; Wang, R.Y.; Chen, J.; Wan, N.; Peng, Y.J.; Suen, M.C. Rendering polypropylene biocomposites antibacterial through modification with oyster shell powder. Polyimide 2019, 160, 265–271. [Google Scholar] [CrossRef]
- Lee, J.I.; Kang, J.K.; Oh, J.S.; Yoo, S.C.; Lee, C.G.; Jho, E.H.; Park, S.J. New insight to the use of oyster shell for removing phosphorus from aqueous solutions and fertilizing rice growth. J. Clean. Prod. 2021, 328, 129536. [Google Scholar] [CrossRef]
- Ok, Y.S.; Oh, S.E.; Ahmad, M.; Hyun, S.; Kim, K.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.T.; Yang, J.E. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth. Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Ok, Y.S.; Lim, J.E.; Moon, D.H. Stabilization of Pb and Cd contaminated soils and soil quality improvements using waster oyster shells. Environ. Geochem. Health 2011, 33, 83–91. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lee, Y.T.; Chiueh, P.T.; Lo, S.L. Microwave calcination of waste oyster shells for CO2 capture. Energy Procedia 2018, 152, 1242–1247. [Google Scholar] [CrossRef]
- Ma, K.W.; Teng, H. CaO powders from oyster shells for efficient CO2 capture in multiple carbonation cycles. J. Am. Ceram. Soc. 2010, 93, 221–227. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, J.J.; Lee, G.W.; Yoo, K.S.; Shon, B.H. Reuse of waste shells as a SO2/NOx removal sorbent. In Material Recycling—Trends and Perspectives; InTech: Rijeka, Croatia, 2012; Volume 13, pp. 301–322. ISBN 978-953-51-0327-1. [Google Scholar]
- Lim, J.; Cho, H.; Kim, J. Optimization of wet flue gas desulfurization system using recycled waste oyster shell as high-grade limeston substitutes. J. Clean. Prod. 2021, 318, 128492. [Google Scholar] [CrossRef]
- MOF (Ministry of Ocean and Fisheries). Available online: www.fips.go.kr (accessed on 14 January 2021).
- Baek, E.Y.; Lee, W.G. A study on the rational recycling of oyster shell. J. Fish. Bus. Adm. 2020, 51, 71–87. [Google Scholar] [CrossRef]
- Sikes, C.S.; Wheeler, A.P.; Wierzbick, A.; Mount, A.S.; Dillaman, R.M. Nucleation and growth of calcite on native versus pyrolyzed oyster shell folia. Biol. Bull. 2000, 198, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, Y.M.; Kim, R.H.; Choi, C.S. Nano-structured biogenic calcite: A thermal and chemical approach to folia in oyster shell. Micron 2008, 39, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Kim, K.; Kim, S.H.; Kim, Y. The effects of marine sediments and NaCl as impurities on the calcination of oyster shell. Econ. Environ. Geol. 2019, 52, 223–230. [Google Scholar]
- Ha, S.; Lee, J.W.; Choi, S.H.; Kim, S.H.; Kim, K.; Kim, Y. Calcination characteristics of oyster shells and their comparison with limestone from the perspective of waste recycling. J. Mater. Cycles Waste Manag. 2019, 21, 1075–1084. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Foelich, P.N. Kinetic control of dissolved phosphate in natural rivers and estuaries: A primer on the phosphate buffer mechanism 1. Limnol. Oceanogr. 1988, 33, 649–668. [Google Scholar]
- Flower, H.; Rains, M.; Tasci, Y.; Zhang, J.Z.; Trout, K.; Lewis, D.; Das, A.; Dalton, R. Why is calcite a strong phosphorus sink in freshwater? Investigating the adsorption mechanism using batch experiments and surface complexation modeling. Chemosphere 2022, 286, 131596. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Song, K.; Xin, Y.; Lv, X. Novel process for deep removal of chlorine and recycling of chlorinated tailings from titanium-bearing blast-furnace slag. Process Saf. Environ. Prot. 2022, 159, 842–849. [Google Scholar] [CrossRef]



| Component (%) | Particle Size (1) (μm) | ||||
|---|---|---|---|---|---|
| 75–150 | 150–300 | 300–600 | 600–1000 | >1000 | |
| CaO | 87.28 | 92.16 | 91.56 | 94.06 | 95.97 |
| SiO2 | 6.869 | 3.811 | 4.189 | 2.398 | 1.581 |
| Fe2O3 | 2.162 | 1.347 | 1.506 | 1.234 | 0.708 |
| Al2O3 | 1.022 | 0.641 | 0.703 | 0.433 | 0.421 |
| SO3 | 0.944 | 0.901 | 0.892 | 1.083 | 0.773 |
| K2O | 0.482 | 0.264 | 0.251 | 0.094 | 0.000 |
| P2O5 | 0.417 | 0.362 | 0.393 | 0.292 | 0.218 |
| SrO | 0.385 | 0.321 | 0.330 | 0.320 | 0.267 |
| MnO | 0.177 | 0.136 | 0.110 | 0.073 | 0.059 |
| Cl | 0.175 | ND | ND | ND | ND |
| ZnO | 0.087 | 0.056 | 0.063 | ND | ND |
| Component (%) | OSW (1) | OSF (2) | ||
|---|---|---|---|---|
| Raw Shell (Unwashed) | Washed Shell | Raw Shell (Unwashed) | Washed Shell | |
| CaO | 94.84 | 98.09 | 94.35 | 98.42 |
| SiO2 | 1.992 | 0.398 | 2.029 | 0.376 |
| Fe2O3 | 0.922 | 0.411 | 1.166 | 0.129 |
| SO3 | 0.812 | 0.473 | 0.820 | 0.459 |
| SrO | 0.436 | 0.390 | 0.462 | 0.384 |
| P2O5 | 0.313 | 0.156 | 0.425 | 0.199 |
| Al2O3 | 0.275 | 0.053 | 0.273 | NA |
| K2O | 0.105 | NA | 0.122 | NA |
| MnO | 0.091 | NA | 0.104 | NA |
| ZnO | 0.088 | NA | 0.115 | NA |
| Na2O | 0.078 | 0.014 | 0.093 | 0.022 |
| Cl | 0.049 | 0.011 | 0.039 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-E.; Kim, S.-H. Evaluation of Washing and Screening for Upgrading the Calcium Content of Oyster Shells Using a Simulated Wet-Type Trommel. Sustainability 2022, 14, 15880. https://doi.org/10.3390/su142315880
Lee S-E, Kim S-H. Evaluation of Washing and Screening for Upgrading the Calcium Content of Oyster Shells Using a Simulated Wet-Type Trommel. Sustainability. 2022; 14(23):15880. https://doi.org/10.3390/su142315880
Chicago/Turabian StyleLee, Sang-Eun, and Seok-Hwi Kim. 2022. "Evaluation of Washing and Screening for Upgrading the Calcium Content of Oyster Shells Using a Simulated Wet-Type Trommel" Sustainability 14, no. 23: 15880. https://doi.org/10.3390/su142315880




