Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Carbon Materials
2.1.1. Materials and Their Carbonization
2.1.2. Activation
2.2. Characterization of Precursors, Carbonisats and Activated Carbons
3. Results and Discussion
3.1. Characterization of Feedstock and Carbonized Materials
3.2. Characterization of Activated Carbon Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plavniece, A.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Kaare, K.; Kruusenberg, I.; Kaprans, K.; Knoks, A.; Kleperis, J. Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application. Sustainability 2021, 13, 9237. [Google Scholar] [CrossRef]
- Plavniece, A.; Dobele, G.; Volperts, A.; Djachkovs, D.; Jashina, L.; Bikovens, O.; Zhurinsh, A. Effect of the pretreatment on the porosity of the hybrid activated carbons prepared from wood-based solid and liquid precursors. Wood Sci. Technol. 2022, 1–17. [Google Scholar] [CrossRef]
- Sundqvist, B. Carbon under pressure. Phys. Rep. 2021, 909, 1–73. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Alevizos, G. Adsorption of Scandium and Neodymium on Biochar Derived after Low-Temperature Pyrolysis of Sawdust. Minerals 2017, 7, 200. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, F.; Zhou, Y.; Li, Z.; Cheng, H.; Li, W.; Ji, R.; Zhang, L.; Bian, Y.; Han, J.; et al. Assisting the carbonization of biowaste with potassium formate to fabricate oxygen-doped porous biochar sorbents for removing organic pollutant from aqueous solution. Bioresour. Technol. 2022, 360, 127546. [Google Scholar] [CrossRef]
