Biostimulants as Innovative Tools to Boost Date Palm (Phoenix dactylifera L.) Performance under Drought, Salinity, and Heavy Metal(Oid)s’ Stresses: A Concise Review
Abstract
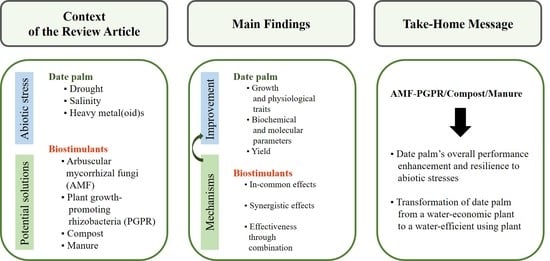
:1. Introduction
2. History and Distribution of Date Palm
3. Date Palm, A Pillar in the Oasis Ecosystem
4. Pests, Diseases, and Anthropogenic Constraints
5. Effects of Abiotic Stresses and Adaptive Strategies in Date Palm
5.1. Drought
5.2. Salinity
5.3. Heavy Metal(Oid)s
6. Biostimulants Attenuate Abiotic Stresses’ Effects in Date Palm
6.1. AMF
6.1.1. Drought
6.1.2. Salinity
6.1.3. Heavy Metal(Oid)s
- Heavy metal(oid)s biofortification/phytoextraction, as AMF improve date palm’s growth traits, water status, and P assimilation, which decreases heavy metal(oid)s through the dilution effect. These roles are facilitated thanks to the mycorrhizal pathway that involves high-affinity metal transporters located at the extraradical mycelium of the symbiotic interface (root hairs and epidermal cells) [112,113,114].
- Phytostabilization, as AMF lead to the immobilization and sorption of heavy metal(oid)s (e.g., Mn, Zn, Cu, Fe, and cesium (Cs)) in mycorrhizal date palm plants, specifically in the level of extra-radicular mycelium structures. Moreover, mycorrhizae bind heavy metal(oid)s against the cell wall, chelate metallic ions within the cytosol, and facilitate their compartmentalization in the vacuoles by stabilizing them with polyP [113,115].
- Detoxification through the antioxidant defense machinery activation (e.g., SOD) [116].
- Overexpression of protective proteins in the level of mycorrhizal roots (e.g., heat-shock proteins and glutathione transferase) [116].
- Exudation of organic acids, which contributes to the reduction in metal mobility in the soil, in addition to chelation by glomalin [117].
6.2. PGPR
6.2.1. Drought
6.2.2. Salinity
6.2.3. Heavy Metal(Oid)s
6.3. Organic Amendments
6.3.1. Drought
6.3.2. Salinity
6.3.3. Heavy Metal(Oid)s
7. AMF–PGPR Co-Inoculation and/or Organic Amendments
- Strengthened in-common effects
- Synergistic and complementarity effects
8. Biostimulants and Plant Hormones: Initiating and Suppressing Effects
8.1. AMF–Plant Hormones
8.2. PGPR–Plant Hormones
9. Research Gaps
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Ghussain, L. Global Warming: Review on Driving Forces and Mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Papalexiou, S.M.; Montanari, A. Global and Regional Increase of Precipitation Extremes Under Global Warming. Water Resour. Res. 2019, 55, 4901–4914. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate Change, Food Security and Mycotoxins: Do We Know Enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Minhas, P.S.; Rane, J.; Pasala, R.K. (Eds.) Abiotic Stress Management for Resilient Agriculture; Springer: Singapore, 2017; ISBN 978-981-10-5743-4. [Google Scholar]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; Mpandeli, S.; Nhamo, L.; Chimonyo, V.G.P.; Nhemachena, C.; Senzanje, A.; Naidoo, D.; Modi, A.T. Prospects for Improving Irrigated Agriculture in Southern Africa: Linking Water, Energy and Food. Water 2018, 10, 1881. [Google Scholar] [CrossRef] [Green Version]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil Salinity, a Serious Environmental Issue and Plant Responses: A Metabolomics Perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli, M.; Ikan, C.; Boutasknit, A.; Slimani, A.; Oufdou, K.; Baslam, M.; Ait Barka, E.; et al. Application of Indigenous Rhizospheric Microorganisms and Local Compost as Enhancers of Lettuce Growth, Development, and Salt Stress Tolerance. Microorganisms 2022, 10, 1625. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Introduction to Soil Salinity, Sodicity and Diagnostics Techniques; Springer: Cham, Switzerland, 2018; ISBN 9783319961897. [Google Scholar]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [PubMed] [Green Version]
- Mia, M.A.T.; Mosaib, M.G.; Khalil, M.I.; Islam, M.A.; Gan, S.H. Potentials and Safety of Date Palm Fruit against Diabetes: A Critical Review. Foods 2020, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.; Rasheed, H.; Naqvi, S.A.; Al-Khayri, J.M.; Lorenzo, J.M.; Alaghbari, M.A.; Manzoor, M.F.; Aadil, R.M. Therapeutic Potential of Date Palm against Human Infertility: A Review. Metabolites 2021, 11, 408. [Google Scholar] [CrossRef]
- Al-Alawi, R.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safronov, O.; Kreuzwieser, J.; Haberer, G.; Alyousif, M.S.; Schulze, W.; Al-Harbi, N.; Arab, L.; Ache, P.; Stempfl, T.; Kruse, J.; et al. Detecting Early Signs of Heat and Drought Stress in Phoenix dactylifera (Date Palm). PLoS ONE 2017, 12, e0177883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ahmed, H.B.; Mitsui, T.; Baslam, M.; Meddich, A. The Native Arbuscular Mycorrhizal Fungi and Vermicompost-Based Organic Amendments Enhance Soil Fertility, Growth Performance, and the Drought Stress Tolerance of Quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hakeem, K.R.; Nahar, K.; Alharby, H.F. Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; Volume 7, pp. 1–490. [Google Scholar] [CrossRef]
- Jogawat, A. Osmolytes and Their Role in Abiotic Stress Tolerance in Plants. In Molecular Plant Abiotic Stress; Wiley: New York, NY, USA, 2019; pp. 91–104. ISBN 9781119463665. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Sarkar, T.; Thankappan, R.; Mishra, G.P.; Nawade, B.D. Advances in the Development and Use of DREB for Improved Abiotic Stress Tolerance in Transgenic Crop Plants. Physiol. Mol. Biol. Plants 2019, 25, 1323–1334. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Mahmud, S.; Paul, G.K.; Afroze, M.; Islam, S.; Gupt, S.B.R.; Razu, M.H.; Biswas, S.; Zaman, S.; Uddin, M.S.; Khan, M.; et al. Efficacy of Phytochemicals Derived from Avicennia Officinalis for the Management of COVID-19: A Combined In Silico and Biochemical Study. Molecules 2021, 26, 2210. [Google Scholar] [CrossRef]
