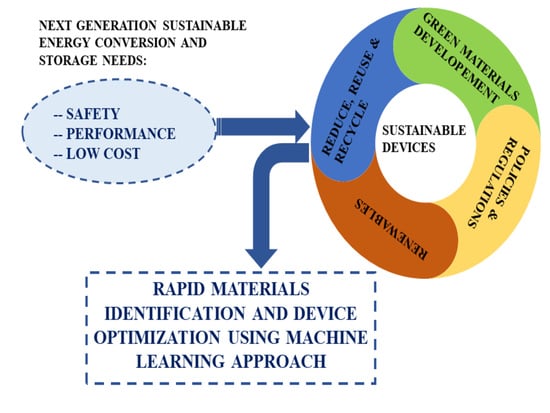
Towards Sustainable Fuel Cells and Batteries with an AI Perspective
Abstract
1. Introduction
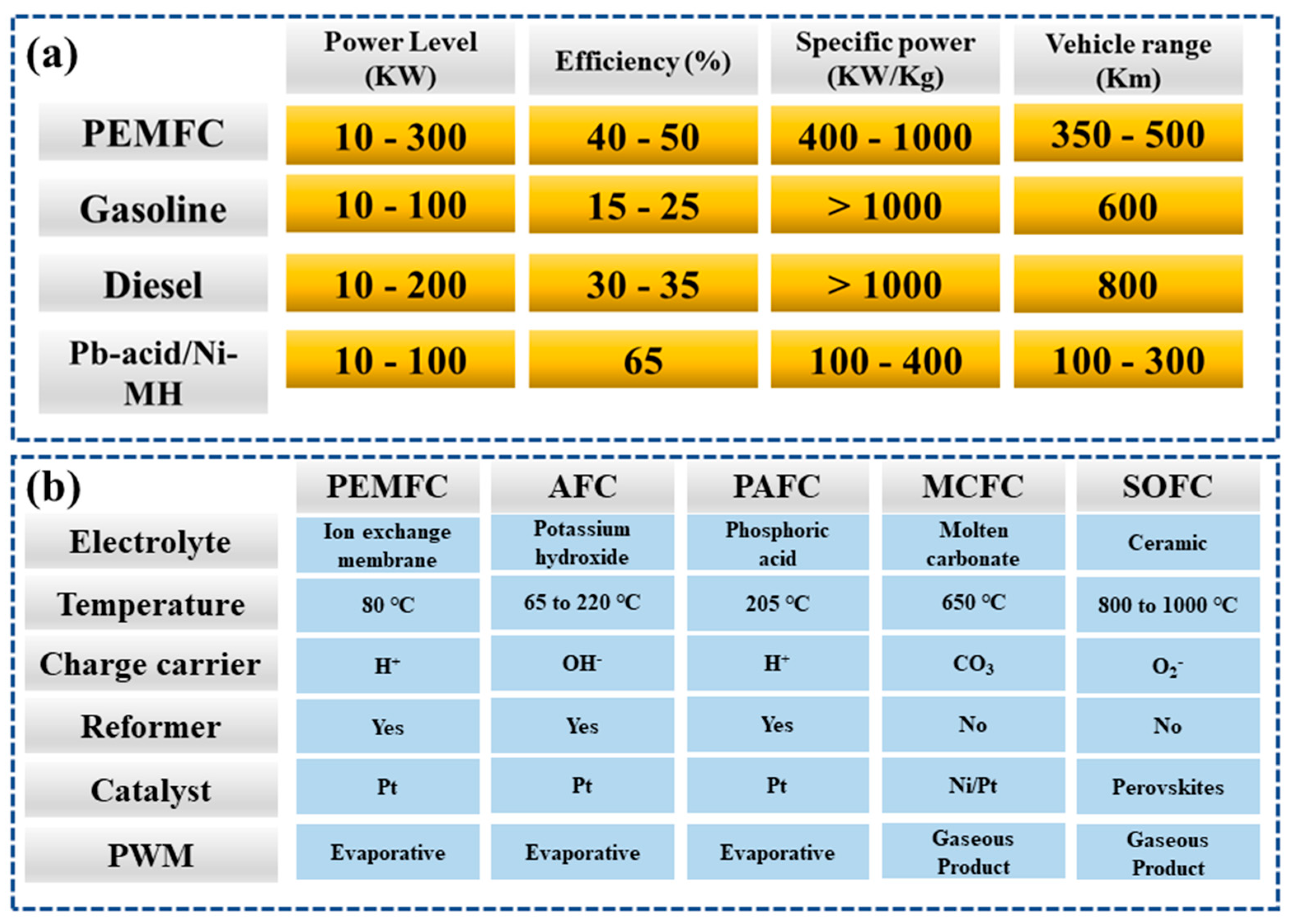
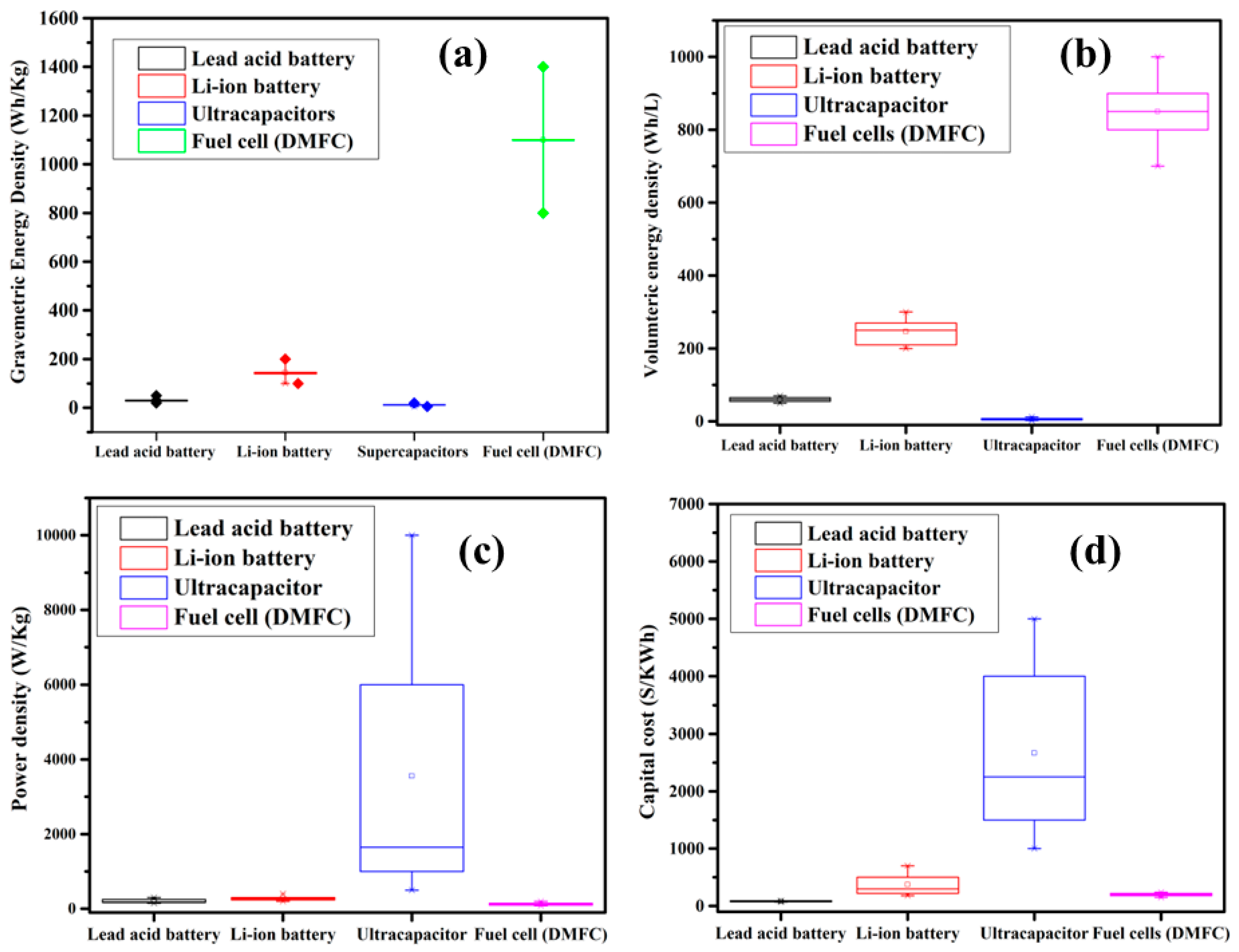
2. Fuel Cell Technologies
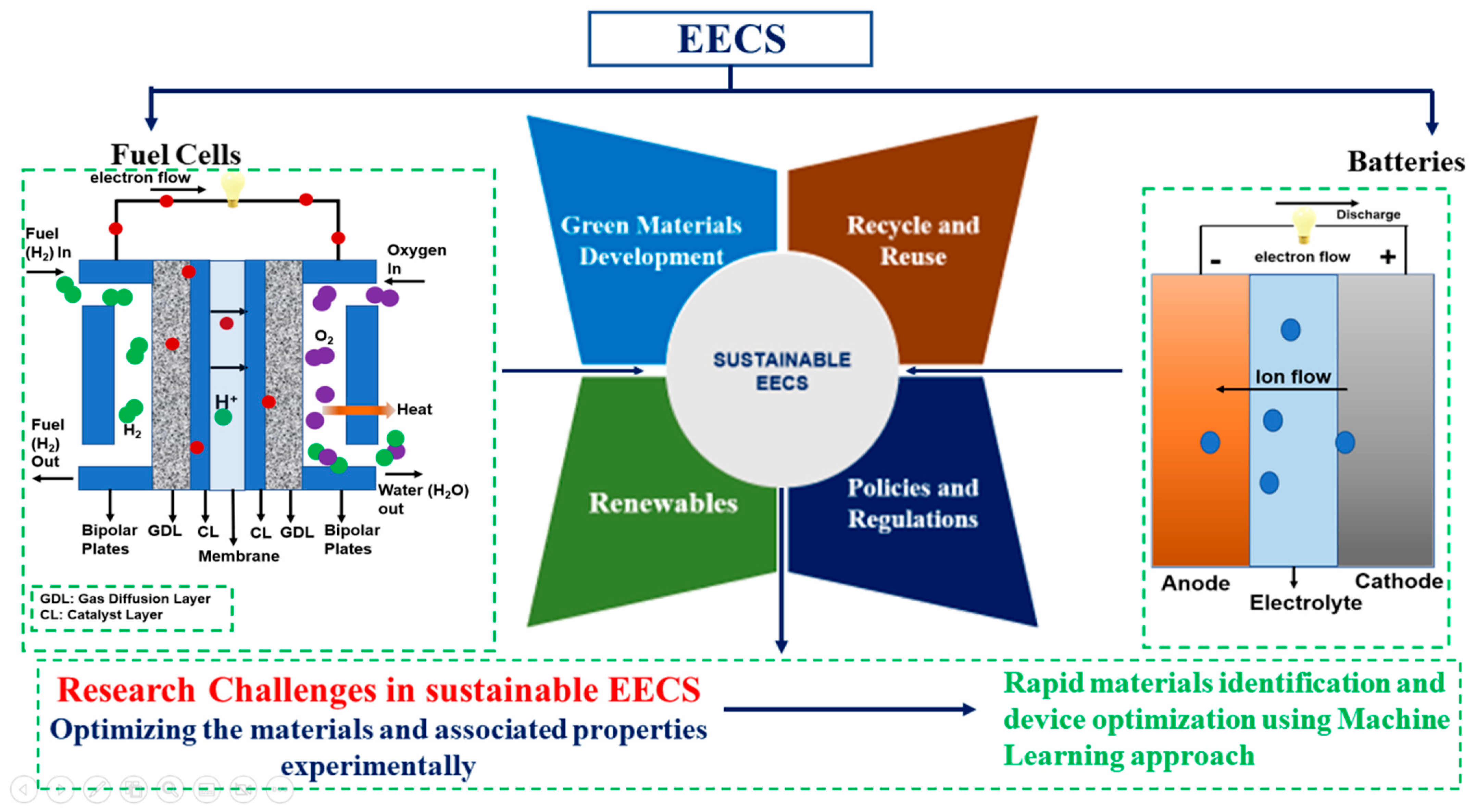
3. Routes for Sustainable EECS
3.1. “Green” Material Development for EECS
3.2. Circular EECS Economy—Reuse, Recycle and Refurbish
3.3. Waste to Energy Materials
3.4. Underpinning Renewables for EECS
3.5. Policies and Regulations
- (i)
- Several feedstocks and techniques may be used to manufacture hydrogen, with varied prices.
- (ii)
- Hydrogen may be utilized in a variety of applications.
- (iii)
- Fuel cells can offer several benefits to the entire electricity requirement of countries.
- (iv)
- Hydrogen technology may be expensive, but there are several inexpensive supplementary approaches that have been recently developed.
- (i)
- Minimizing the expenses associated with producing hydrogen using industrial technologies that emit less carbon dioxide.
- (ii)
- Making use of every available collaboration with practical facilities that already exist to spread hydrogen’s possibilities logistically.
- (iii)
- Lowering the cost of producing renewable power, which includes lowering the price of producing hydrogen thanks to manufacturing methods using minimal carbon dioxide emissions.
- (iv)
- Maturing green hydrogen technology and making it viable in the long term (beyond 2030).
- (v)
- Subsidizing blue hydrogen investments via pilot projects andnational and EU funding.
- (vi)
- Reducing the cost of electrolyzers and membranes.
4. Proposed Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and Fuel Cells for Emerging Electric Vehicle Markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Mohamed, N.; Allam, N.K. Recent Advances in the Design of Cathode Materials for Li-Ion Batteries. RSC Adv. 2020, 10, 21662–21685. [Google Scholar] [CrossRef] [PubMed]
- Brindha, R.; Ajith, S.S.R.; Nandhini, M.; Selvam, M.; Subannajui, K.; Khotmungkhun, K.; Sakthipandi, K. Evaluation of Anticorrosive Behaviour of ZnO Nanotetra-Pods on a AZ91-Grade Mg Alloy. Bull. Mater. Sci. 2019, 42, 221. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.X.; Turiansky, M.E.; van de Walle, C.G. Minimizing Hydrogen Vacancies to Enable Highly Efficient Hybrid Perovskites. Nat. Mater. 2021, 20, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Global EV Outlook 2021—Analysis—IEA. Available online: https://www.iea.org/reports/global-ev-outlook-2021 (accessed on 29 July 2022).
- Ramasubramanian, B.; Reddy, M.V.; Zaghib, K.; Armand, M.; Ramakrishna, S. Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review. Nanomaterials 2021, 11, 2476. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Brindha, R.; Nandhini, M.; Selvam, M.; Saminathan, K.; Sakthipandi, K. Water-Suspended Graphene as Electrolyte Additive in Zinc-Air Alkaline Battery System. Ionics 2019, 25, 1699–1706. [Google Scholar] [CrossRef]