- Halysh, V.; Sevastyanova, O.; Pikus, S.; Dobele, G.; Pasalskiy, B.; Gun’ko, V.M.; Kartel, M. Sugarcane bagasse and straw as low-cost lignocellulosic sorbents for the removal of dyes and metal ions from water. Cellulose 2020, 27, 8181–8197. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Volperts, A.; Dobele, G.; Zhurinsh, A.; Vervikishko, D.; Shkolnikov, E.; Ozolinsh, J. Wood-based activated carbons for supercapacitor electrodes with a sulfuric acid electrolyte. Xinxing Tan Cailiao/New Carbon Mater. 2017, 32, 319–326. [Google Scholar] [CrossRef]
- Shao, Y.; Sui, J.; Yin, G.; Gao, Y. Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2008, 79, 89–99. [Google Scholar] [CrossRef]
- Han, T.; Wu, Y.; Li, L.; Xie, Z.; Xie, Y.; Zhang, J.; Meng, X.; Yu, F.; Yang, N. A high-performance direct carbon solid oxide fuel cell powered by barium-based catalyst-loaded biochar derived from sunflower seed shell. Int. J. Hydrog. Energy 2022, 47, 38747–38756. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Kaare, K.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I. Influence of Chemical Activation Temperatures on Nitrogen-Doped Carbon Material Structure, Pore Size Distribution and Oxygen Reduction Reaction Activity. Catalysts 2021, 11, 1460. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass based activated carbons for fuel cells. Renew. Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Chung, S.; Kang, H.; Ocon, J.D.; Lee, J.K.; Lee, J. Enhanced electrical and mass transfer characteristics of acid-treated carbon nanotubes for capacitive deionization. Curr. Appl. Phys. 2015, 15, 1539–1544. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R.M. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, Q.; Li, M.; Zhao, H.; Jin, S.; Huang, Y.; Fan, J.; Chen, J. Analysis of factors influencing pore structure development of agricultural and forestry waste-derived activated carbon for adsorption application in gas and liquid phases: A review. J. Environ. Chem. Eng. 2021, 9, 105905. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Lin, H.; Li, Y.; Lu, H.; Wang, Y. A green technology for the preparation of high capacitance rice husk-based activated carbon. J. Clean. Prod. 2016, 112, 1190–1198. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Z.; Li, B.; Zhang, C. High performance electrode materials for electric double-layer capacitors based on biomass-derived activated carbons. Electrochim. Acta 2015, 173, 377–384. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Yue, Q.; Gao, B.; Sun, Y.; Kong, J.; Zhao, P. Simple synthesis of hierarchical porous carbon from Enteromorpha prolifera by a self-template method for supercapacitor electrodes. J. Power Sources 2014, 270, 403–410. [Google Scholar] [CrossRef]