- Ghirardo, A.; Nosenko, T.; Kreuzwieser, J.; Winkler, J.B.; Kruse, J.; Albert, A.; Schnitzler, J.P. Protein Expression Plasticity Contributes to Heat and Drought Tolerance of Date Palm. Oecologia 2021, 197, 903–919. [Google Scholar] [PubMed]
- Al-Harrasi, I.; Jana, G.A.; Patankar, H.V.; Al-Yahyai, R.; Rajappa, S.; Kumar, P.P.; Yaish, M.W. A Novel Tonoplast Na+/H+ Antiporter Gene from Date Palm (PdNHX6) Confers Enhanced Salt Tolerance Response in Arabidopsis. Plant Cell Rep. 2020, 39, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Rekik, I.; Chaâbene, Z.; Kriaa, W.; Rorat, A.; Franck, V.; Hafedh, M.; Elleuch, A. Transcriptome Assembly and Abiotic Related Gene Expression Analysis of Date Palm Reveal Candidate Genes Involved in Response to Cadmium Stress. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2019, 225, 108569. [Google Scholar] [CrossRef]
- Jana, G.A.; Yaish, M.W. Genome-Wide Identification and Functional Characterization of Glutathione Peroxidase Genes in Date Palm (Phoenix dactylifera L.) under Stress Conditions. Plant Gene 2020, 23, 100237. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as Strategies to Improve Photosynthetic Apparatus, Growth, and Drought Stress Tolerance in the Date Palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight Into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 183. [Google Scholar] [CrossRef]
- Lahbouki, S.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Ait-El-Mokhtar, M.; El Gabardi, S.; Douira, A.; Wahbi, S.; Outzourhit, A.; et al. Arbuscular Mycorrhizal Fungi and/or Organic Amendment Enhance the Tolerance of Prickly Pear (Opuntia Ficus-indica) under Drought Stress. J. Arid Environ. 2022, 199, 104703. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of Arbuscular Mycorrhizal Fungi on Soil Fertility: Contribution in the Improvement of Physical, Chemical, and Biological Properties of the Soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
- Slimani, A.; Raklami, A.; Oufdou, K.; Meddich, A. Isolation and Characterization of PGPR and Their Potenzial for Drought Alleviation in Barley Plants. Gesunde Pflanz. 2022, 1–15. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting Parameters and Compost Quality: A Literature Review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Whalen, J.K.; Thomas, B.W.; Sharifi, M. Novel Practices and Smart Technologies to Maximize the Nitrogen Fertilizer Value of Manure for Crop Production in Cold Humid Temperate Regions. Adv. Agron. 2019, 153, 1–85. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Flowers, J.M.; Nelson, D.; Lemansour, A.; Masmoudi, K.; Amiri, K.M.A. Prospects for the Study and Improvement of Abiotic Stress Tolerance in Date Palms in the Post-Genomics Era. Front. Plant Sci. 2020, 11, 293. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Chao, C.C.T.; Krueger, R.R. The Date Palm (Phoenix dactylifera L.): Overview of Biology, Uses, and Cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Gros-Balthazard, M.; Hazzouri, K.; Flowers, J. Genomic Insights into Date Palm Origins. Genes 2018, 9, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Simchoni, O.; Kislev, M. Germination, Genetics, and Growth of an Ancient Date Seed. Science 2008, 320, 1464. [Google Scholar] [CrossRef]
- El-Juhany, L.I. Degradation of Date Palm Trees and Date Production in Arab Countries: Causes and Potential Rehabilitation. Aust. J. Basic Appl. Sci. 2010, 4, 3998–4010. [Google Scholar] [CrossRef]
- Sghaier-Hammami, B.; Baazaoui, N.; Drira, R.; Drira, N.; Jorrín-Novo, J.V. Proteomic Insights of Date Palm Embryogenesis and Responses to Environmental Stress. In The Date Palm Genome; Springer: Cham, Switzerland, 2021; Volume 2, pp. 85–99. ISBN 978-3-030-73746-7. [Google Scholar]
- Hussain, M.I.; Farooq, M.; Syed, Q.A. Nutritional and Biological Characteristics of the Date Palm Fruit (Phoenix dactylifera L.)—A Review. Food Biosci. 2020, 34, 100509. [Google Scholar] [CrossRef]
- Mihi, A.; Tarai, N.; Chenchouni, H. Can Palm Date Plantations and Oasification Be Used as a Proxy to Fight Sustainably against Desertification and Sand Encroachment in Hot Drylands? Ecol. Indic. 2019, 105, 365–375. [Google Scholar] [CrossRef]
- Raho, O.; Boutasknit, A.; Anli, M.; Ben-Laouane, R.; Rahou, Y.A.; Ouhaddou, R.; Duponnois, R.; Douira, A.; Modafar, C.; El Meddich, A. Impact of Native Biostimulants/Biofertilizers and Their Synergistic Interactions On the Agro-Physiological and Biochemical Responses of Date Palm Seedlings. Gesunde Pflanz. 2022, 74, 1053–1069. [Google Scholar] [CrossRef]
- Al-Dosary, N.M.N.; Al-Dobai, S.; Faleiro, J.R. Review on the Management of Red Palm Weevil Rhynchophorus ferrugineus Olivier in Date Palm Phoenix dactylifera L. Emir. J. Food Agric. 2016, 28, 34–44. [Google Scholar] [CrossRef]
- Ibrahim, K.M. The Role of Date Palm Tree in Improvement of the Environment. Acta Hortic. 2010, 882, 777–778. [Google Scholar] [CrossRef]
- Al-Hammadi, M.S.; Al-Shariqi, R.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M. Molecular Identification of Fungal Pathogens Associated with Date Palm Root Diseases in the United Arab Emirates. J. Plant Pathol. 2019, 101, 141–147. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Alikhani-Koupaei, M.; Fatahi, R.; Zamani, Z.; Salimi, S. Effects of Deficit Irrigation on Some Physiological Traits, Production and Fruit Quality of ‘Mazafati’ Date Palm and the Fruit Wilting and Dropping Disorder. Agric. Water Manag. 2018, 209, 219–227. [Google Scholar] [CrossRef]
