Effects of Sleep Deprivation on Functional Connectivity of Brain Regions after High-Intensity Exercise in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Participants
2.1.2. Random Groups
2.2. Research Methods
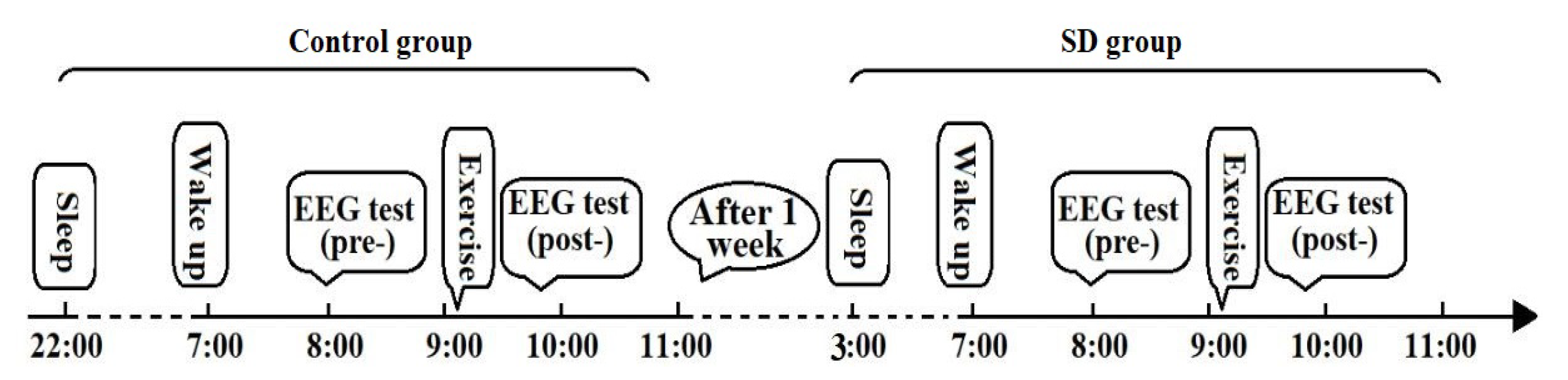
2.2.1. Development of a Sleep-Deprivation Model
2.2.2. Exercise Protocol and EEG Testing
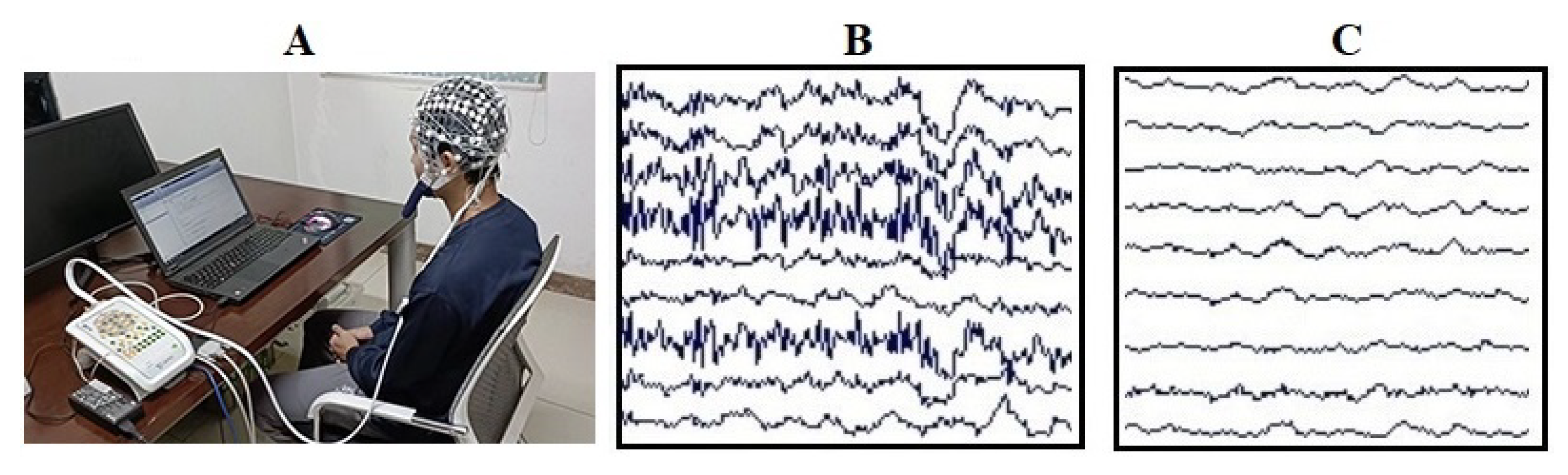
2.2.3. EEG Data Collection and Processing
2.2.4. Mathematical and Statistical Methods
- (1)
- Selection of threshold values
- (2)
- Statistical analysis
3. Results
3.1. Evaluation of the Motion Model
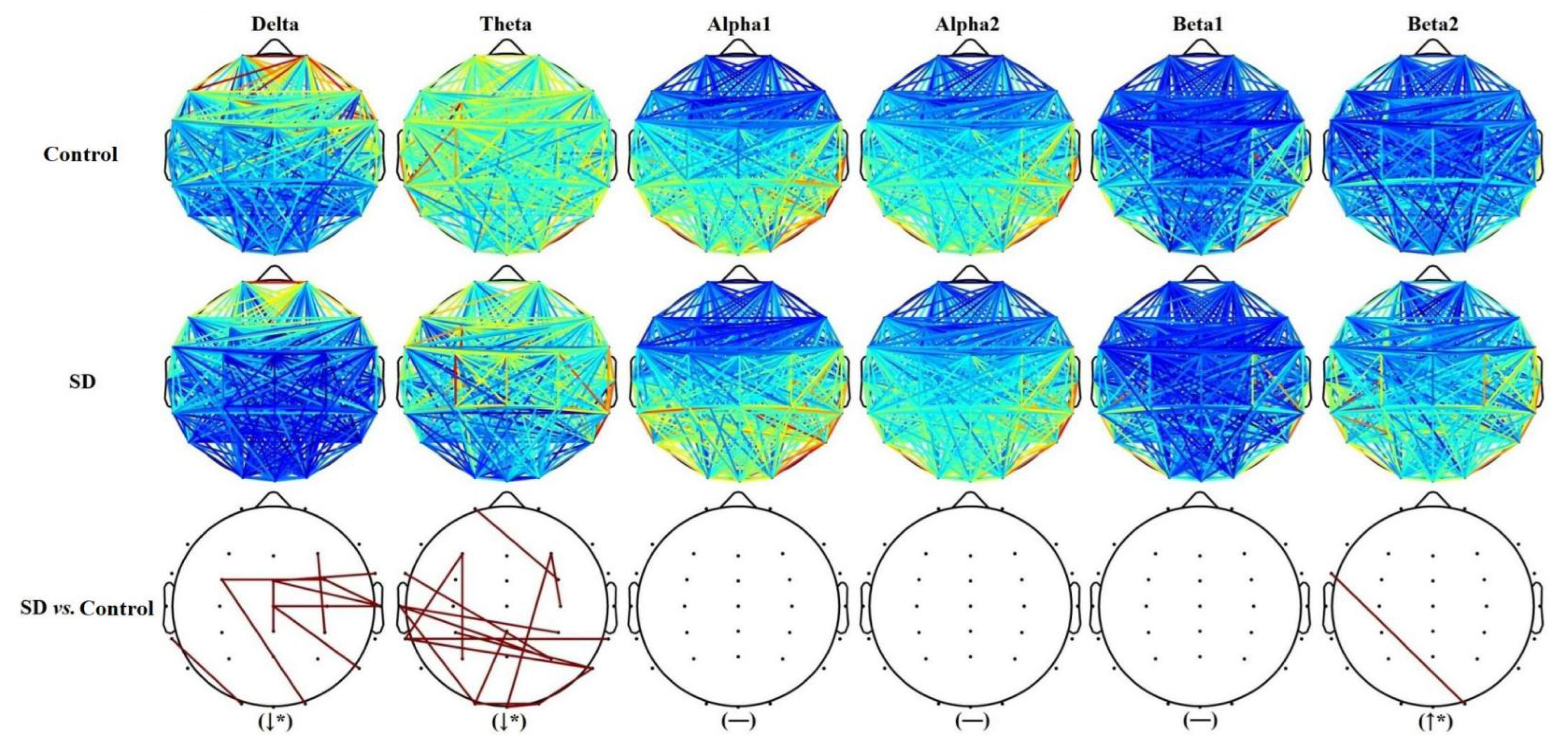
3.2. Results of the Network Distribution Map Comparison
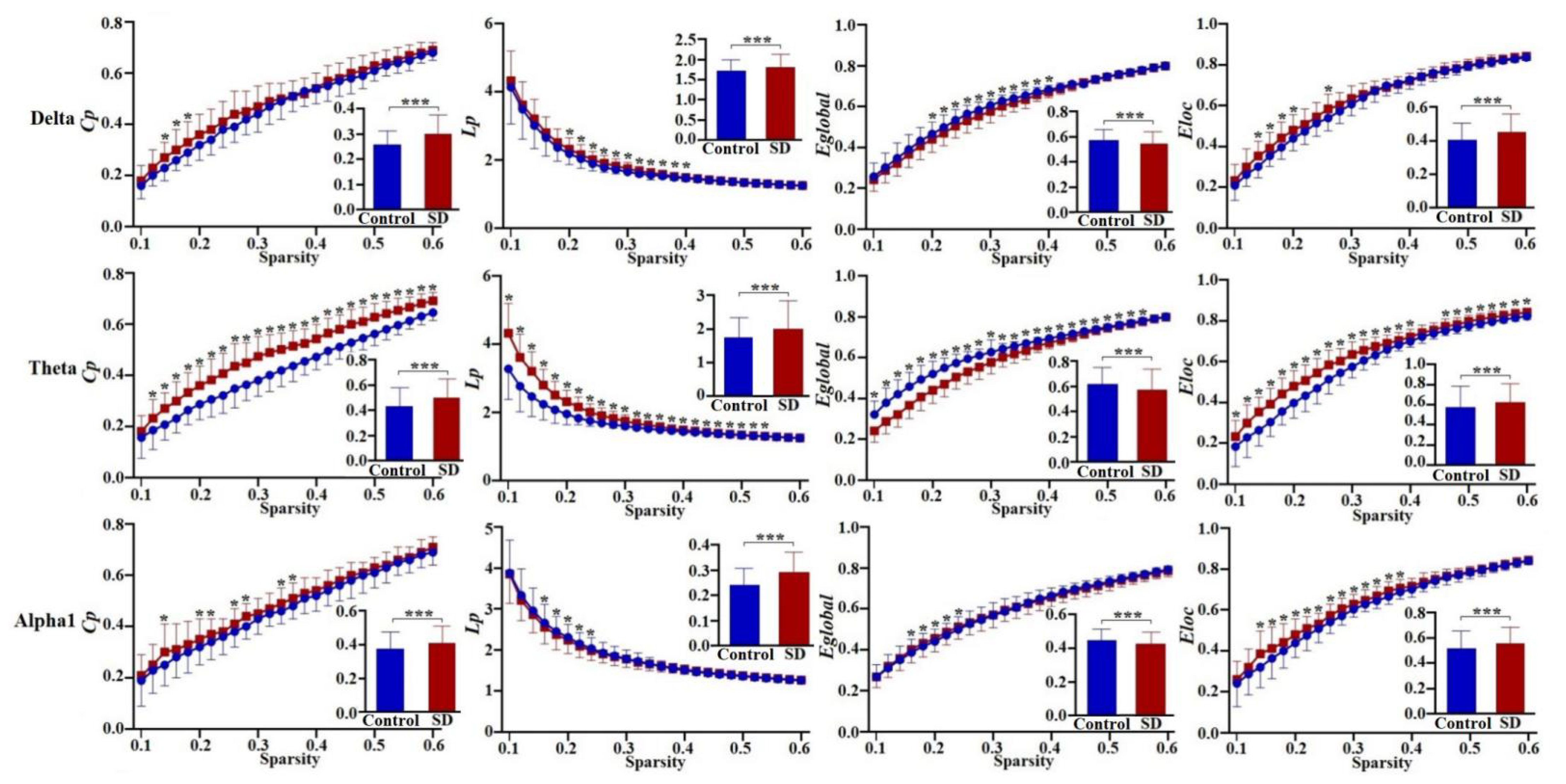
3.3. Results of the Graphical Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullen, T.; Thomas, G.; Wadley, A.J.; Myers, T. The effects of a single night of complete and partial sleep deprivation on physical and cognitive performance: A Bayesian analysis. J. Sport. Sci. 2019, 37, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.S.H.; Teo, W.P.; Aisbett, B.; Warmington, S.A. Extended Sleep Maintains Endurance Performance Better than Normal or Restricted Sleep. Med. Sci. Sport. Exerc. 2019, 51, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukos, E.; Maillis, A.; Papageorgiou, C.; Gatzonis, S.; Stefanis, C.; Angelopoulos, E. The persistent and broadly distributed EEG synchronization might inhibit the normal processing capability of the human brain. Neurosci. Lett. 2015, 609, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Peraza, L.R.; Asghar, A.U.; Green, G.; Halliday, D.M. Volume conduction effects in brain network inference from electroencephalographic recordings using phase lag index. J. Neurosci. Methods 2012, 207, 189–199. [Google Scholar] [CrossRef]