- Wu, Y.; Ghalkhani, M.; Afshar, E.A.; Karimi, F.; Xia, C.; van Le, Q.; Vasseghian, Y. Recent Progress in Biomass-Derived Nanoelectrocatalysts for the Sustainable Energy Development. Fuel 2022, 323, 124349. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and Fuel Cell Technologies for Heating: A Review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, Technological Status, and Fundamentals of PEM Fuel Cells—A Review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Hameer, S.; van Niekerk, J.L. A Review of Large-Scale Electrical Energy Storage. Int. J. Energy Res. 2015, 39, 1179–1195. [Google Scholar] [CrossRef]
- Smdani, G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I.B. Performance Evaluation of Advanced Energy Storage Systems: A Review. Energy Environ. 2022. [Google Scholar] [CrossRef]
- Whittingham, M.S. History, Evolution, and Future Status of Energy Storage. Proc. IEEE 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- Khaligh, A.; Li, Z. Battery, Ultracapacitor, Fuel Cell, and Hybrid Energy Storage Systems for Electric, Hybrid Electric, Fuel Cell, and Plug-in Hybrid Electric Vehicles: State of the Art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. Recent Advancements in Applications of Alkaline Anion Exchange Membranes for Polymer Electrolyte Fuel Cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Markgraf, S.; Hörenz, M.; Schmiel, T.; Jehle, W.; Lucas, J.; Henn, N. Alkaline Fuel Cells Running at Elevated Temperature for Regenerative Fuel Cell System Applications in Spacecrafts. J. Power Source 2012, 201, 236–242. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H. Alkaline Fuel Cell Technology—A Review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Chen, D.; Zou, Y.; Shi, W.; Serbin, S.; You, H. Proton Exchange Membrane Fuel Cells Using New Cathode Field Designs of Multi-Inlet Shunt Intake Design. Int. J. Energy Res. 2021, 45, 9948–9960. [Google Scholar] [CrossRef]
- Son, T.Y.; Kim, D.J.; Vijayakumar, V.; Kim, K.; Kim, D.S.; Nam, S.Y. Anion Exchange Membrane Using Poly(Ether Ether Ketone) Containing Imidazolium for Anion Exchange Membrane Fuel Cell (AEMFC). J. Ind. Eng. Chem. 2020, 89, 175–182. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Jin, C. Development of a High-Performance Anion Exchange Membrane Using Poly(Isatin Biphenylene) with Flexible Heterocyclic Quaternary Ammonium Cations for Alkaline Fuel Cells. J. Mater. Chem. A 2019, 7, 6883–6893. [Google Scholar] [CrossRef]
- Kimura, T.; Matsumoto, A.; Inukai, J.; Miyatake, K. Highly Anion Conductive Polymers: How Do Hexafluoroisopropylidene Groups Affect Membrane Properties and Alkaline Fuel Cell Performance? ACS Appl. Energy Mater. 2020, 3, 469–477. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B.; Fuel Cell Fundamentals. Prinz—Google Books. Available online: https://books.google.com.sg/books?hl=en&lr=&id=O2JYCwAAQBAJ&oi=fnd&pg=PR11&dq=hydrogen+fuel+cells+types&ots=RRDRTTJZpk&sig=lxVfE_wQSLaJV-Mvh4JnnJhUoi8&redir_esc=y#v=onepage&q=hydrogen%20fuel%20cells%20types&f=false (accessed on 3 November 2022).