- Sakran, M.I.; El Rabey, H.A.; Almulaiky, Y.Q.; Al-Duais, M.A.; Elbakry, M.; Faridi, U. The Antioxidant Enzymatic Activity of Date Palm Seedlings under Abiotic Drought Stress. Indian J. Pharm. Educ. Res. 2018, 52, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Al-Khateeb, S.A.; Al-Khateeb, A.A.; El-Beltagi, H.S.; Sattar, M.N. Genotypic Variation for Drought Tolerance in Three Date Palm (Phoenix dactylifera L.) Cultivars. Fresenius Environ. Bull. 2019, 28, 4671–4683. [Google Scholar]
- Kruse, J.; Adams, M.; Winkler, B.; Ghirardo, A.; Alfarraj, S.; Kreuzwieser, J.; Hedrich, R.; Schnitzler, J.P.; Rennenberg, H. Optimization of Photosynthesis and Stomatal Conductance in the Date Palm Phoenix dactylifera during Acclimation to Heat and Drought. New Phytol. 2019, 223, 1973–1988. [Google Scholar] [CrossRef]
- Zein El Din, A.F.M.; Ibrahim, M.F.M.; Farag, R.; Abd El-Gawad, H.G.; El-Banhawy, A.; Alaraidh, I.A.; Rashad, Y.M.; Lashin, I.; Abou El-Yazied, A.; Elkelish, A.; et al. Influence of Polyethylene Glycol on Leaf Anatomy, Stomatal Behavior, Water Loss, and Some Physiological Traits of Date Palm Plantlets Grown In Vitro and Ex Vitro. Plants 2020, 9, 1440. [Google Scholar] [CrossRef]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarraj, S.; Albasher, G.; Schnitzler, J.P.; Ache, P.; Hedrich, R.; Rennenberg, H. Metabolic Responses of Date Palm (Phoenix dactylifera L.) Leaves to Drought Differ in Summer and Winter Climate. Tree Physiol. 2021, 41, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Mattar, M.A.; Soliman, S.S.; Al-Obeed, R.S. Effects of Various Quantities of Three Irrigation Water Types on Yield and Fruit Quality of ‘Succary’ Date Palm. Agronomy 2021, 11, 796. [Google Scholar] [CrossRef]
- Alnaim, M.A.; Mohamed, M.S.; Mohammed, M.; Munir, M. Effects of Automated Irrigation Systems and Water Regimes on Soil Properties, Water Productivity, Yield and Fruit Quality of Date Palm. Agriculture 2022, 12, 343. [Google Scholar] [CrossRef]
- Al Kharusi, L.; Assaha, D.; Al-Yahyai, R.; Yaish, M. Screening of Date Palm (Phoenix dactylifera L.) Cultivars for Salinity Tolerance. Forests 2017, 8, 136. [Google Scholar] [CrossRef]
- Bouhouch, S.; Eshelli, M.; Slama, H.B.; Bouket, A.C.; Oszako, T.; Okorski, A.; Rateb, M.E.; Belbahri, L. Morphological, Biochemical, and Metabolomic Strategies of the Date Palm (Phoenix dactylifera L., Cv. Deglet Nour) Roots Response to Salt Stress. Agronomy 2021, 11, 2389. [Google Scholar] [CrossRef]
- Dghaim, R.; Hammami, Z.; Al Ghali, R.; Smail, L.; Haroun, D. The Mineral Composition of Date Palm Fruits (Phoenix dactylifera L.) under Low to High Salinity Irrigation. Molecules 2021, 26, 7361. [Google Scholar] [CrossRef]
- Yaish, M.W.; Patankar, H.V.; Assaha, D.V.M.; Zheng, Y.; Al-Yahyai, R.; Sunkar, R. Genome-Wide Expression Profiling in Leaves and Roots of Date Palm (Phoenix dactylifera L.) Exposed to Salinity. BMC Genom. 2017, 18, 246. [Google Scholar] [CrossRef] [Green Version]
- Al-Harrasi, I.; Al-Yahyai, R.; Yaish, M.W. Differential DNA Methylation and Transcription Profiles in Date Palm Roots Exposed to Salinity. PLoS ONE 2018, 13, e0191492. [Google Scholar] [CrossRef] [Green Version]
- Al Kharusi, L.; Sunkar, R.; Al-Yahyai, R.; Yaish, M.W. Comparative Water Relations of Two Contrasting Date Palm Genotypes under Salinity. Int. J. Agron. 2019, 2019, 4262013. [Google Scholar] [CrossRef]
- Al Kharusi, L.; Al Yahyai, R.; Yaish, M.W. Antioxidant Response to Salinity in Salt-Tolerant and Salt-Susceptible Cultivars of Date Palm. Agriculture 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Jana, G.A.; Al Kharusi, L.; Sunkar, R.; Al-Yahyai, R.; Yaish, M.W. Metabolomic Analysis of Date Palm Seedlings Exposed to Salinity and Silicon Treatments. Plant Signal. Behav. 2019, 14, 1663112. [Google Scholar] [CrossRef] [PubMed]
- Al-Muaini, A.; Green, S.; Dakheel, A.; Abdullah, A.H.; Sallam, O.; Abou Dahr, W.A.; Dixon, S.; Kemp, P.; Clothier, B. Water Requirements for Irrigation with Saline Groundwater of Three Date-Palm Cultivars with Different Salt-Tolerances in the Hyper-Arid United Arab Emirates. Agric. Water Manag. 2019, 222, 213–220. [Google Scholar] [CrossRef]
- Abdelhadi, A.W.; Salih, A.A.; Sultan, K.; Alsafi, A.; Tashtoush, F. Actual Water Use of Young Date Palm Trees As Affected By Aminolevulinic Acid Application and Different Irrigation Water Salinities. Irrig. Drain. 2020, 69, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Serret, M.D.; Al-Dakheel, A.J.; Yousfi, S.; Fernáandez-Gallego, J.A.; Elouafi, I.A.; Araus, J.L. Vegetation Indices Derived from Digital Images and Stable Carbon and Nitrogen Isotope Signatures as Indicators of Date Palm Performance under Salinity. Agric. Water Manag. 2020, 230, 105949. [Google Scholar] [CrossRef]
- Al Kharusi, L.; Jana, G.A.; Patankar, H.V.; Yaish, M.W. Comparative Metabolic Profiling of Two Contrasting Date Palm Genotypes Under Salinity. Plant Mol. Biol. Rep. 2021, 39, 351–363. [Google Scholar] [CrossRef]
- Zemni, N.; Slama, F.; Bouksila, F.; Bouhlila, R. Simulating and Monitoring Water Flow, Salinity Distribution and Yield Production under Buried Diffuser Irrigation for Date Palm Tree in Saharan Jemna Oasis (North Africa). Agric. Ecosyst. Environ. 2022, 325, 107772. [Google Scholar] [CrossRef]