- Linares-Solano, Á.; Lillo-Ródenas, M.; Lozar, J.; Kunowsky, M.; Anaya, A.J.R. NaOH and KOH for preparing activated carbons used in energy and environmental applications. Int. J. Energy Environ. Econ. 2012, 20, 59–91. [Google Scholar]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-Efficient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2012, 27, 454–460. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Liu, F.; Yu, R.; Ji, X.; Guo, M. Hydrothermal carbonization of holocellulose into hydrochar: Structural, chemical characteristics, and combustion behavior. Bioresour. Technol. 2018, 263, 508–516. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Braghiroli, F.L.; Izquierdo, M.T.; Parmentier, J.; Celzard, A.; Fierro, V. Synthesis and properties of carbon microspheres based on tannin–sucrose mixtures treated in hydrothermal conditions. Ind. Crops Prod. 2020, 154, 112564. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Rabinovich, M.L.; Fedoryak, O.; Dobele, G.; Andersone, A.; Gawdzik, B.; Lindström, M.E.; Sevastyanova, O. Carbon adsorbents from industrial hydrolysis lignin: The USSR/Eastern European experience and its importance for modern biorefineries. Renew. Sustain. Energy Rev. 2016, 57, 1008–1024. [Google Scholar] [CrossRef]
- Ponomarev, N.; Sillanpää, M. Combined chemical-templated activation of hydrolytic lignin for producing porous carbon. Ind. Crops Prod. 2019, 135, 30–38. [Google Scholar] [CrossRef]
- Liu, X.; Song, P.; Wang, B.; Wu, Y.; Jiang, Y.; Xu, F.; Zhang, X. Lignosulfonate-Directed Synthesis of Consubstantial Yolk-Shell Carbon Microspheres with Pollen-Like Surface from Sugar Biomass. ACS Sustain. Chem. Eng. 2018, 6, 16315–16322. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Fang, W.; Yang, S.; Wang, X.-L.; Yuan, T.-Q.; Sun, R.-C. Manufacture and application of lignin-based carbon fibers (LCFs) and lignin-based carbon nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827. [Google Scholar] [CrossRef]
- Li, H.; Shi, F.; An, Q.; Zhai, S.; Wang, K.; Tong, Y. Three-dimensional hierarchical porous carbon derived from lignin for supercapacitors: Insight into the hydrothermal carbonization and activation. Int. J. Biol. Macromol. 2021, 166, 923–933. [Google Scholar] [CrossRef]
- Brazdausks, P.; Paze, A.; Rizhikovs, J.; Puke, M.; Meile, K.; Vedernikovs, N.; Tupciauskas, R.; Andzs, M. Effect of aluminium sulphate-catalysed hydrolysis process on furfural yield and cellulose degradation of Cannabis sativa L. shives. Biomass Bioenergy 2016, 89, 98–104. [Google Scholar] [CrossRef]