- Chua, B.L.; Dai, Z.; Thakor, N.; Bezerianos, A.; Sun, Y. Connectome pattern alterations with increment of mental fatigue in one-hour driving simulation. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 2017, 4355–4358. [Google Scholar]
- Kar, S.; Routray, A. Effect of Sleep Deprivation on Functional Connectivity of EEG Channels. IEEE Trans. Syst. Man Cybern.-Syst. 2013, 43, 666–672. [Google Scholar] [CrossRef]
- Yang, L.; Lei, Y.; Wang, L.; Chen, P.; Cheng, S.; Chen, S.; Sun, J.; Li, Y.; Wang, Y.; Hu, W.; et al. Abnormal functional connectivity density in sleep-deprived subjects. Brain Imaging Behav. 2018, 12, 1650–1657. [Google Scholar] [CrossRef]
- Ghorbani, M.; Clark, C.C.T. Brain function during central fatigue induced by intermittent high-intensity cycling. Neurol. Sci. 2021, 42, 3655–3661. [Google Scholar] [CrossRef]
- Pi, Y.L.; Wu, X.H.; Wang, F.J.; Liu, K.; Wu, Y.; Zhu, H.; Zhang, J. Motor skill learning induces brain network plasticity: A diffusion-tensor imaging study. PLoS ONE 2019, 14, e0210015. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Wu, C.H.; Karageorghis, C.I.; Wang, C.C.; Chu, C.-H.; Kao, S.-C.; Hung, T.-M.; Chang, Y.-K. Effects of acute aerobic and resistance exercise on executive function: An ERP study. J. Sci. Med. Sport 2019, 22, 1367–1372. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9, 386–401. [Google Scholar] [PubMed] [Green Version]