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.G. Developments in Fuel Cell Technologies in the Transport Sector. Int. J. Hydrogen Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- Chai, L.; Hu, Z.; Wang, X.; Zhang, L.; Li, T.T.; Hu, Y.; Pan, J.; Qian, J.; Huang, S. Fe7C3 Nanoparticles with in Situ Grown CNT on Nitrogen Doped Hollow Carbon Cube with Greatly Enhanced Conductivity and ORR Performance for Alkaline Fuel Cell. Carbon 2021, 174, 531–539. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Hassan, N.U.; Peng, X.; Gu, T.; Brooks-Starks, A.H.; Bahar, B.; Mustain, W.E.; Kohl, P.A. The Importance of Water Transport in High Conductivity and High-Power Alkaline Fuel Cells. J. Electrochem. Soc. 2020, 167, 054501. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, W.; Shen, Y.; Liu, L.; Dai, S.; Li, N. N-Cyclic Quaternary Ammonium-Functionalized Anion Exchange Membrane with Improved Alkaline Stability Enabled by Aryl-Ether Free Polymer Backbones for Alkaline Fuel Cells. J. Memb. Sci. 2019, 587, 117135. [Google Scholar] [CrossRef]
- Patil, S.S.; Madhura, V.; Kammakakam, I.; Swamy, M.H.; Patil, K.S.; Lai, Z.; HN, A.R. Quinuclidinium-Piperidinium Based Dual Hydroxide Anion Exchange Membranes as Highly Conductive and Stable Electrolyte Materials for Alkaline Fuel Cell Applications. Electrochim. Acta 2022, 426, 140826. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, D.; Lee, Y.N.; Yoon, Y.S.; Kim, D.J. Solid Solution Palladium-Nickel Bimetallic Anode Catalysts by Co-Sputtering for Direct Urea Fuel Cells (DUFC). J. Power Source 2019, 431, 259–264. [Google Scholar] [CrossRef]
- Pothaya, S.; Regalbuto, J.R.; Monnier, J.R.; Punyawudho, K. Preparation of Pt/Graphene Catalysts for Polymer Electrolyte Membrane Fuel Cells by Strong Electrostatic Adsorption Technique. Int. J. Hydrogen Energy 2019, 44, 26361–26372. [Google Scholar] [CrossRef]
- Radenahmad, N.; Azad, A.T.; Saghir, M.; Taweekun, J.; Bakar, M.S.A.; Reza, M.S.; Azad, A.K. A Review on Biomass Derived Syngas for SOFC Based Combined Heat and Power Application. Renew. Sustain. Energy Rev. 2020, 119, 109560. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Recent Progress in Design and Fabrication of SOFC Cathodes for Efficient Catalytic Oxygen Reduction. Catal. Today 2022, 374. [Google Scholar] [CrossRef]
- Pasierb, P.; Drożdż-Cieśla, E.; Gajerski, R.; Łabuś, S.; Komornicki, S.; Rękas, M. Chemical Stability of Ba(Ce1−xTix)1−yYyO3 Proton-Conducting Solid Electrolytes. J. Therm. Anal. Calorim. 2009, 96, 475–480. [Google Scholar] [CrossRef]
- Lenka, R.K.; Patro, P.K.; Patel, V.; Muhmood, L.; Mahata, T. Comparative Investigation on the Functional Properties of Alkaline Earth Metal (Ca, Ba, Sr) Doped Nd2NiO4+δ Oxygen Electrode Material for SOFC Applications. J. Alloys Compd. 2021, 860, 158490. [Google Scholar] [CrossRef]
- Lee, F.C.; Ismail, M.S.; Ingham, D.B.; Hughes, K.J.; Ma, L.; Lyth, S.M.; Pourkashanian, M. Alternative Architectures and Materials for PEMFC Gas Diffusion Layers: A Review and Outlook. Renew. Sustain. Energy Rev. 2022, 166, 112640. [Google Scholar] [CrossRef]
- Belenov, S.; Alekseenko, A.; Pavlets, A.; Nevelskaya, A.; Danilenko, M. Architecture Evolution of Different Nanoparticles Types: Relationship between the Structure and Functional Properties of Catalysts for PEMFC. Catalysts 2022, 12, 638. [Google Scholar] [CrossRef]
- Manojkumar, K.; Kandeeban, R.; Brindha, R.; Sangeetha, V.; Saminathan, K. Non-Precious Metal-Based Integrated Electrodes for Overall Alkaline Water Splitting. J. Indian Chem. Soc. 2022, 99, 100775. [Google Scholar] [CrossRef]
- Rajagopalan, K.; Ramasubramanian, B.; Manojkumar, K.; Ramakrishna, S.; Marappan, P.; Saminathan, R.K. Organo-Metallic Electrolyte Additive for Regulating Hydrogen Evolution and Self-Discharge in Mg–Air Aqueous Battery. New J. Chem. 2022, 46, 19950–19962. [Google Scholar] [CrossRef]
- Ott, S.; Du, F.; Luna, M.L.; Dao, T.A.; Cuenya, B.R.; Orfanidi, A.; Strasser, P. Understanding the Performance Increase of Catalysts Supported on N-Functionalized Carbon in PEMFC Catalyst Layers. J. Electrochem. Soc. 2022, 169, 054520. [Google Scholar] [CrossRef]
- Ungan, H.; Yurtcan, A.B. PEMFC Catalyst Layer Modification with the Addition of Different Amounts of PDMS Polymer in Order to Improve Water Management. Int. J. Energy Res. 2019, 43, 5946–5958. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zhao, J.; Liu, J.; Wang, L.; Lei, J.; Xue, R. Enhanced Proton Conductivity of Nafion Membrane Induced by Incorporation of MOF-Anchored 3D Microspheres: A Superior and Promising Membrane for Fuel Cell Applications. Chem. Commun. 2022, 58, 2906–2909. [Google Scholar] [CrossRef]
- Xie, X.; Shang, L.; Xiong, X.; Shi, R.; Zhang, T. Fe Single-Atom Catalysts on MOF-5 Derived Carbon for Efficient Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2022, 12, 2102688. [Google Scholar] [CrossRef]
- Lim, B.H.; Majlan, E.H.; Tajuddin, A.; Husaini, T.; Daud, W.R.W.; Radzuan, N.A.M.; Haque, M.A. Comparison of Catalyst-Coated Membranes and Catalyst-Coated Substrate for PEMFC Membrane Electrode Assembly: A Review. Chin. J. Chem. Eng. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Balogun, E.O.; Hussain, N.; Chamier, J.; Barendse, P. Performance and Durability Studies of Perfluorosulfonic Acid Ionomers as Binders in PEMFC Catalyst Layers Using Electrochemical Impedance Spectroscopy. Int. J. Hydrogen Energy 2019, 44, 32219–32230. [Google Scholar] [CrossRef]
- Kravos, A.; Ritzberger, D.; Tavčar, G.; Hametner, C.; Jakubek, S.; Katrašnik, T. Thermodynamically Consistent Reduced Dimensionality Electrochemical Model for Proton Exchange Membrane Fuel Cell Performance Modelling and Control. J. Power Source 2020, 454, 227930. [Google Scholar] [CrossRef]
- Oroujzadeh, M.; Nikouei, M.A.; Mehdipour-Ataei, S.; Amiri, M. Materials Selection for Choosing the Best Composite Blend Polymeric Membrane for Hydrogen/Oxygen Proton Exchange Membrane Fuel Cell. J. Power Source 2022, 538, 231566. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Chinglenthoiba, C.; Huiqing, X.; Xiping, N.; Hui, H.K.; Valiyaveettil, S.; Ramakrishna, S.; Chellappan, V. Sustainable Fe-MOF@carbon Nanocomposite Electrode for Supercapacitor. Surf. Interfaces 2022, 34, 102397. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Wang, G.; Hou, L.; Yuan, C. Eco-Friendly and Scalable Synthesis of Micro-/Mesoporous Carbon Sub-Microspheres as Competitive Electrodes for Supercapacitors and Sodium-Ion Batteries. Appl. Surf. Sci. 2020, 533, 147511. [Google Scholar] [CrossRef]