- Dobele, G.; Zhurinsh, A.; Volperts, A.; Jurkjane, V.; Pomilovskis, R.; Meile, K. Study of levoglucosenone obtained in analytical pyrolysis and screw-type reactor, separation and distillation. Wood Sci. Technol. 2020, 54, 383–400. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Fang, Z.; Sato, T.; Smith, R.L.; Inomata, H.; Arai, K.; Kozinski, J.A. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water. Bioresour. Technol. 2008, 99, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9781420028812. [Google Scholar]
- Marsh, H.; Yan, D.S.; O’Grady, T.M.; Wennerberg, A. Formation of active carbons from cokes using potassium hydroxide. Carbon 1984, 22, 603–611. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-08-097035-6. [Google Scholar]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 2015, 273, 622–629. [Google Scholar] [CrossRef]
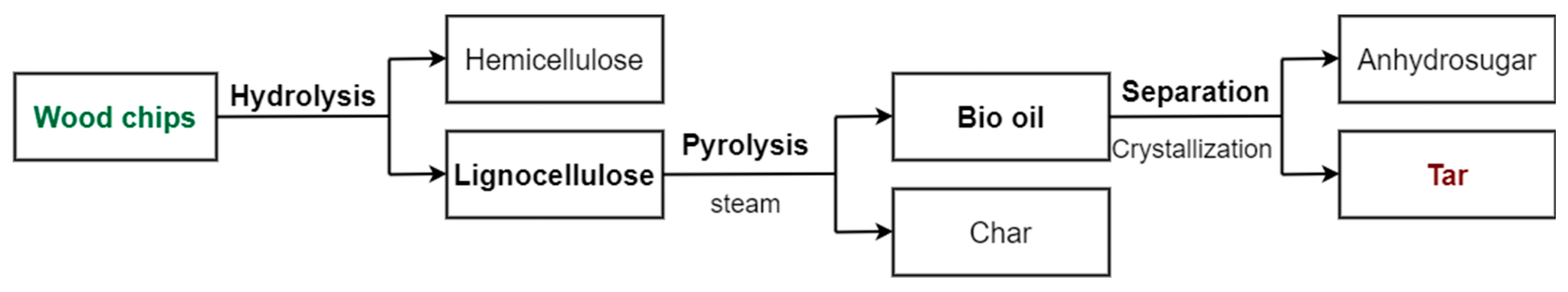
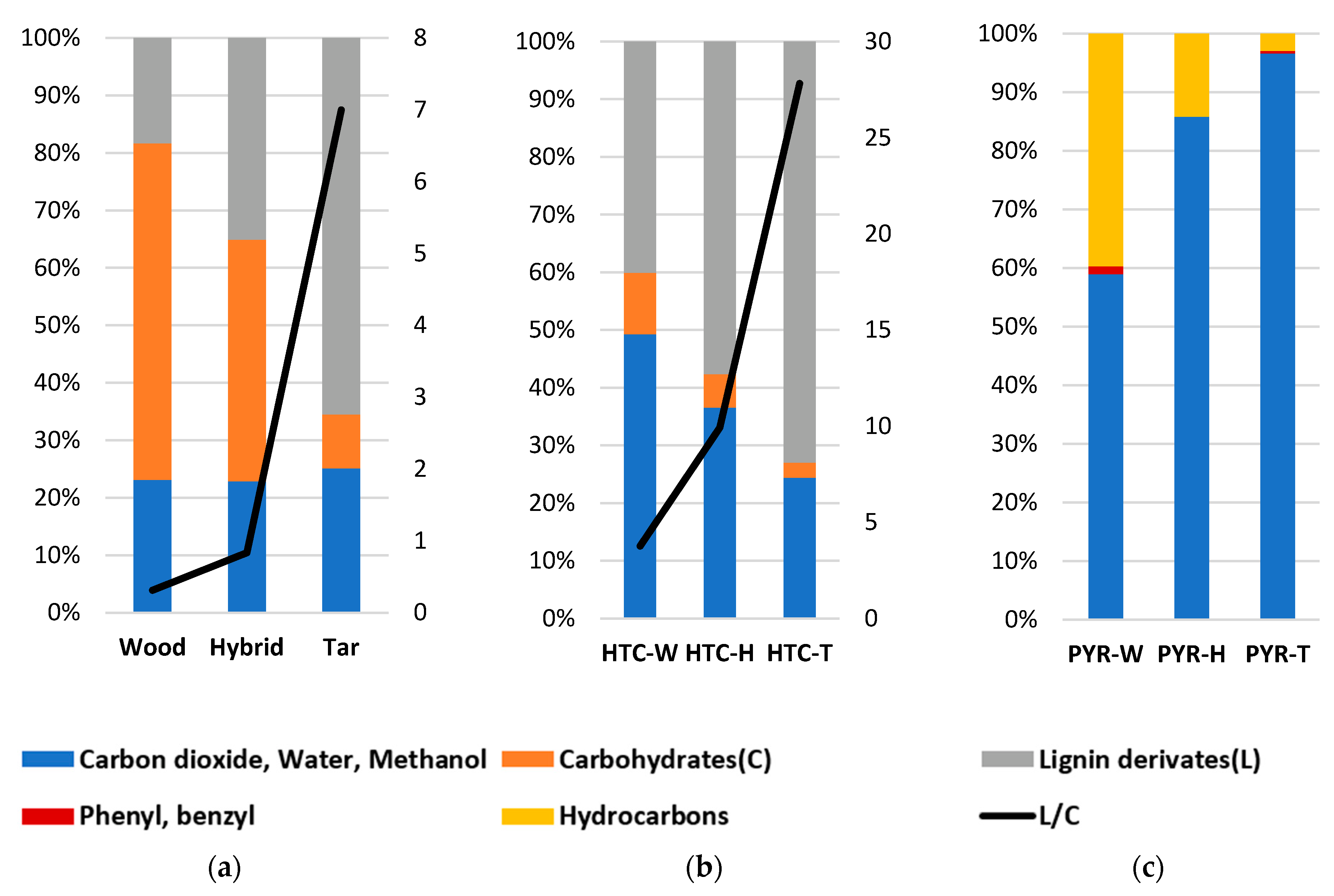
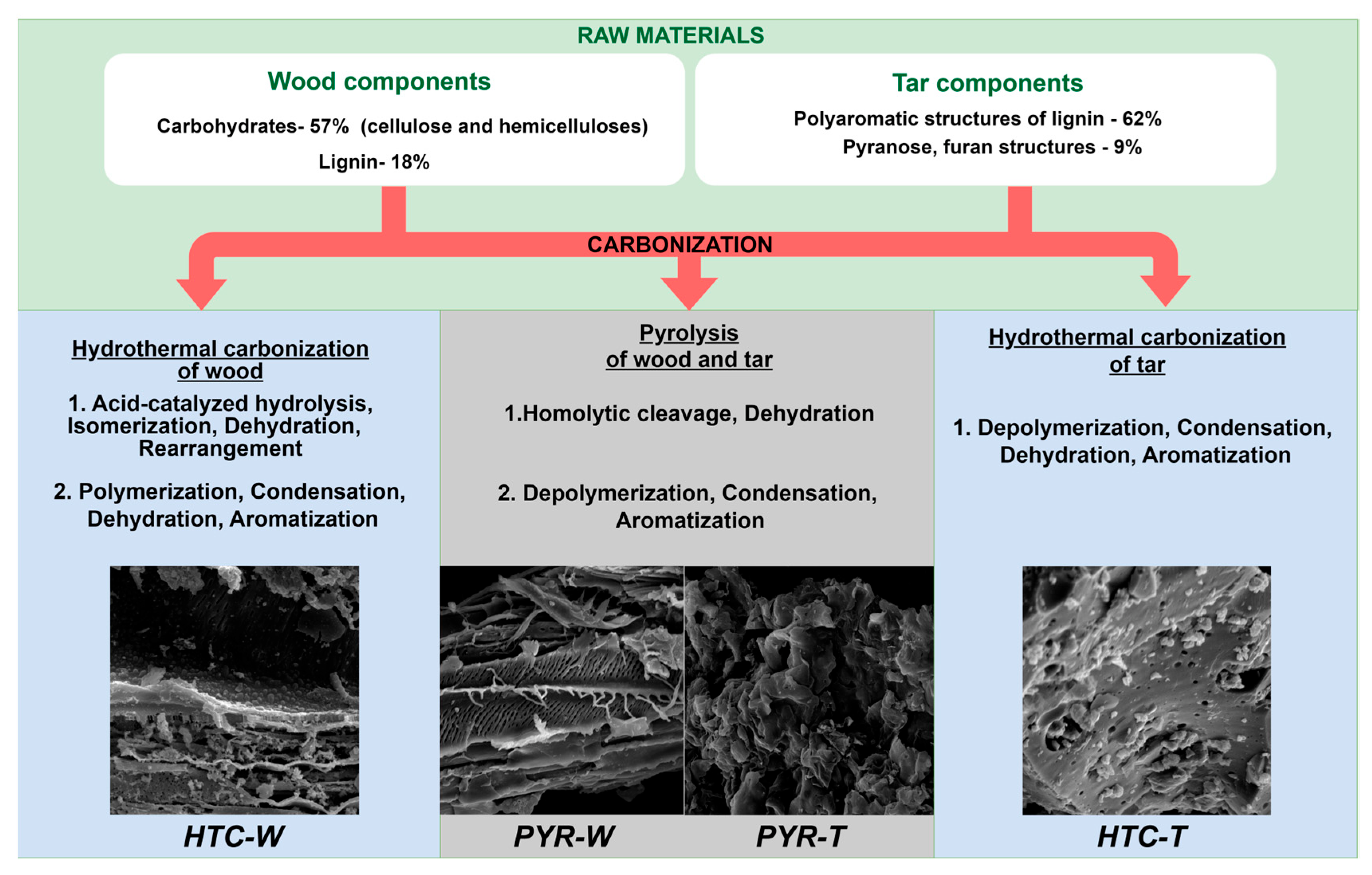
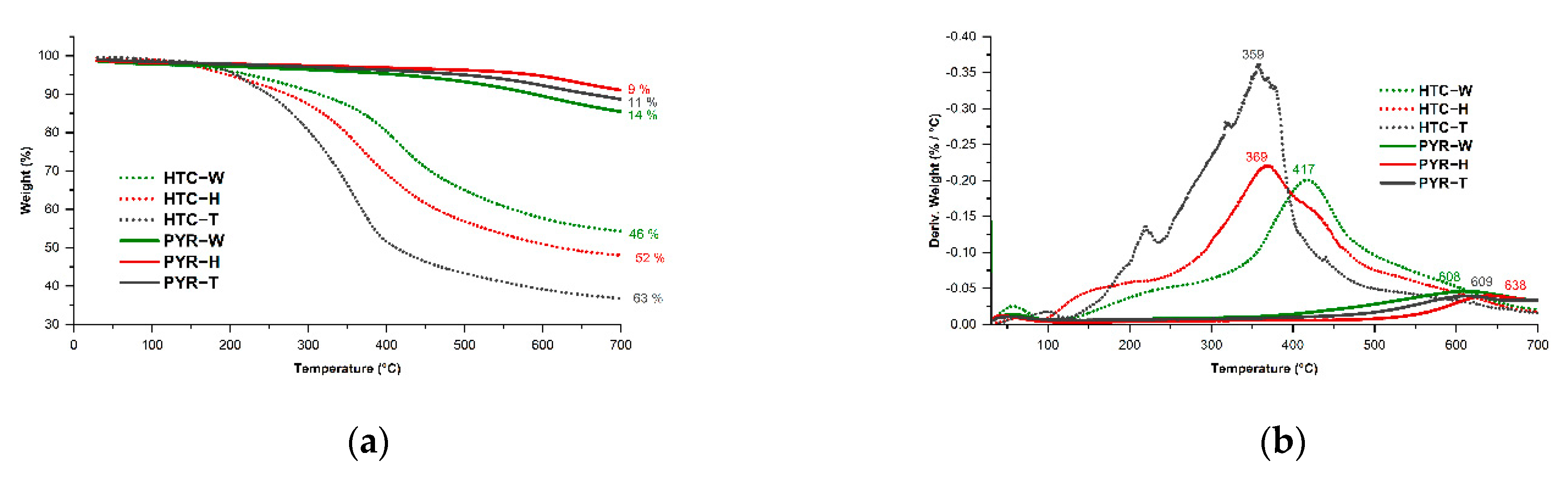
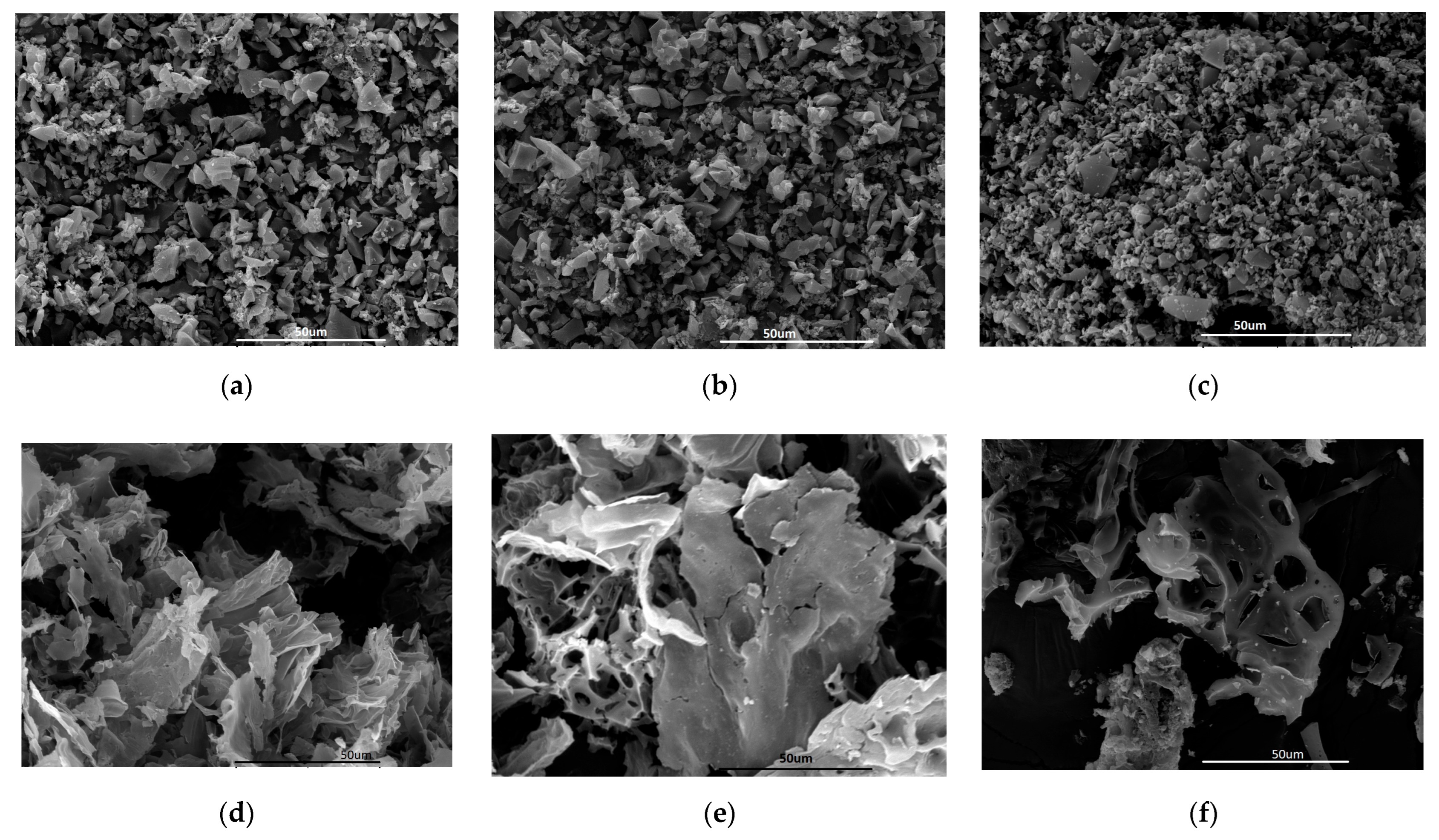
| Wood | Hybrid | Tar | HTC-W | HTC-H | HTC-T | |
|---|---|---|---|---|---|---|
| Carbohydrates derivates | 56.82 | 41.71 | 8.87 | 10.20 | 5.60 | 2.44 |
| Acid, Ester | 13.58 | 10.85 | 0.91 | 2.99 | 1.58 | 0.88 |
| Alcohol | 0.78 | 0.67 | - | - | - | - |
| Aldehyde, Ketone | 17.27 | 12.42 | 0.47 | 2.32 | 1.37 | 0.40 |