- Roberts, S.S.H.; Teo, W.P.; Aisbett, B.; Warmington, S.A. Effects of total sleep deprivation on endurance cycling performance and heart rate indices used for monitoring athlete readiness. J. Sport. Sci. 2019, 37, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Abedelmalek, S.; Chtourou, H.; Aloui, A.; Aouichaoui, C.; Souissi, N.; Tabka, Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur. J. Appl. Physiol. 2013, 113, 241–248. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Q.; Li, J.; Chen, Y.; Shao, S.; Xiao, Y. Decreased resting-state alpha-band activation and functional connectivity after sleep deprivation. Sci. Rep. 2021, 11, 484–857. [Google Scholar] [CrossRef]
- Abutalebi, J.; Canini, M.; Della Rosa, P.A.; Green, D.W.; Weekes, B.S. The neuroprotective effects of bilingualism upon the inferior parietal lobule: A Structural Neuroimaging Study in Aging Chinese Bilinguals. J. Neurolinguist. 2015, 33, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Yong, Y.; Jovicich, J.; De Pisapia, N.; Wang, X.; Zuo, C.S.; Levitt, J.J. Static and dynamic posterior cingulate cortex nodal topology of default mode network predicts attention task performance. Brain Imaging Behav. 2015, 10, 212–225. [Google Scholar]
- Shao, Y.; Wang, L.; Ye, E.; Jin, X.; Ni, W.; Yang, Y.; Wen, B.; Hu, D.; Yang, Z. Decreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: Evidence from resting state FMRI. PLoS ONE 2013, 8, e78830–e78835. [Google Scholar] [CrossRef]
- Schmitt, A.; Upadhyay, N.; Martin, J.A.; Rojas, S.; Strüder, H.K.; Boecker, H. Modulation of Distinct Intrinsic Resting State Brain Networks by Acute Exercise Bouts of Differing Intensity. Brain Plast. 