- Chaâbene, Z.; Rorat, A.; Rekik Hakim, I.; Bernard, F.; Douglas, G.C.; Elleuch, A.; Vandenbulcke, F.; Mejdoub, H. Insight into the Expression Variation of Metal-Responsive Genes in the Seedling of Date Palm (Phoenix dactylifera). Chemosphere 2018, 197, 123–134. [Google Scholar] [CrossRef]
- Salama, K.F.; Randhawa, M.A.; Al Mulla, A.A.; Labib, O.A. Heavy Metals in Some Date Palm Fruit Cultivars in Saudi Arabia and Their Health Risk Assessment. Int. J. Food Prop. 2019, 22, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Seleiman, M.F.; Al-suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Al-Khateeb, S.A.; Al-Khateeb, A.A.; Sattar, M.N.; Mohmand, A.S. Induced in Vitro Adaptation for Salt Tolerance in Date Palm (Phoenix dactylifera L.) Cultivar Khalas. Biol. Res. 2020, 53, 37. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarray, S.; Schnitzler, J.-P.; Ache, P.; Hedrich, R.; Rennenberg, H. Climate and Development Modulate the Metabolome and Antioxidative System of Date Palm Leaves. J. Exp. Bot. 2019, 70, 5959–5969. [Google Scholar] [CrossRef] [Green Version]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, J.; Lazarovitch, N.; Tripler, E. Effects of Fruit Load Intensity and Irrigation Level on Fruit Quality, Water Productivity and Net Profits of Date Palms. Agric. Water Manag. 2020, 241, 106385. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of Detrimental Effects of Salt Stress on Date Palm (Phoenix dactylifera L.) by the Application of Arbuscular Mycorrhizal Fungi and/or Compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Toubali, S.; Tahiri, A.; Anli, M.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Oufdou, K.; Ait-Rahou, Y.; Ben-Ahmed, H.; et al. Physiological and Biochemical Behaviors of Date Palm Vitroplants Treated with Microbial Consortia and Compost in Response to Salt Stress. Appl. Sci. 2020, 10, 8665. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Laouane, R.B.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of Mycorrhizal Fungi in Improving Tolerance of the Date Palm (Phoenix dactylifera L.) Seedlings to Salt Stress. Sci. Hortic. 2019, 253, 429–438. [Google Scholar] [CrossRef]
- Yaish, M.W. Proline Accumulation Is a General Response to Abiotic Stress in the Date Palm Tree (Phoenix dactylifera L.). Genet. Mol. Res. 2015, 14, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-Mediated Alleviation of Combined Salinity and Cadmium Stress in Date Palm (Phoenix dactylifera L.) by Regulating Physio-Hormonal Alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Shareef, H.J.; Al-Tememi, I.H.; Abdi, G. Foliar Nutrition of Date Palm: Advances and Applications. A Review. Folia Oecologica 2021, 48, 82–99. [Google Scholar] [CrossRef]
- Harkousse, O.; Slimani, A.; Jadrane, I.; Aitboulahsen, M.; Mazri, M.A.; Zouahri, A.; Ouahmane, L.; Koussa, T.; Najib, M.; Feddy, A. Role of Local Biofertilizer in Enhancing the Oxidative Stress Defence Systems of Date Palm Seedling (Phoenix dactylifera) against Abiotic Stress. Appl. Environ. Soil Sci. 2021, 2021, 6628544. [Google Scholar] [CrossRef]
- Akensous, F.-Z.; Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Ait-Rahou, Y.; Ahmed, H.B.; Nasri, N.; Hafidi, M.; Meddich, A. Boosting Date Palm (Phoenix dactylifera L.) Growth under Drought Stress: Effects of Innovative Biostimulants. Gesunde Pflanz. 2022, 74, 961–982. [Google Scholar] [CrossRef]
- Glick, B.; Glick, B.; Glick, B.; Eid, A.; Salem, S.S.; Glick, B.R. Isolation and Characterization of Endophytic Plant Growth- Promoting Bacteria from Date Palm Tree (Phoenix dactylifera L.) and Their Potential Role in Salinity Tolerance. Antonie Van Leeuwenhoek 2015, 107, 1519–1532. [Google Scholar] [CrossRef]
- Chebaane, A.; Symanczik, S.; Oehl, F.; Azri, R.; Gargouri, M.; Mäder, P.; Mliki, A.; Fki, L. Arbuscular Mycorrhizal Fungi Associated with Phoenix dactylifera L. Grown in Tunisian Sahara Oases of Different Salinity Levels. Symbiosis 2020, 81, 173–186. [Google Scholar] [CrossRef]
- Outamamat, E.; Bourhia, M.; Dounas, H.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Al Feddy, M.N.; Mnasri, B.; Ouahmane, L. Application of Native or Exotic Arbuscular Mycorrhizal Fungi Complexes and Monospecific Isolates from Saline Semi-Arid Mediterranean Ecosystems Improved Phoenix dactylifera’s Growth and Mitigated Salt Stress Negative Effects. Plants 2021, 10, 2501. [Google Scholar] [CrossRef]
- Elsadig, E.H.; Aljuburi, H.J.; Elamin, A.H.B.; Gafar, M.O. Impact of Organic Manure and Combination of N P K S, on Yield, Fruit Quality and Fruit Mineral Content of Khenazi Date Palm (Phoenix dactylifera L.) Cultivar. J. Sci. Agric. 2017, 1, 335. [Google Scholar] [CrossRef] [Green Version]
- Ghadbane, M.; Medjekal, S.; Benderradji, L.; Belhadj, H.; Daoud, H. Assessment of Arbuscular Mycorrhizal Fungi Status and Rhizobium on Date Palm (Phoenix dactylifera L.) Cultivated in a Pb Contaminated Soil. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, 2nd ed.; Springer: Cham, Switzerland, 2021; pp. 703–707. [Google Scholar] [CrossRef]
- Sugumar, T.; Srinivasan, P.; Muthukumar, B.; Natarajan, E. Whole Genome Sequencing and Genome Annotation of PGPR ‘Exiguobacterium Sp. TNDT2’ Isolated from Dates Palm Tree Rhizospheric Soil. Indian J. Agric. Res. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Baslam, M.; Qaddoury, A. Role of Native and Exotic Mycorrhizal Symbiosis to Develop Morphological, Physiological and Biochemical Responses Coping with Water Drought of Date Palm, Phoenix dactylifera. Trees 2013, 28, 161–172. [Google Scholar] [CrossRef]