| Cyclopentane derivates | 3.53 | 2.83 | 0.38 | 3.63 | 1.80 | 0.70 |
| Furan | 9.91 | 7.27 | 1.23 | 1.26 | 0.85 | 0.46 |
| Pyran | 3.11 | 1.71 | 0.36 | - | - | - |
| Sugars | 8.64 | 5.96 | 5.52 | - | - | - |
| Lignin derivates | 17.79 | 34.76 | 62.08 | 38.41 | 55.62 | 67.91 |
| Phenyl and benzyl derivates | 0.73 | 3.15 | 7.46 | 6.48 | 9.59 | 13.84 |
| Guaiacyl derivates | 5.42 | 11.50 | 18.67 | 8.94 | 13.08 | 17.56 |
| Syringyl derivates | 11.64 | 20.11 | 35.95 | 22.99 | 32.95 | 36.51 |
| Carbon dioxide, Water, Methanol | 22.39 | 22.65 | 23.78 | 47.26 | 35.31 | 22.72 |
| Sample | Precursor | Carbonization | Yield, % | C, % | O, % | NaOH, mmol g−1 (Total Acidic Groups) |
|---|---|---|---|---|---|---|
| PYR-W | Wood | 500 °C | 27 | 84.1 | 12.1 | >0.001 |
| PYR-H | Wood and tar | 35 | 88.2 | 8.1 | 0.001 | |
| PYR-T | Tar | 37 | 82.0 | 14.3 | 0.003 | |
| HTC-W | Wood | 250 °C, H2O | 60 | 71.1 | 23.9 | 0.008 |
| HTC-H | Wood and tar | 44 | 72.4 | 22.1 | 0.011 | |
| HTC-T | Tar | 50 | 74.8 | 19.0 | 0.010 |
| Sample | Yield (after Activation), % | Yield (after Carbonization and Activation), % | N, % | C, % | H, % | O, % |
|---|---|---|---|---|---|---|
| APYR-W | 36 | 10 | 0.9 | 92.0 | 0.6 | 6.4 |
| APYR-H | 40 | 14 | 0.7 | 92.5 | 0.7 | 6.1 |
| APYR-T | 37 | 14 | 0.8 | 93.0 | 0.7 | 5.5 |
| AHTC-W | 15 | 9 | 1.7 | 91.7 | 0.6 | 6.0 |