2019, 5, 39–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coco, M.; Perciavalle, V.; Cavallari, P.; Perciavalle, V. Effects of an Exhaustive Exercise on Motor Skill Learning and on the Excitability of Primary Motor Cortex and Supplementary Motor Area. Medicine 2016, 95, e2978–e2986. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701–198704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miraglia, F.; Tomino, C.; Vecchio, F.; Gorgoni, M.; De Gennaro, L.; Rossini, P.M. The brain network organization during sleep onset after deprivation. Clin. Neurophysiol. 2021, 132, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. The Research of Brain Functional Network Characteristics under Mental Fatigue Based on EEG; Academy of Military Sciences: Beijing, China, 2018. [Google Scholar]
- Tamburro, G.; di Fronso, S.; Robazza, C.; Bertollo, M.; Comani, S. Modulation of Brain Functional Connectivity and Efficiency During an Endurance Cycling Task: A Source-Level EEG and Graph Theory Approach. Front. Hum. Neurosci. 2020, 14, 243–252. [Google Scholar] [CrossRef]
- Wang, J.; Lu, M.; Fan, Y.; Wen, X.; Zhang, R.; Wang, B.; Ma, Q.; Song, Z.; He, Y.; Wang, J.; et al. Exploring brain functional plasticity in world class gymnasts: A network analysis. Brain Struct. Funct. 2016, 221, 3503–3519. [Google Scholar] [CrossRef]


| Variable | Results |
|---|---|
| Age (years) | 21.97 ± 2.14 |
| Height (Cm) | 178 ± 5.33 |
| Weight (Kg) | 70.21 ± 8.37 |
| BMI (Kg/m2) | 22.02 ± 2.14 |
| Skeletal Muscle (%) | 39.48 ± 3.72 |
| Body Fat (%) | 13.92 ± 4.63 |
| Basal metabolism/d (Kcal) | 1883 ± 187.2 |
| Training time (years) | 3.52 ± 0.45 |
| Parameters | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Level 6 | Level 7 |
|---|---|---|---|---|---|---|---|
| Speed (km/h) | 2.7 | 4.0 | 5.4 | 6.7 | 8.0 | 8.8 | 9.6 |