- Benhiba, L.; Fouad, M.O.; Essahibi, A.; Ghoulam, C.; Qaddoury, A. Arbuscular Mycorrhizal Symbiosis Enhanced Growth and Antioxidant Metabolism in Date Palm Subjected to Long-Term Drought. Trees-Struct. Funct. 2015, 29, 1725–1733. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, N.; Fan, J.; Wang, F.; George, T.S.; Feng, G. Arbuscular Mycorrhizal Fungi Stimulate Organic Phosphate Mobilization Associated with Changing Bacterial Community Structure under Field Conditions. Environ. Microbiol. 2018, 20, 2639–2651. [Google Scholar] [CrossRef] [PubMed]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of Mycorrhizal Fungi as a Strategy for Improving the Drought Tolerance in Date Palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic Regulation Under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture. PGPR Amelior. Sustain. Agric. 2019, 129–157. [Google Scholar] [CrossRef]
- Patankar, H.V.; Al-Harrasi, I.; Al Kharusi, L.; Jana, G.A.; Al-Yahyai, R.; Sunkar, R.; Yaish, M.W. Overexpression of a Metallothionein 2A Gene from Date Palm Confers Abiotic Stress Tolerance to Yeast and Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 2871. [Google Scholar] [CrossRef]
- Estrada, B.; Barea, J.M.; Aroca, R.; Ruiz-Lozano, J.M. A Native Glomus Intraradices Strain from a Mediterranean Saline Area Exhibits Salt Tolerance and Enhanced Symbiotic Efficiency with Maize Plants under Salt Stress Conditions. Plant Soil 2013, 366, 333–349. [Google Scholar] [CrossRef]
- Patankar, H.V.; Assaha, D.V.M.; Al-Yahyai, R.; Sunkar, R.; Yaish, M.W. Identification of Reference Genes for Quantitative Real-Time PCR in Date Palm (Phoenix dactylifera L.) Subjected to Drought and Salinity. PLoS ONE 2016, 11, e0166216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrol, N.; Tamayo, E.; Vargas, P. The Heavy Metal Paradox in Arbuscular Mycorrhizas: From Mechanisms to Biotechnological Applications. J. Exp. Bot. 2016, 67, 6253–6565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular Mycorrhizal Fungi-Induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Meier, S.; Borie, F.; Bolan, N.; Cornejo, P. Phytoremediation of Metal-Polluted Soils by Arbuscular Mycorrhizal Fungi. Crit. Rev. Environ. Sci. Technol. 2012, 42, 741–775. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, S. Arbuscular Mycorrhizal Fungi (AMF) from Heavy Metal-Contaminated Soils: Molecular Approach and Application in Phytoremediation. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2019; Volume 2, pp. 489–500. ISBN 978-981-10-6592-7. [Google Scholar]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular Mechanisms Underlying Heavy Metal Uptake, Translocation and Tolerance in Hyperaccumulators-an Analysis: Heavy Metal Tolerance in Hyperaccumulators. Environ. Challenges 2021, 4, 100197. [Google Scholar] [CrossRef]
- Khalid, M.; Ur-rahman, S.; Hassani, D.; Hayat, K.; Zhou, P.; Hui, N. Advances in Fungal-Assisted Phytoremediation of Heavy Metals: A Review. Pedosphere 2021, 31, 475–495. [Google Scholar] [CrossRef]
- Hussain, S. Mineral Biofortification and Metal/Metalloid Accumulation in Food Crops: Recent Research and Trends (Part I). Crop Pasture Sci. 2022, 73, 1. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of Heavy Metals: Understanding of Principles and Practices. In Plant-Metal Interactions; Springer International Publishing: Cham, Switzerland, 2019; pp. 263–282. ISBN 9783030207328. [Google Scholar]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Gao, W.Q.; Wang, P.; Wu, Q.S. Functions and Application of Glomalin-Related Soil Proteins: A Review. Sains Malays. 2019, 48, 111–119. [Google Scholar] [CrossRef]
- Javan Gholiloo, M.; Yarnia, M.; Ghorttapeh, A.H.; Farahvash, F.; Daneshian, A.M. Evaluating Effects of Drought Stress and Bio-Fertilizer on Quantitative and Qualitative Traits of Valerian (Valeriana officinalis L.). J. Plant Nutr. 2019, 42, 1417–1429. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Mahmood-Ur-Rahman; Fakhar, A.; Rafique, M.; et al. Plant Growth-Promoting Rhizobacteria Eliminate the Effect of Drought Stress in Plants: A Review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, G.A.; Al-yahyai, R.; Yaish, W. Genome Sequencing of Microbacterium from the Rhizosphere of Date Palm Trees Affected by Salinity. Genome Announc. 2017, 5, 44–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwal, S.; Ilyas, N.; Batool, N.; Arshad, M. Amelioration of Drought Stress in Wheat by Combined Application of PGPR, Compost, and Mineral Fertilizer. J. Plant Nutr. 2017, 40, 1250–1260. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; El-Samnoudi, I.M.; Ibrahim, A.E.-A.M.; Abd El Tawwab, A.R. Compost and Mulching Modulates Morphological, Physiological Responses and Water Use Efficiency in Sorghum bicolor L. (Moench) under Low Moisture Regime. Agric. Water Manag. 2018, 208, 431–439. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.R.; Amiri, H.; Ismaili, A. Evaluation of Photosynthesis, Physiological, and Biochemical Responses of Chickpea (Cicer arietinum L. Cv. Pirouz) under Water Deficit Stress and Use of Vermicompost Fertilizer. J. Integr. Agric. 2018, 17, 2426–2437. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Iron, Magnesium, Nitrogen and Potassium Deficiency Symptom Discrimination by Reflectance Spectroscopy in Grapevine Leaves. Sci. Hortic. 2018, 241, 152–159. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Elise, R.; Pluchon, S.; Ali, N.; Billiot, B.; Yvin, J.-C. Calcium Application Enhances Drought Stress Tolerance in Sugar Beet and Promotes Plant Biomass and Beetroot Sucrose Concentration. Int. J. Mol. Sci. 2019, 20, 3777. [Google Scholar] [CrossRef]