| AHTC-H | 16 | 7 | 1.2 | 92.0 | 0.6 | 6.1 |
| AHTC-T | 14 | 7 | 0.6 | 93.4 | 1.3 | 4.5 |
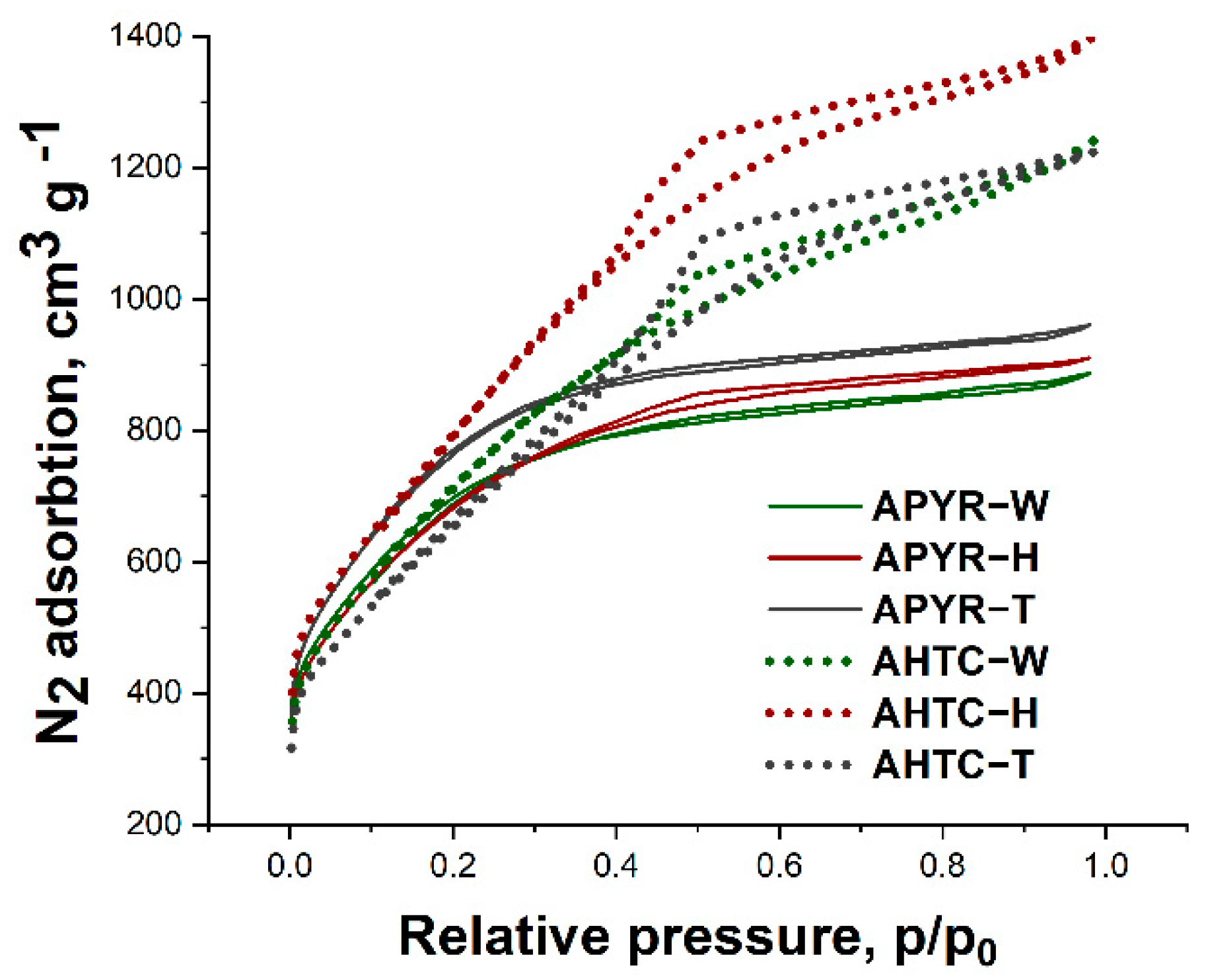
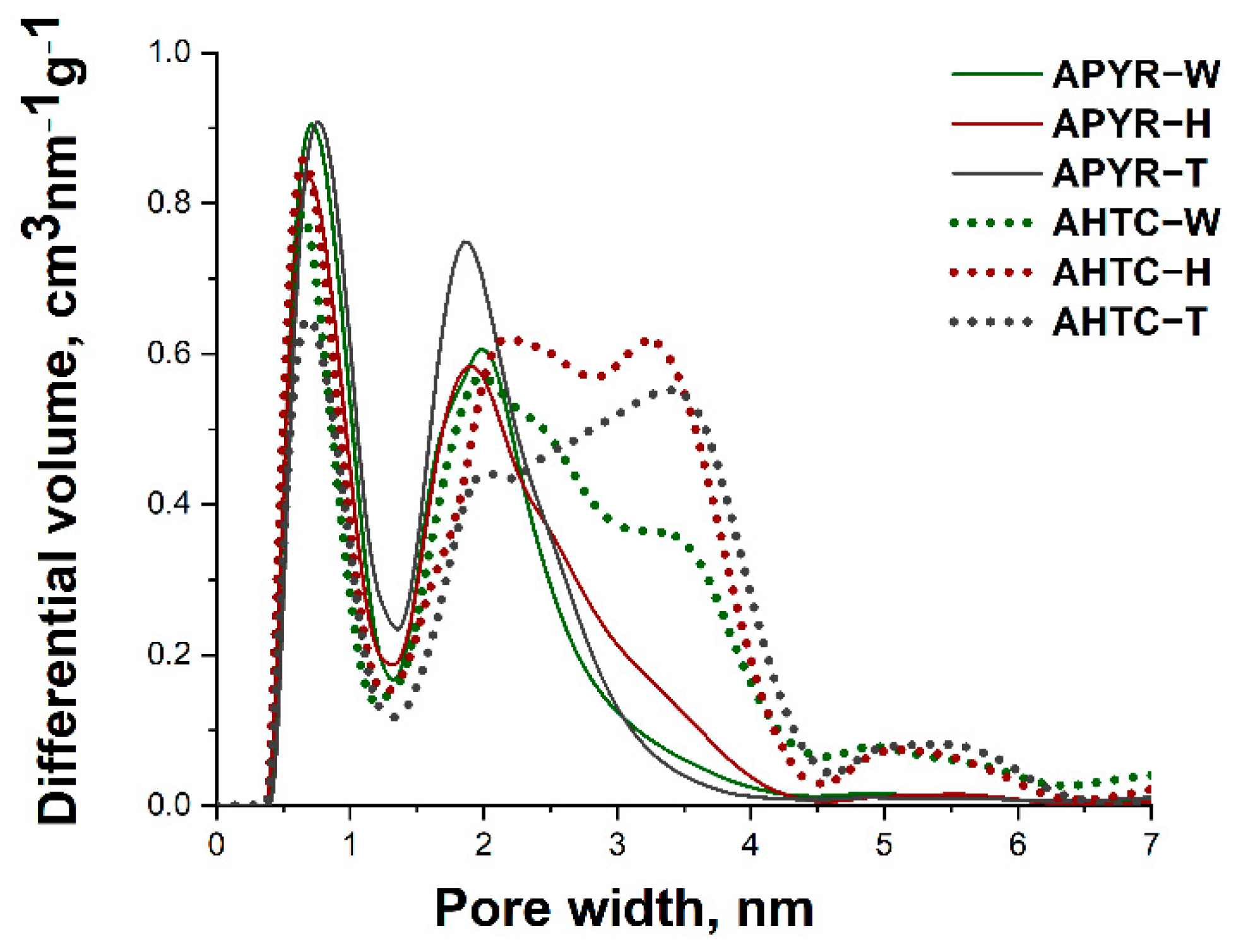
| Sample | SBET, m2 g−1 | Vtotal, cm3 g−1 | Vmicro, cm3 g−1 | Vmeso, cm3 g−1 | Vmeso from Vt, % | Average Pore Size, nm |
|---|---|---|---|---|---|---|
| APYR-W | 2553 | 1.4 | 0.8 | 0.6 | 43 | 2.2 |
| APYR-H | 2494 | 1.4 | 0.8 | 0.6 | 43 | 2.3 |
| APYR-T | 2804 | 1.5 | 0.9 | 0.6 | 40 | 2.1 |
| AHTC-W | 2629 | 1.9 | 0.8 | 1.1 | 58 | 2.9 |
| AHTC-H | 2919 | 2.2 | 0.9 | 1.3 | 59 | 3.0 |
| AHTC-T | 2401 | 1.9 | 0.8 | 1.1 | 58 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A. Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials. Sustainability 2022, 14, 15982. https://doi.org/10.3390/su142315982
Plavniece A, Dobele G, Volperts A, Zhurinsh A. Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials. Sustainability. 2022; 14(23):15982. https://doi.org/10.3390/su142315982
Chicago/Turabian StylePlavniece, Ance, Galina Dobele, Aleksandrs Volperts, and Aivars Zhurinsh. 2022. "Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials" Sustainability 14, no. 23: 15982. https://doi.org/10.3390/su142315982
APA StylePlavniece, A., Dobele, G., Volperts, A., & Zhurinsh, A. (2022). Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials. Sustainability, 14(23), 15982. https://doi.org/10.3390/su142315982