| Slope (%) | 10 | 12 | 14 | 16 | 18 | 20 | 22 |
| Duration (min) | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Sleep |
Sleep Time (h) | Condition of Exerciser Termination |
Exercise Duration (min) | ||||
|---|---|---|---|---|---|---|---|
| Behavior | SBP (mmHg) | DBP (mmHg) | HRmax | RPE > 18 | |||
| Control | ≥7 h | Breath difficulty | 156.47 ± 18.56 | 77.56 ± 14.12 | 189.47 ± 12.35 | 19.24 ± 1.15 | 19.20 ± 3.07 |
| SD | <4 h | Breath difficulty | 159.71 ± 27.45 | 87.77 ± 25.12 | 196.85 ± 17.48 | 19.93 ± 0.90 | 16.95 ± 2.77 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Chi, P.; Song, J.; Pang, Y.; Wu, Q.; Liu, Y.; Chi, A. Effects of Sleep Deprivation on Functional Connectivity of Brain Regions after High-Intensity Exercise in Adolescents. Sustainability 2022, 14, 16175. https://doi.org/10.3390/su142316175
Niu X, Chi P, Song J, Pang Y, Wu Q, Liu Y, Chi A. Effects of Sleep Deprivation on Functional Connectivity of Brain Regions after High-Intensity Exercise in Adolescents. Sustainability. 2022; 14(23):16175. https://doi.org/10.3390/su142316175
Chicago/Turabian StyleNiu, Xiaodan, Puyan Chi, Jing Song, Yaohui Pang, Qianqian Wu, Yang Liu, and Aiping Chi. 2022. "Effects of Sleep Deprivation on Functional Connectivity of Brain Regions after High-Intensity Exercise in Adolescents" Sustainability 14, no. 23: 16175. https://doi.org/10.3390/su142316175
APA StyleNiu, X., Chi, P., Song, J., Pang, Y., Wu, Q., Liu, Y., & Chi, A. (2022). Effects of Sleep Deprivation on Functional Connectivity of Brain Regions after High-Intensity Exercise in Adolescents. Sustainability, 14(23), 16175. https://doi.org/10.3390/su142316175