- Ahanger, A.M.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving Growth and Photosynthetic Performance of Drought Stressed Tomato by Application of Nano-Organic Fertilizer Involves up-Regulation of Nitrogen, Antioxidant and Osmolyte Metabolism. Ecotoxicol. Environ. Saf. 2021, 216, 112195. [Google Scholar] [CrossRef]
- Mamnabi, S.; Nasrollahzadeh, S.; Ghassemi-golezani, K.; Raei, Y. Improving Yield-Related Physiological Characteristics of Spring Rapeseed by Integrated Fertilizer Management under Water Deficit Conditions. Saudi J. Biol. Sci. 2020, 27, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mageed, T.A.; El-Sherif, A.M.A.; Abd El-Mageed, S.A.; Abdou, N.M. A Novel Compost Alleviates Drought Stress for Sugar Beet Production Grown in Cd-Contaminated Saline Soil. Agric. Water Manag. 2019, 226, 105831. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, J.; Zhao, R.; Dai, H.; Zhang, Z. Application of Vermicompost Improves Strawberry Growth and Quality through Increased Photosynthesis Rate, Free Radical Scavenging and Soil Enzymatic Activity. Sci. Hortic. 2018, 233, 132–140. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Zia ur Rehman, M.; Zubair, M.; Riaz, M.; Qayyum, M.F.; Alharby, H.F.; Bamagoos, A.A.; Ali, S. Application of Co-Composted Farm Manure and Biochar Increased the Wheat Growth and Decreased Cadmium Accumulation in Plants under Different Water Regimes. Chemosphere 2020, 246, 125809. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Hafez, M.; Abdallah, A.M.; Mohamed, A.E.; Rashad, M. Influence of Environmental-Friendly Bio-Organic Ameliorants on Abiotic Stress to Sustainable Agriculture in Arid Regions: A Long Term Greenhouse Study in Northwestern Egypt. J. King Saud Univ.-Sci. 2022, 34, 102212. [Google Scholar] [CrossRef]
- Bonini, P.; Rouphael, Y.; Miras-Moreno, B.; Lee, B.; Cardarelli, M.; Erice, G.; Cirino, V.; Lucini, L.; Colla, G. A Microbial-Based Biostimulant Enhances Sweet Pepper Performance by Metabolic Reprogramming of Phytohormone Profile and Secondary Metabolism. Front. Plant Sci. 2020, 11, 567388. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Masters-Clark, E.; Shone, E.; Paradelo, M.; Hirsch, P.R.; Clark, I.M.; Otten, W.; Brennan, F.; Mauchline, T.H. Development of a Defined Compost System for the Study of Plant-Microbe Interactions. Sci. Rep. 2020, 10, 7521. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Lephatsi, M.M.; Meyer, V.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Plant Responses to Abiotic Stresses and Rhizobacterial Biostimulants: Metabolomics and Epigenetics Perspectives. Metabolites 2021, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of Reactive Oxygen Species and Hormone Signaling during Abiotic Stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S. Contribution of Strigolactone in Plant Physiology, Hormonal Interaction and Abiotic Stresses. Planta 2021, 254, 28. [Google Scholar] [CrossRef]
- Saeed, W.; Naseem, S.; Ali, Z. Strigolactones Biosynthesis and Their Role in Abiotic Stress Resilience in Plants: A Critical Review. Front. Plant Sci. 2017, 8, 1487. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lizarazo, J.C.; Moreno-Fonseca, L.P. Mechanisms for Tolerance to Water-Deficit Stress in Plants Inoculated with Arbuscular Mycorrhizal Fungi: A Review Mecanismos de Tolerancia Al Estrés Por Déficit Hídrico En Plantas. Agron. Colomb. 2016, 34, 179–189. [Google Scholar] [CrossRef]
- Bakshi, P.; Handa, N.; Gautam, V.; Kaur, P.; Sareen, S.; Mir, B.A.; Bhardwaj, R. Role and Regulation of Plant Hormones as a Signal Molecule in Response to Abiotic Stresses. Plant Signal. Mol. Role Regul. Stress. Environ. 2019, 303–317. [Google Scholar] [CrossRef]
- Rehman, A.; Azhar, M.T.; Hinze, L.; Qayyum, A.; Li, H.; Peng, Z.; Qin, G.; Jia, Y.; Pan, Z.; He, S.; et al. Insight into Abscisic Acid Perception and Signaling to Increase Plant Tolerance to Abiotic Stress. J. Plant Interact. 2021, 16, 222–237. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Ruan, Y.; Xu, L.; Hu, Y.; Hao, Z.; Zhang, X.; Li, H.; Wang, Y.; Yang, L.; et al. Potential Role of D-Myo-Inositol-3-Phosphate Synthase and 14-3-3 Genes in the Crosstalk between Zea mays and Rhizophagus intraradices under Drought Stress. Mycorrhiza 2016, 26, 879–893. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Chebotar, V.; Meyer, J.J.M.; Bibikova, T.N. Effect of Plant Growth-Promoting Rhizobacteria on Plant Hormone Homeostasis. S. African J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Sami, F.; Faizan, M.; Faraz, A.; Siddiqui, H.; Yusuf, M.; Hayat, S. Nitric Oxide-Mediated Integrative Alterations in Plant Metabolism to Confer Abiotic Stress Tolerance, NO Crosstalk with Phytohormones and NO-Mediated Post Translational Modifications in Modulating Diverse Plant Stress. Nitric Oxide-Biol. Chem. 2018, 73, 22–38. [Google Scholar] [CrossRef] [PubMed]
- İpek, M.; Mutluay, E. Enhancing the Physiological and Molecular Responses of Horticultural Plants to Drought Stress through Plant Growth–Promoting Rhizobacterias. In Sustainable Horticulture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 185–199. ISBN 9780323918619. [Google Scholar]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of Shape During Stress: A Key Role for Auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xin, P.; Ma, X.; Chu, J.; Wang, G. Gibberellin Metabolism in Flowering Plants: An Update and Perspectives. Front. Plant Sci. 2020, 11, 5–10. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant. Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. Nitric Oxide (NO) and Salicylic Acid (SA): A Framework for Their Relationship in Plant Development under Abiotic Stress. Plant Biol. 2021, 23, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Prasad, S.M.; Singh, V.P. A Brief Appraisal of Ethylene Signaling under Abiotic Stress in Plants. Plant Signal. Behav. 2020, 15, 1782051. [Google Scholar] [CrossRef]