- Chabhadiya, K.; Srivastava, R.R.; Pathak, P. Two-Step Leaching Process and Kinetics for an Eco-Friendly Recycling of Critical Metals from Spent Li-Ion Batteries. J. Environ. Chem. Eng. 2021, 9, 105232. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, J.; Jung, S.K.; Kang, D.; Park, M.S.; Cha, G.D.; Cho, K.W.; Song, J.H.; Moon, S.; Yun, Y.S.; et al. A Biodegradable Secondary Battery and Its Biodegradation Mechanism for Eco-Friendly Energy-Storage Systems. Adv. Mater. 2021, 33, 2004902. [Google Scholar] [CrossRef]
- Zhu, G.; Luo, W.; Wang, L.; Jiang, W.; Yang, J. Silicon: Toward Eco-Friendly Reduction Techniques for Lithium-Ion Battery Applications. J. Mater. Chem. A Mater. 2019, 7, 24715–24737. [Google Scholar] [CrossRef]
- Zhang, X.; Su, K.; Mohamed, A.G.A.; Liu, C.; Sun, Q.; Yuan, D.; Wang, Y.; Xue, W.; Wang, Y. Photo-Assisted Charge/Discharge Li-Organic Battery with a Charge-Separated and Redox-Active C60@porous Organic Cage Cathode. Energy Environ. Sci. 2022, 15, 780–785. [Google Scholar] [CrossRef]
- Hoffknecht, J.P.; Atik, J.; Krause, C.; Thienenkamp, J.; Brunklaus, G.; Winter, M.; Paillard, E. Beyond Fluorine: Sustainable Ternary Polymer Electrolytes for Lithium Batteries. Green Chem. 2021, 23, 9935–9944. [Google Scholar] [CrossRef]
- Deng, K.; Xu, Z.; Zhou, S.; Zhao, Z.; Zeng, K.; Xiao, M.; Meng, Y.; Xu, Y. Nonflammable Highly-Fluorinated Polymer Electrolytes with Enhanced Interfacial Compatibility for Dendrite-Free Lithium Metal Batteries. J. Power Source 2021, 510, 230411. [Google Scholar] [CrossRef]
- Li, Q.; Li, D.; Wang, H.; Wang, H.G.; Li, Y.; Si, Z.; Duan, Q. Conjugated Carbonyl Polymer-Based Flexible Cathode for Superior Lithium-Organic Batteries. ACS Appl. Mater. Interfaces 2019, 11, 28801–28808. [Google Scholar] [CrossRef]
- Weeraratne, K.S.; Alzharani, A.A.; El-Kaderi, H.M. Redox-Active Porous Organic Polymers as Novel Electrode Materials for Green Rechargeable Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 23520–23526. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Shi, Y.; Chen, Z.; Bai, P.; Su, H.; Xiong, P.; Cheng, M.; Zhao, J.; Xu, Y. Soluble Organic Cathodes Enable Long Cycle Life, High Rate, and Wide-Temperature Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2107226. [Google Scholar] [CrossRef]
- Dong, X.; Guo, Z.; Guo, Z.; Wang, Y.; Xia, Y. Organic Batteries Operated at −70 °C. Joule 2018, 2, 902–913. [Google Scholar] [CrossRef]
- Shea, J.J.; Luo, C. Organic Electrode Materials for Metal Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5361–5380. [Google Scholar] [CrossRef]
- Esser, B.; Dolhem, F.; Becuwe, M.; Poizot, P.; Vlad, A.; Brandell, D. A Perspective on Organic Electrode Materials and Technologies for next Generation Batteries. J. Power Source 2021, 482, 228814. [Google Scholar] [CrossRef]
- Sun, T.; Xie, J.; Guo, W.; Li, D.S.; Zhang, Q. Covalent–Organic Frameworks: Advanced Organic Electrode Materials for Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 1904199. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J. Prospects of Organic Electrode Materials for Practical Lithium Batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef]
- Stropnik, R.; Lotrič, A.; Montenegro, A.B.; Sekavčnik, M.; Mori, M. Critical Materials in PEMFC Systems and a LCA Analysis for the Potential Reduction of Environmental Impacts with EoL Strategies. Energy Sci. Eng. 2019, 7, 2519–2539. [Google Scholar] [CrossRef]
- Rodríguez-Garnica, P.; Alatorre-Ordaz, A.; Pierna, Á.R.; Guereño, M.S.; Martín, A.L. Silica Based Hybrid Organic-Inorganic Materials for PEMFC Application. Int. J. Hydrogen Energy 2020, 45, 16698–16707. [Google Scholar] [CrossRef]
- González-Espasandín, Ó.; Leo, T.J.; Raso, M.A.; Navarro, E. Direct Methanol Fuel Cell (DMFC) and H2 Proton Exchange Membrane Fuel (PEMFC/H2) Cell Performance under Atmospheric Flight Conditions of Unmanned Aerial Vehicles. Renew. Energy 2019, 130, 762–773. [Google Scholar] [CrossRef]
- Carvalho, R.P.; Marchiori, C.F.N.; Brandell, D.; Araujo, C.M. Artificial Intelligence Driven In-Silico Discovery of Novel Organic Lithium-Ion Battery Cathodes. Energy Storage Mater. 2022, 44, 313–325. [Google Scholar] [CrossRef]
- Liedel, C. Sustainable Battery Materials from Biomass. ChemSusChem 2020, 13, 2110–2141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Liu, H.; Yuan, H.; Peng, H.-J.; Tang, C.; Huang, J.-Q.; Zhang, Q.; Qiang Zhang, C. A Perspective on Sustainable Energy Materials for Lithium Batteries. SusMat 2021, 1, 38–50. [Google Scholar] [CrossRef]
- Jin, C.; Nai, J.; Sheng, O.; Yuan, H.; Zhang, W.; Tao, X.; Lou, X.W. Biomass-Based Materials for Green Lithium Secondary Batteries. Energy Environ. Sci. 2021, 14, 1326–1379. [Google Scholar] [CrossRef]
- Rajagopalan, K.; Ramasubramanian, B.; Velusamy, S.; Ramakrishna, S.; Kannan, A.M.; Kaliyannan, M.; Kulandaivel, S. Examining the Economic and Energy Aspects of Manganese Oxide in Li-Ion Batteries. Mater. Circ. Econ. 2022, 4, 1–22. [Google Scholar] [CrossRef]
- Mohanraj, R.; Brindha, R.; Kandeeban, R.; Mahendhar, M.; Saminathan, K.; Ayyappadasan, G. Electrochemical Detection of 5-Hydroxytryptamine Using Sustainable SnO2-Graphite Nanocomposite Modified Electrode. Mater. Lett. 2021, 305, 130796. [Google Scholar] [CrossRef]
- Brindha, R.; Mohanraj, R.; Manojkumar, P.; Selvam, M.; Sakthipandi, K. Hybrid Electrochemical Behaviour of La1-XCaxMnO3 Nano Perovskites and Recycled Polar Interspersed Graphene for Metal-Air Battery System. J. Electrochem. Soc. 2020, 167, 120539. [Google Scholar] [CrossRef]
- He, J.; Bhargav, A.; Manthiram, A. High-Energy-Density, Long-Life Lithium-Sulfur Batteries with Practically Necessary Parameters Enabled by Low-Cost Fe-Ni Nanoalloy Catalysts. ACS Nano 2021, 15, 8583–8591. [Google Scholar] [CrossRef]
- Zhang, B.W.; Sheng, T.; Liu, Y.D.; Wang, Y.X.; Zhang, L.; Lai, W.H.; Wang, L.; Yang, J.; Gu, Q.F.; Chou, S.L.; et al. Atomic Cobalt as an Efficient Electrocatalyst in Sulfur Cathodes for Superior Room-Temperature Sodium-Sulfur Batteries. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From Rocking Chair—Li-Ion to Solid-State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ren, Z.; Singh, G. Beyond Graphene Anode Materials for Emerging Metal Ion Batteries and Supercapacitors. Nanomicro Lett. 2018, 10, 1–27. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Subramanian, S.; Rayavarapu, P.R.P.; Reddy, M.V.; Chellappan, V.; Ramakrishna, S. Novel Low-Carbon Energy Solutions for Powering Emerging Wearables, Smart Textiles, and Medical Devices. Energy Environ. Sci. 2022. [Google Scholar] [CrossRef]
- Li, T.; Zhi, D.D.; Guo, Z.H.; Li, J.Z.; Chen, Y.; Meng, F.B. 3D Porous Biomass-Derived Carbon Materials: Biomass Sources, Controllable Transformation and Microwave Absorption Application. Green Chem. 2022, 24, 647–674. [Google Scholar] [CrossRef]
- Gao, M.; Lin, Y.-C.; Shih, C.-C.; Lee, W.-Y.; Chueh, C.-C.; Chen, W.-C. (Invited) A Nature-Inspired Porous Electrode for Flexible, Stretchable Supercapacitors and Lithium-Ion Batteries. ECS Meet. Abstr. 2018, MA2018-01, 1539. [Google Scholar] [CrossRef]
- Ani, P.C.; Nzereogu, P.U.; Agbogu, A.C.; Ezema, F.I.; Nwanya, A.C. Cellulose from Waste Materials for Electrochemical Energy Storage Applications: A Review. Appl. Surf. Sci. Adv. 2022, 11, 100298. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.; Zhao, H.; Li, L.; Deng, P.; Wu, Y.; Luo, S.; Chen, B. Ultrathin Amorphous MnO2 Modified Prawn Shells-Derived Porous Carbon towards Robust Oxygen Electrocatalyst for Rechargeable Zn-Air Battery. Ceram. Int. 2022, 48, 6506–6511. [Google Scholar] [CrossRef]
- Edward, M.L.; Dharanibalaji, K.C.; Kumar, K.T.; Chandrabose, A.R.S.; Shanmugharaj, A.M.; Jaisankar, V. Preparation and Characterisation of Chitosan Extracted from Shrimp Shell (Penaeus Monodon) and Chitosan-Based Blended Solid Polymer Electrolyte for Lithium-Ion Batteries. Polym. Bull. 2021, 79, 587–604. [Google Scholar] [CrossRef]
- Jin, W.W.; Li, H.J.; Zou, J.Z.; Inguva, S.; Zhang, Q.; Zeng, S.Z.; Xu, G.Z.; Zeng, X.R. Metal Organic Framework-Derived Carbon Nanosheets with Fish-Scale Surface Morphology as Cathode Materials for Lithium–Selenium Batteries. J. Alloys Compd. 2020, 820, 153084. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Sun, X.; Wei, K.; Wang, W.; Wang, A.; Huang, Y.; Guan, Y. Hierarchical Porous Carbon with Nano-MgO as Efficient Sulfur Species Micro-Reactors for Lithium-Sulfur Battery. J. Electrochem. Soc. 2021, 168, 040506. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Huang, D.; Liu, J.; Chen, C.; Li, D.; Zhu, M.; Chen, Y. An Ultra-Strong, Water Stable and Antimicrobial Chitosan Film with Interdigitated Bouligand Structure. Adv. Sustain. Syst. 2022, 6, 2200033. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.; Qiu, J. Hierarchical Porous Carbon Sheets Derived from Biomass Containing an Activation Agent and In-Built Template for Lithium Ion Batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.D.; Ghangrekar, M.M. A Novel Proton Exchange Membrane Developed from Clay and Activated Carbon Derived from Coconut Shell for Application in Microbial Fuel Cell. Biochem. Eng. J. 2019, 148, 170–177. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-Derived Biochar Materials as Sustainable Energy Sources for Electrochemical Energy Storage Devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Guo, D.; Li, Z.; Liu, P.; Sun, M. N, P, S Co-Doped Biomass-Derived Hierarchical Porous Carbon through Simple Phosphoric Acid-Assisted Activation for High-Performance Electrochemical Energy Storage. Int. J. Hydrogen Energy 2021, 46, 8197–8209. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Muruganantham, R.; Tai, S.H.; Chang, B.K.; Wu, S.C.; Chueh, Y.L.; Liu, W.R. Coffee Grounds-Derived Carbon as High Performance Anode Materials for Energy Storage Applications. J. Taiwan Inst. Chem. Eng. 2019, 97, 178–188. [Google Scholar] [CrossRef]
- Escobar, B.; Martínez-Casillas, D.C.; Pérez-Salcedo, K.Y.; Rosas, D.; Morales, L.; Liao, S.J.; Huang, L.L.; Shi, X. Research Progress on Biomass-Derived Carbon Electrode Materials for Electrochemical Energy Storage and Conversion Technologies. Int. J. Hydrogen Energy 2021, 46, 26053–26073. [Google Scholar] [CrossRef]
- Schonvogel, D.; Nowotny, M.; Woriescheck, T.; Multhaupt, H.; Wagner, P.; Dyck, A.; Agert, C.; Wark, M. Hydrothermal Carbonization-Derived Carbon from Waste Biomass as Renewable Pt Support for Fuel Cell Applications: Role of Carbon Activation. Energy Technol. 2019, 7, 1900344. [Google Scholar] [CrossRef]
- Xie, F.; Zhao, B.; Cui, Y.; Ma, X.; Zhang, X.; Yue, X. Reutilize Tire in Microbial Fuel Cell for Enhancing the Nitrogen Removal of the Anammox Process Coupled with Iron-Carbon Micro-Electrolysis. Front. Environ. Sci. Eng. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Unknown, S.; Chand, P.; Joshi, A. Biomass Derived Carbon for Supercapacitor Applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Tang, X.; Liu, D.; Wang, Y.J.; Cui, L.; Ignaszak, A.; Yu, Y.; Zhang, J. Research Advances in Biomass-Derived Nanostructured Carbons and Their Composite Materials for Electrochemical Energy Technologies. Prog. Mater. Sci. 2021, 118, 100770. [Google Scholar] [CrossRef]
- Sun, S.; Yan, Q.; Wu, M.; Zhao, X. Carbon Aerogel Based Materials for Secondary Batteries. Sustain. Mater. Technol. 2021, 30, e00342. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Tian, Z.; Zhao, X.; Shen, X. Low-Temperature Synthesis of LiFePO4Nanoplates/C Composite for Lithium Ion Batteries. Energy Fuels 2020, 34, 11597–11605. [Google Scholar] [CrossRef]
- Golmohammadi, F.; Amiri, M. Fabrication of MEA from Biomass-Based Carbon Nanofibers Composited with Nickel-Cobalt Oxides as a New Electrocatalyst for Oxygen Reduction Reaction in Passive Direct Methanol Fuel Cells. Electrocatalysis 2020, 11, 485–496. [Google Scholar] [CrossRef]
- Che, S.; Li, C.; Wang, C.; Zaheer, W.; Ji, X.; Phillips, B.; Gurbandurdyyev, G.; Glynn, J.; Guo, Z.H.; Al-Hashimi, M.; et al. Solution-Processable Porous Graphitic Carbon from Bottom-up Synthesis and Low-Temperature Graphitization. Chem. Sci. 2021, 12, 8438–8444. [Google Scholar] [CrossRef]
- Rahayu, I.; Suci, U.A.; Taufiqulhadi, F. Modification of Cathode Material Lithium Iron Phosphate by Silicon Doping Using Solid State Reaction. Mater. Sci. Forum 2021, 1044, 73–79. [Google Scholar] [CrossRef]
- Song, B.F.; Dhanabalan, A.; Biswal, S.L. Evaluating the Capacity Ratio and Prelithiation Strategies for Extending Cyclability in Porous Silicon Composite Anodes and Lithium Iron Phosphate Cathodes for High Capacity Lithium-Ion Batteries. J. Energy Storage 2020, 28, 101268. [Google Scholar] [CrossRef]