| Abiotic Stress | Stress Level | Growth Stage | Cultivar/Variety | Main Effects | References |
|---|---|---|---|---|---|
| Drought | Watering cessation for 7–8 days before harvest | Seedlings | - | Heat-shock proteins (HSPs), chaperone proteins, and heat stress Transcription Factors (TFs) genes’ expression Cell death elimination Enrichment of phytohormones- related, wax, secondary metabolism, fatty acids biosynthesis, and plant cell wall pathways | [17] |
| Drought | 70%, 100% evapotranspiration (ETc) | 10–12-year-old orchards | “Mazafati” | Increase in bunch weight, fruit weight, fruit starch, yielding, and water-use efficiency (WUE) Increase in soluble solids and sugar content A rise in total phenolic compounds, peroxidase (POX), polyphenol Amelioration of (PPO) activities A rise in calcium (Ca), iron (Fe), and zinc (Zn) | [52] |
| Drought | 6.9, 13.95, 27.5% of polyethylene glycol 6000 (PEG) | 3 month seedlings | “Sagie” | Enhancement of phenolic and flavonoid content Rise of Catalase (CAT), peroxidase (POX), and polyphenol oxidase (PPO) activities | [53] |
| Drought | 0 (control), −0.41, −0.82, −1.23, −1.63 MPa of mannitol | 4–5-year-old suckers | “Barhee”, “Ruziz”, “Sukary” | Decrease in leaf and root numbers, leaf, and root dry weights, and total dry weight A decline in relative water content (RWC), photosynthetic and rate of transpiration, water-use efficiency (WUE), and mesophyll conductance Increase in [CO2]i | [54] |
| Drought | Irrigation reduced to 50% of the control | 2 year old seedlings | - | Decrease in shoot growth A decline in leaf gas exchange Decrease in intrinsic leaf water-use efficiency (WUEi) | [55] |
| Drought | Gradual decline in humidity | Plantlets | “Sewi” | Dehydration A decline in photosynthetic pigments Decline in surviving chances | [56] |
| Drought | Irrigation reduced to 50% of the control | 2-year-old seedlings | - | Decrease in leaf hydration, foliar total and reduced ascorbate, chlorophyll a/b ratio, sugars, and organic acids Increase in total reduced glutathione (GSH), oxidized glutathione (GSSG), the GSSG/GSH ratio, amino acids, and 5,8,11,14- eicosatetraenoic acid | [57] |
| Drought | Irrigation reduced to 50% and 25% of the control | 2-year-old plants | - | A rise in isoprene emission rates and a decline in soil water content (SWC) Upregulation of primary metabolism, stress response, photosynthesis, and antioxidant-related proteins Downregulation of gene expression, metabolic, and secondary metabolism-related proteins | [25] |
| Drought | 50, 100, 150% of evapotranspiration levels | Trees | “Succary” | A decline in date palm yielding Affected fruit traits An overall decline in fruit metabolites | [58] |
| Drought | 50, 75, 100% of watering demand | Trees | “Khalas” | Affected fruit yielding as well as quality | [59] |
| Salinity | 0, 240 mM NaCl | Seedlings | “Khalas”, “Manoma”, “Barni”, “Nashukharma”, “Hilali-Omani”, “Fard”, “Abunarenja”, “Nagal”, “Umsila”, “Zabad” | Reduction in shoot as well as root dry weights, and leaf area Decrease in photosynthetic properties | [60] |
| Salinity | 50, 100, 150 mM NaCl | 2 month seedlings | “Khalas” | Enhancement of proline content and thiobarbituric acid reactive substances (TBARS) A rise in Catalase (CAT) as well as Superoxide Dismutase (SOD) activities Variation within cDNA start codon-targeted (cDNASCoT) marker genes’ expression | [61] |
| Salinity | 5, 10, 15 dS m−1 of salt water | Trees | “Ajwat AlMadinah”, “Naghal”, “Khnizi”, “Barhi”, “Makhtoumi”, “Farad”, “Khisab”, “Nabtat-Saif“, “Shagri”, “Abu-Maan”, “Jabri”, “Sukkari”, “Rothan” | Excluding of Na+ Retaining of K+ Decease in osmotic potential | [62] |
| Salinity | 0, 300 mM NaCl | Seedlings | “Khalas” | Decline in photosynthetic capacity, stomatal conductance (gs), rate of transpiration (E), as well as internal carbon dioxide concentration [CO2]i Variation within genes expression Transcripts enrichment implicated in metabolism pathways | [63] |
| Salinity | 50, 300 mM NaCl | Seedlings | “Khalas” | Decease in photosynthetic capacity, stomatal conductance (gs), rate of transpiration (E), and root system traits Hypermethylated and hypomethylated DNA regions, coupled with insignificant genes expression | [64] |
| Salinity | 0, 240 mM NaCl | Seedlings | “Umsila”, “Zabad” | Decrease in leaf area, physiological traits, and leaf water potential (LWP) Increase in leaf total soluble sugars, proline and glycine betaine | [65] |
| Salinity | 0, 240 mM NaCl | Seedlings | “Umsila”, “Zabad” | A decline in leaf fresh and dry weights | [66] |
| Salinity | 0, 300 mM NaCl | Seedlings | “Khalas” | Decrease in leaf area, leaf and root dry weights, K+ accumulation, and roots’ Casparian strips Enhancement of stress-related metabolites (e.g., osmolytes and antioxidant enzymes) | [67] |
| Salinity | 5 dS m−1, 15 dS m−1 of saline water | Trees | “Lulu”, “Khalas”, “Shahlah” | Negative effect on height Decrease in tree water use (ETc) Variation in the consumed water productivity (CWP) | [68] |
| Salinity | <1, 12–15, 18–20 dS m−1 of saline water | 4-year-old trees | - | A decline in actual water use | [69] |
| Salinity | 5, 10, 15 dS m−1 of salt water | Trees | - | Decrease in trunk height and diameter, brunch total number, yielding of dates Increase in canopy temperature (CT) | [70] |