- An, Z.; Gong, Y.; Fang, W.; Zhao, K.; Ye, D.; Zhao, H.; Xu, J.; Zhang, L.; Zhang, J. Biomineralization-Inspired Synthesis of Na3V2(PO4)3 Nanoparticles Wrapped with 3D Porous Carbon as High-Performance Cathode for Sodium-Ion Batteries. Ionics 2021, 27, 1165–1175. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent Advances in Biomass Derived Activated Carbon Electrodes for Hybrid Electrochemical Capacitor Applications: Challenges and Opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Liu, W.; Cui, Z. Life Cycle Assessment of Power Batteries Used in Electric Bicycles in China. Renew. Sustain. Energy Rev. 2021, 139, 110596. [Google Scholar] [CrossRef]
- Arya, S.; Rautela, R.; Chavan, D.; Kumar, S. Evaluation of Soil Contamination Due to Crude E-Waste Recycling Activities in the Capital City of India. Process Saf. Environ. Prot. 2021, 152, 641–653. [Google Scholar] [CrossRef]
- Wu, X.; Ma, J.; Wang, J.; Zhang, X.; Zhou, G.; Liang, Z.; Wu, X.; Wang, J.; Liang, Z.; Ma, J.; et al. Progress, Key Issues, and Future Prospects for Li-Ion Battery Recycling. Glob. Chall. 2022, 38, 2200067. [Google Scholar] [CrossRef]
- Geng, X.; Ru, J.; Hua, Y.; Zhang, W. The Recovery of Lead from Spent Lead Acid Battery Paste by Electrodeposition in Deep Eutectic Solvent. J. Sustain. Metall. 2022, 8, 1257–1268. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical Review of Bioadsorption on Modified Cellulose and Removal of Divalent Heavy Metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, J.; Jin, W.; Xu, F.; Jiang, L.; Xi, D.; Wen, Y.; Li, J.; Yu, Z.; Li, Z.; et al. Size Effect of γ-MnO2 Precoated Anode on Lead-Containing Pollutant Reduction and Its Controllable Fabrication in Industrial-Scale for Zinc Electrowinning. Chemosphere 2022, 287, 132457. [Google Scholar] [CrossRef]
- Rahman, S.A.; Shaikh, F.U.A.; Sarker, P.K. A Comprehensive Review of Properties of Concrete Containing Lithium Refinery Residue as Partial Replacement of Cement. Constr. Build. Mater. 2022, 328, 127053. [Google Scholar] [CrossRef]
- Noruzi, F.; Nasirpour, N.; Vakilchap, F.; Mousavi, S.M. Complete Bioleaching of Co and Ni from Spent Batteries by a Novel Silver Ion Catalyzed Process. Appl. Microbiol. Biotechnol. 2022, 106, 5301–5316. [Google Scholar] [CrossRef]
- Määttä, L.; Hajdu-Rahkama, R.; Oinonen, C.; Puhakka, J.A. Effects of Metal Extraction Liquors from Electric Vehicle Battery Materials Production on Iron and Sulfur Oxidation by Heap Bioleaching Microorganisms. Miner. Eng. 2022, 178, 107409. [Google Scholar] [CrossRef]
- Naseri, T.; Pourhossein, F.; Mousavi, S.M.; Kaksonen, A.H.; Kuchta, K. Manganese Bioleaching: An Emerging Approach for Manganese Recovery from Spent Batteries. Rev. Environ. Sci. Bio/Technol. 2022, 21, 447–468. [Google Scholar] [CrossRef]
- Moosakazemi, F.; Ghassa, S.; Jafari, M.; Chelgani, S.C. Bioleaching for Recovery of Metals from Spent Batteries—A Review. Miner. Process. Extr. Metall. Rev. 2022, 1–31. [Google Scholar] [CrossRef]
- Yang, H.; Lee, J.; Cheong, J.Y.; Wang, Y.; Duan, G.; Hou, H.; Jiang, S.; Kim, I.D. Molecular Engineering of Carbonyl Organic Electrodes for Rechargeable Metal-Ion Batteries: Fundamentals, Recent Advances, and Challenges. Energy Environ. Sci. 2021, 14, 4228–4267. [Google Scholar] [CrossRef]
- Lyu, H.; Sun, X.-G.; Dai, S. Organic Cathode Materials for Lithium-Ion Batteries: Past, Present, and Future. Adv. Energy Sustain. Res. 2021, 2, 2000044. [Google Scholar] [CrossRef]
- Bubbico, R.; Greco, V.; Menale, C. Hazardous Scenarios Identification for Li-Ion Secondary Batteries. Saf. Sci. 2018, 108, 72–88. [Google Scholar] [CrossRef]
- Zaghib, K.; Charest, P.; Guerfi, A.; Shim, J.; Perrier, M.; Striebel, K. LiFePO4 Safe Li-Ion Polymer Batteries for Clean Environment. J. Power Source 2005, 146, 380–385. [Google Scholar] [CrossRef]
- Rajoba, S.J.; Jadhav, L.D.; Kalubarme, R.S.; Patil, P.S.; Varma, S.; Wani, B.N. Electrochemical Performance of LiFePO4/GO Composite for Li-Ion Batteries. Ceram. Int. 2018, 44, 6886–6893. [Google Scholar] [CrossRef]
- Sridharpanday, M.; Brindha, R.; Vinoth, M.; Narthana, K.; Rajendran, V. Investigation on Temperature-Dependent Structural, Dielectric and Impedance Characteristics of Cu-Doped CaFexTi1-XO3-δ Nanotitanates. J. Mater. Sci. Mater. Electron. 2021, 32, 22076–22092. [Google Scholar] [CrossRef]
- Yu, Z.; Jiao, S.; Li, S.; Chen, X.; Song, W.L.; Teng, T.; Tu, J.; Chen, H.S.; Zhang, G.; Fang, D.N. Flexible Stable Solid-State Al-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1806799. [Google Scholar] [CrossRef]
- Perea, A.; Dontigny, M.; Zaghib, K. Safety of Solid-State Li Metal Battery: Solid Polymer versus Liquid Electrolyte. J. Power Source 2017, 359, 182–185. [Google Scholar] [CrossRef]
- Stenina, I.; Minakova, P.; Kulova, T.; Yaroslavtsev, A. Electrochemical Properties of LiFePO4 Cathodes: The Effect of Carbon Additives. Batteries 2022, 8, 111. [Google Scholar] [CrossRef]
- Ling, J.; Karuppiah, C.; Krishnan, S.G.; Reddy, M.V.; Misnon, I.I.; Rahim, M.H.A.; Yang, C.C.; Jose, R. Phosphate Polyanion Materials as High-Voltage Lithium-Ion Battery Cathode: A Review. Energy Fuels 2021, 35, 10428–10450. [Google Scholar] [CrossRef]
- Yang, S.; Zavalij, P.Y.; Whittingham, M.S. Hydrothermal Synthesis of Lithium Iron Phosphate Cathodes. Electrochem. Commun 2001, 3, 505–508. [Google Scholar] [CrossRef]
- Zhang, H.; Oteo, U.; Judez, X.; Eshetu, G.G.; Martinez-Ibañez, M.; Carrasco, J.; Li, C.; Armand, M. Designer Anion Enabling Solid-State Lithium-Sulfur Batteries. Joule 2019, 3, 1689–1702. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Arulraj, A.; Khalid, M.; Reddy, M.V.; Jose, R. Energy Storage in Metal Cobaltite Electrodes: Opportunities & Challenges in Magnesium Cobalt Oxide. Renew. Sustain. Energy Rev. 2021, 141, 110798. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Z.; Zhou, Z.; Xi, S.; Xia, Y.; Zhang, Q.; Huang, L.; Mei, L.; Jiang, Y.; Gao, J.; et al. A Safe Flexible Self-Powered Wristband System by Integrating Defective MnO2-X Nanosheet-Based Zinc-Ion Batteries with Perovskite Solar Cells. ACS Nano 2021, 15, 10597–10608. [Google Scholar] [CrossRef]
- Wu, K.; Huang, J.; Yi, J.; Liu, X.; Liu, Y.; Wang, Y.; Zhang, J.; Xia, Y. Recent Advances in Polymer Electrolytes for Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. Adv. Energy Mater. 2020, 10, 1903977. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-Free Zinc Deposition Induced by Multifunctional CNT Frameworks for Stable Flexible Zn-Ion Batteries. Adv. Mater. 2019, 31, 1903675. [Google Scholar] [CrossRef]
- Municipal Solid Waste Management in Developed Countries and India—An Overview of Current Practices, Challenges, Opportunities, and Threats|Specialusis Ugdymas. Available online: https://www.sumc.lt/index.php/se/article/view/639 (accessed on 30 July 2022).
- Sun, Y.; Shi, X.-L.; Yang, Y.-L.; Suo, G.; Zhang, L.; Lu, S.; Chen, Z.-G.; Sun, Y.; Yang, Y.-L.; Suo, G.; et al. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Adv. Funct. Mater. 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Lai, W.L.; Sharma, S.; Roy, S.; Maji, P.K.; Sharma, B.; Ramakrishna, S.; Goh, K.L.; Blanco, A. Roadmap to Sustainable Plastic Waste Management: A Focused Study on Recycling PET for Triboelectric Nanogenerator Production in Singapore and India. Environ. Sci. Pollut. Res. 2022, 29, 51234–51268. [Google Scholar] [CrossRef]
- Veldevi, T.; Raghu, S.; Kalaivani, R.A.; Shanmugharaj, A.M. Waste Tire Derived Carbon as Potential Anode for Lithium-Ion Batteries. Chemosphere 2022, 288, 132438. [Google Scholar] [CrossRef]
- Zhao, P.; Han, Y.; Dong, X.; Zhang, C.; Liu, S. Application of Activated Carbons Derived from Scrap Tires as Electrode Materials for Supercapacitors. ECS J. Solid State Sci. Technol. 2015, 4, M35–M40. [Google Scholar] [CrossRef]
- Naskar, A.K.; Bi, Z.; Li, Y.; Akato, S.K.; Saha, D.; Chi, M.; Bridges, C.A.; Paranthaman, M.P. Tailored Recovery of Carbons from Waste Tires for Enhanced Performance as Anodes in Lithium-Ion Batteries. RSC Adv. 2014, 4, 38213–38221. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A. Morphologically Tailored Activated Carbon Derived from Waste Tires as High-Performance Anode for Li-Ion Battery. J. Appl. Electrochem. 2017, 48, 1–13. [Google Scholar] [CrossRef]
- Parasuram, B.; Sundaram, S.; Sathiskumar, C.; Karthikeyan, S. Synthesis of Multi-Walled Carbon Nanotubes Using Tire Pyrolysis Oil as a Carbon Precursor by Spray Pyrolysis Method. Inorg. Nano-Met. Chem. 2018, 48, 103–106. [Google Scholar] [CrossRef]
- Gao, R.; Liu, Y.; Liu, B.; Xu, Z. Novel Utilization of Pyrolysis Products Produced from Waste Printed Circuit Boards: Catalytic Cracking and Synthesis of Graphite Carbon. J. Clean. Prod. 2019, 236, 117662. [Google Scholar] [CrossRef]
- Kan, Y.; Yue, Q.; Liu, S.; Gao, B. Effects of Cu and CuO on the Preparation of Activated Carbon from Waste Circuit Boards by H3PO4 Activation. Chem. Eng. J. 2018, 331, 93–101. [Google Scholar] [CrossRef]
- US Ep; Office of International Affairs. Handout 10 Workshop Materials on WEEE Management in Taiwan Printed Circuit Board Recycling Methods; EPA: Washington, DC, USA, 2012.
- Sahajwalla, V.; Cayumil, R.; Khanna, R.; Ikram-Ul-Haq, M.; Rajarao, R.; Mukherjee, P.S.; Hill, A. Recycling Polymer-Rich Waste Printed Circuit Boards at High Temperatures: Recovery of Value-Added Carbon Resources. J. Sustain. Metall. 2015, 1, 75–84. [Google Scholar] [CrossRef]
- Bhattacharya, G.; Fishlock, S.J.; Roy, J.S.; Pritam, A.; Banerjee, D.; Deshmukh, S.; Ghosh, S.; McLaughlin, J.A.; Roy, S.S.; Bhattacharya, G.; et al. Effective Utilization of Waste Red Mud for High Performance Supercapacitor Electrodes. Glob. Chall. 2019, 3, 1800066. [Google Scholar] [CrossRef]
- Salunkhe, T.T.; Varma, R.S.; Kadam, A.N.; Lee, S.W.; Lee, Y.C.; Hur, J.; Kim, I.T. Scraps to Superior Anodes for Li-Ion Batteries: Sustainable and Scalable Upgrading of Waste Rust. J. Hazard Mater. 2021, 410, 124571. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y.; Xu, D.; Li, W.; Zhao, X.; Tao, X.; Zeng, S. Mesoporous Fe2O3 Nanomaterials from Natural Rust for Lithium Storage. J. Mater. Sci. Mater. Electron. 2017, 28, 19098–19104. [Google Scholar] [CrossRef]
- Konikkara, N.; Kennedy, L.J.; Vijaya, J.J. Preparation and Characterization of Hierarchical Porous Carbons Derived from Solid Leather Waste for Supercapacitor Applications. J. Hazard Mater. 2016, 318, 173–185. [Google Scholar] [CrossRef]
- Martínez-Casillas, D.C.; Alonso-Lemus, I.L.; Mascorro-Gutiérrez, I.; Cuentas-Gallegos, A.K. Leather Waste-Derived Biochar with High Performance for Supercapacitors. J. Electrochem. Soc. 2018, 165, A2061–A2068. [Google Scholar] [CrossRef]
- Soni, R.; Bhange, S.N.; Kurungot, S. A 3-D Nanoribbon-like Pt-Free Oxygen Reduction Reaction Electrocatalyst Derived from Waste Leather for Anion Exchange Membrane Fuel Cells and Zinc-Air Batteries. Nanoscale 2019, 11, 7893–7902. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, L.; Zhan, J.; Du, N.; Liu, W.; Ma, J.; Su, L.; Wang, L. Recycling Silicon-Based Industrial Waste as Sustainable Sources of Si/SiO2 Composites for High-Performance Li-Ion Battery Anodes. J. Power Source 2020, 449, 227513. [Google Scholar] [CrossRef]
- Creutzig, F.; Agoston, P.; Goldschmidt, J.C.; Luderer, G.; Nemet, G.; Pietzcker, R.C. The Underestimated Potential of Solar Energy to Mitigate Climate Change. Nat. Energy 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar Water Splitting by Photovoltaic-Electrolysis with a Solar-to-Hydrogen Efficiency over 30%. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Talebian, H.; Herrera, O.E.; Mérida, W. Policy Effectiveness on Emissions and Cost Reduction for Hydrogen Supply Chains: The Case for British Columbia. Int. J. Hydrogen Energy 2021, 46, 998–1011. [Google Scholar] [CrossRef]
- Fazioli, R.; Pantaleone, F. Macroeconomic Factors Influencing Public Policy Strategies for Blue and Green Hydrogen. Energies 2021, 14, 7938. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Ling, W. Policy Optimization of Hydrogen Energy Industry Considering Government Policy Preference in China. Sustain. Prod. Consum. 2022, 33, 890–902. [Google Scholar] [CrossRef]
- Browne, S.; Waghmare, U.V.; Singh, A. Opportunities and Challenges for 2D Heterostructures in Battery Applications: A Computational Perspective. Nanotechnology 2022, 33, 272501. [Google Scholar] [CrossRef] [PubMed]
- Wieder, B.J.; Bradlyn, B.; Cano, J.; Wang, Z.; Vergniory, M.G.; Elcoro, L.; Soluyanov, A.A.; Felser, C.; Neupert, T.; Regnault, N.; et al. Topological Materials Discovery from Crystal Symmetry. Nat. Rev. Mater. 2021, 7, 196–216. [Google Scholar] [CrossRef]
- Pyzer-Knapp, E.O.; Pitera, J.W.; Staar, P.W.J.; Takeda, S.; Laino, T.; Sanders, D.P.; Sexton, J.; Smith, J.R.; Curioni, A. Accelerating Materials Discovery Using Artificial Intelligence, High Performance Computing and Robotics. Npj Comput. Mater. 2022, 8, 1–9. [Google Scholar] [CrossRef]
- Bash, D.; Cai, Y.; Chellappan, V.; Wong, S.L.; Yang, X.; Kumar, P.; Tan, J.D.; Abutaha, A.; Cheng, J.J.W.; Lim, Y.F.; et al. Multi-Fidelity High-Throughput Optimization of Electrical Conductivity in P3HT-CNT Composites. Adv. Funct. Mater. 2021, 31, 2102606. [Google Scholar] [CrossRef]