| Salinity | 4 g/L, 8 g/L, 12 g/L, 16 g/L NaCl | Seedlings | “Deglet Nour” | Drop in seeds’ germination, radicle length, and Catalase (CAT) activity A rise in total protein content, superoxide dismutase (SOD), and secondary metabolites | [61] |
| Salinity | 5, 10, 15 dS m−1 of salt water | Trees | “Ajwat Al Madinah”, “Naghal”, “Barhi”, “Shagri”, “Abu Maan”, “Jabri”, “Sukkari”, “Rothan”, “Khinizi”, “Maktoumi” | Increase in minerals, mainly K, P, and Ca | [62] |
| Salinity | Irrigation levels based on crop evapotranspiration (ETc) at 50%, 100%, and 150% of saline water | Trees | “Succary” | Decrease in dates yielding, fruit weight and size, total soluble solids (TTS), acidity, fruit moisture content, and total sugar and non-reducing sugar content in fruits | [58] |
| Salinity | 0, 240 mM NaCl | Seedlings | “Umsila”, “Zabad” | Production of salinity- related metabolites | [71] |
| Salinity | 3.2–4.5 dS m−1 salt water (ECw) | Trees | - | Increase in transpiration, soil evaporation, percolation, and salt accumulation | [72] |
| Heavy metal(oid)s | Cadmium (Cd), chromium (Cr) | Seedlings | “Deglet Nour” | Decrease in phytochelatin synthase (pcs) and metallothionein (mt) genes’ expression | [73] |
| Heavy metal(oid)s | Antimony (Sb), cadmium (Cd), lead (Pb), chromium (Cr), arsenic (As), aluminum (Al) | Date fruits | “Sakay Mabroum”, “Kadary”, “Safawy Al-Madina”, “Eklas Al-Hassa”, “Barny Al-Madina”, “Rashadya Al-qaseem”, “Sakay Normal” | As along with Pb surpassed the maximal allowable levels (MAL) | [74] |
| Abiotic Stress | Level of Stress | Biostimulants | Main Effects | References | ||
|---|---|---|---|---|---|---|
| AMF | PGPR | Organic Amendment | ||||
| Drought | 75, 25% FC | Rhizoglomus irregulare Aoufous consortium | PGPR consortium | Grass-based compost Green waste-based compost | Enhancement of growth traits and physiological parameters Improvement of N and P content Increase in sugar and protein content Decrease in MDA and H2O2 Decrease in soil pH and boosting of electrical conductivity (EC), organic matter (OM), and total organic carbon (TOC), | [30] |
| Drought | 100, 75, 50, 25% FC | A complex of 28 different species | Bacillus S48 | - | Improvement of RWC Enhancement of proline content Decrease in SOD, CAT, POX, and glutathione S-transferase (GST) Increase in soil EC | [89] |
| Drought | Water regimes: 32 L/h for well-watered (WW); 16 L/h for drought stress (DS) | Aoufous consortium | PGPR consortium | Organic waste-based compost | Improvement of plant biomass Amelioration of plant-water relations Enhancement of P uptake A rise in total soluble sugar and protein content Decrease in MDA as well as H2O2 Improvement of soil traits, such as OM, P, and glomalin content | [90] |
| Salinity | 0, 50, 100, 200 mM NaCl | - | Endophytic bacteria | - | Ferric ion (Fe3+) chelation, K+ solubilization, phosphate ion (PO43−) and zinc ion (Zn2+), and ammonia (NH3) production 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase together with IAA production capacity | [91] |
| Salinity | 0, 240 mM NaCl | Aoufous consortium | - | - | Improvement of growth and physiological traits Enhancement of water potential Amelioration of P, K as well as Ca content Decrease in MDA and H2O2 and rise in SOD, CAT, POX as well as APX activities | [82] |
| Salinity | 0, 240 mM NaCl | Aoufous consortium | - | Green waste-based compost | Amelioration of physiological parameters Improvement of P, potassium ion (K+), and calcium ion (Ca2+) content Enhancement of proline Reduction in the effect of lipid peroxidation and H2O2 | [80] |
| Salinity | Up to 7.6 dS m−1 NaCl | Identification of Albahypha drummondii, Dominikia disticha, Funneliformis coronatus, Rhizoglomus irregular | - | - | Positive correlation of soil salinity and intensity of mycorrhization Negative correlation of soil salinity and easily extractable glomalin | [92] |
| Salinity | 0, 120, 240 mM NaCl | Aoufous consortium Rhizophagus irregularis | PGPR consortium | Green waste-based compost | Enhancement of growth traits and antioxidant defensive machinery | [81] |
| Salinity | 0, 10, 20 g·L−1 NaCl | Autochthonous AMF Exogenous AMF | - | - | Negatively impacted growth as well as physiological properties | [93] |
| Heavy metal(oid)s | - | - | - | Organic manure | Enhancement of heavy metal(oid)s content in date palm fruits | [94] |
| Heavy metal(oid)s | Pb(NO3)2 200 mg/L | Glomus spp. | Rhizobium leguminosarum | - | Improvement of root length, root fresh weight, shoot height, shoot fresh weight as well as the germination index Enhancement of seedling length, root basal diameter, and dry biomass | [95] |
| Heavy metal(oid)s | - | - | Exiguobacterium sp. | - | Identification of proteins/ enzymes involved in reducing heavy metal(oid)s contamination | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akensous, F.-Z.; Anli, M.; Meddich, A. Biostimulants as Innovative Tools to Boost Date Palm (Phoenix dactylifera L.) Performance under Drought, Salinity, and Heavy Metal(Oid)s’ Stresses: A Concise Review. Sustainability 2022, 14, 15984. https://doi.org/10.3390/su142315984
Akensous F-Z, Anli M, Meddich A. Biostimulants as Innovative Tools to Boost Date Palm (Phoenix dactylifera L.) Performance under Drought, Salinity, and Heavy Metal(Oid)s’ Stresses: A Concise Review. Sustainability. 2022; 14(23):15984. https://doi.org/10.3390/su142315984
Chicago/Turabian StyleAkensous, Fatima-Zahra, Mohamed Anli, and Abdelilah Meddich. 2022. "Biostimulants as Innovative Tools to Boost Date Palm (Phoenix dactylifera L.) Performance under Drought, Salinity, and Heavy Metal(Oid)s’ Stresses: A Concise Review" Sustainability 14, no. 23: 15984. https://doi.org/10.3390/su142315984