- Jalem, R.; Nakayama, M.; Kasuga, T. An Efficient Rule-Based Screening Approach for Discovering Fast Lithium Ion Conductors Using Density Functional Theory and Artificial Neural Networks. J. Mater. Chem. A Mater. 2013, 2, 720–734. [Google Scholar] [CrossRef]
- Ahmad, Z.; Xie, T.; Maheshwari, C.; Grossman, J.C.; Viswanathan, V. Machine Learning Enabled Computational Screening of Inorganic Solid Electrolytes for Suppression of Dendrite Formation in Lithium Metal Anodes. ACS Cent. Sci. 2018, 4, 996–1006. [Google Scholar] [CrossRef]
- Bhowmik, A.; Castelli, I.E.; Garcia-Lastra, J.M.; Jørgensen, P.B.; Winther, O.; Vegge, T. A Perspective on Inverse Design of Battery Interphases Using Multi-Scale Modelling, Experiments and Generative Deep Learning. Energy Storage Mater. 2019, 21, 446–456. [Google Scholar] [CrossRef]
- Wu, H.; Lorenson, A.; Anderson, B.; Witteman, L.; Wu, H.; Meredig, B.; Morgan, D. Robust FCC Solute Diffusion Predictions from Ab-Initio Machine Learning Methods. Comput. Mater. Sci. 2017, 134, 160–165. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Hemmati-Sarapardeh, A.; Ameli, F.; Naderi, F.; Dastgahi, M. A Computational Intelligence Scheme for Estimating Electrical Conductivity of Ternary Mixtures Containing Ionic Liquids. J. Mol. Liq. 2016, 221, 624–632. [Google Scholar] [CrossRef]
- Chemali, E.; Kollmeyer, P.J.; Preindl, M.; Emadi, A. State-of-Charge Estimation of Li-Ion Batteries Using Deep Neural Networks: A Machine Learning Approach. J. Power Source 2018, 400, 242–255. [Google Scholar] [CrossRef]
- Li, Z.; Outbib, R.; Giurgea, S.; Hissel, D.; Li, Y. Fault Detection and Isolation for Polymer Electrolyte Membrane Fuel Cell Systems by Analyzing Cell Voltage Generated Space. Appl. Energy 2015, 148, 260–272. [Google Scholar] [CrossRef]
- Legala, A.; Zhao, J.; Li, X. Machine Learning Modeling for Proton Exchange Membrane Fuel Cell Performance. Energy AI 2022, 10, 100183. [Google Scholar] [CrossRef]
- Meng, T.; Cui, D.; Ji, Y.; Cheng, M.; Tu, B.; Lan, Z. Optimization and Efficiency Analysis of Methanol SOFC-PEMFC Hybrid System. Int. J. Hydrogen Energy 2022, 47, 27690–27702. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, H.; Liu, J.; Zhou, H.; Wang, H.; Yang, W. Machine Learning-Assisted Discovery of Strong and Conductive Cu Alloys: Data Mining from Discarded Experiments and Physical Features. Mater. Des. 2021, 197, 109248. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Zhao, H.; Wang, Y. Durability Estimation and Short-Term Voltage Degradation Forecasting of Vehicle PEMFC System: Development and Evaluation of Machine Learning Models. Appl. Energy 2022, 326, 119975. [Google Scholar] [CrossRef]
- Ue, M.; Sakaushi, K.; Uosaki, K. Basic Knowledge in Battery Research Bridging the Gap between Academia and Industry. Mater. Horiz. 2020, 7, 1937–1954. [Google Scholar] [CrossRef]
- Chun, H.; Lee, E.; Nam, K.; Jang, J.H.; Kyoung, W.; Noh, S.H.; Han, B. First-Principle-Data-Integrated Machine-Learning Approach for High-Throughput Searching of Ternary Electrocatalyst toward Oxygen Reduction Reaction. Chem. Catal. 2021, 1, 855–869. [Google Scholar] [CrossRef]
- Karim, M.R.; Ferrandon, M.; Medina, S.; Sture, E.; Kariuki, N.; Myers, D.J.; Holby, E.F.; Zelenay, P.; Ahmed, T. Coupling High-Throughput Experiments and Regression Algorithms to Optimize PGM-Free ORR Electrocatalyst Synthesis. ACS Appl. Energy Mater. 2020, 3, 9083–9088. [Google Scholar] [CrossRef]
- Houchins, G.; Viswanathan, V. An Accurate Machine-Learning Calculator for Optimization of Li-Ion Battery Cathodes. J. Chem. Phys. 2020, 153, 054124. [Google Scholar] [CrossRef]
- Attia, P.M.; Grover, A.; Jin, N.; Severson, K.A.; Markov, T.M.; Liao, Y.H.; Chen, M.H.; Cheong, B.; Perkins, N.; Yang, Z.; et al. Closed-Loop Optimization of Fast-Charging Protocols for Batteries with Machine Learning. Nature 2020, 578, 397–402. [Google Scholar] [CrossRef]
- Dave, A.; Mitchell, J.; Kandasamy, K.; Wang, H.; Burke, S.; Paria, B.; Póczos, B.; Whitacre, J.; Viswanathan, V. Autonomous Discovery of Battery Electrolytes with Robotic Experimentation and Machine Learning. Cell Rep. Phys. Sci. 2020, 1, 100264. [Google Scholar] [CrossRef]
- Manna, S.; Roy, D.; Das, S.; Pathak, B. Capacity Prediction of K-Ion Batteries: A Machine Learning Based Approach for High Throughput Screening of Electrode Materials. Mater. Adv. 2022, 3, 7833–7845. [Google Scholar] [CrossRef]
- Benayad, A.; Diddens, D.; Heuer, A.; Krishnamoorthy, A.N.; Maiti, M.; Cras, F.L.; Legallais, M.; Rahmanian, F.; Shin, Y.; Stein, H.; et al. High-Throughput Experimentation and Computational Freeway Lanes for Accelerated Battery Electrolyte and Interface Development Research. Adv. Energy Mater. 2022, 12, 2102678. [Google Scholar] [CrossRef]
- Ong, S.P. Accelerating Materials Science with High-Throughput Computations and Machine Learning. Comput. Mater. Sci. 2019, 161, 143–150. [Google Scholar] [CrossRef]
- Zhou, M.; Gallegos, A.; Liu, K.; Dai, S.; Wu, J. Insights from Machine Learning of Carbon Electrodes for Electric Double Layer Capacitors. Carbon 2020, 157, 147–152. [Google Scholar] [CrossRef]
- Zhou, S.; Shearing, P.R.; Brett, D.J.L.; Jervis, R. Machine Learning as an Online Diagnostic Tool for Proton Exchange Membrane Fuel Cells. Curr. Opin. Electrochem. 2022, 31, 100867. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasubramanian, B.; Rao, R.P.; Chellappan, V.; Ramakrishna, S. Towards Sustainable Fuel Cells and Batteries with an AI Perspective. Sustainability 2022, 14, 16001. https://doi.org/10.3390/su142316001
Ramasubramanian B, Rao RP, Chellappan V, Ramakrishna S. Towards Sustainable Fuel Cells and Batteries with an AI Perspective. Sustainability. 2022; 14(23):16001. https://doi.org/10.3390/su142316001
Chicago/Turabian StyleRamasubramanian, Brindha, Rayavarapu Prasada Rao, Vijila Chellappan, and Seeram Ramakrishna. 2022. "Towards Sustainable Fuel Cells and Batteries with an AI Perspective" Sustainability 14, no. 23: 16001. https://doi.org/10.3390/su142316001
APA StyleRamasubramanian, B., Rao, R. P., Chellappan, V., & Ramakrishna, S. (2022). Towards Sustainable Fuel Cells and Batteries with an AI Perspective. Sustainability, 14(23), 16001. https://doi.org/10.3390/su142316001