A Circular Economy Model to Improve Phosphate Rock Fertiliser Using Agro-Food By-Products
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Hellal, F.; El-Sayed, S.; Zewainy, R.; Amer, A. Importance of phosphate pock application for sustaining agricultural production in Egypt. Bull. Natl. Res. Cent. 2019, 43, 11. [Google Scholar] [CrossRef]
- Hiddink, J.G.; Kaiser, M.J. Implications of Liebig’s Law of the Minimum for the Use of Ecological Indicators Based on Abundance. Ecography 2005, 28, 264–271. [Google Scholar] [CrossRef]
- Wang, J.; Baerenklau, K.A. Crop response functions integrating water, nitrogen, and salinity. Agric. Water Manag. 2014, 139, 17–30. [Google Scholar] [CrossRef]
- Ferreira, I.E.P.; Zocchi, S.S.; Baron, D. Reconciling the Mitscherlich’s law of diminishing returns with Liebig’s law of the minimum. Some results on crop modeling. Math. Biosci. 2017, 293, 29–37. [Google Scholar] [CrossRef]
- Rogers, D.; Weaver, D.; Summers, R.; Dobbe, E.; Master, R.; McFerran, R.; Mussell, G.; Dawson, L.; Mercy, J.; Richards, P.; et al. Critical phosphorus values from the Better Fertiliser Decisions for Pastures project: Early insights from validation trials. Crop Pasture Sci. 2021, 72, 731–741. [Google Scholar] [CrossRef]
- Withers, P.J.; Sylvester-Bradley, R.; Jones, D.L.; Healey, J.R.; Talboys, P.J. Feed the crop not the soil: Rethinking phosphorus management in the food chain. Environ. Sci. Technol. 2014, 48, 6523–6530. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Lin, S.S.; Shen, S.L.; Zhou, A.; Lyu, H.A. Assessment and management of lake eutrophication: A case study in Lake Erhai, China. Sci. Total Environ. 2021, 751, 141618. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef]
- Approaching peak phosphorus. Nat. Plants 2022, 8, 979. [CrossRef]
- Urrutia, O.; Javier Erro, J.; Guardado, I.; San Francisco, S.; Mandado, M.; Baigorri, R.; Yvin, J.C.; Garcia-Mina, J.M. Physico-chemical characterization of humic-metal-phosphate complexes and their potential application to the manufacture of new types of phosphate-based fertilizers. J. Plant Nutr. Soil Sci. 2013, 1–9. [Google Scholar] [CrossRef]
- Erro, J.; Urrutia, O.; Baigorri, R.; Aparicio-Tejo, P.; Irigoyen, I.; Torino, F.; Mandado, M.; Yvin, J.C.; Garcia-Mina, J.M. Organic Complexed Superphosphates (CSP): Physicochemical Characterization and Agronomical Properties. J. Agric. Food Chem. 2008, 60, 2008–2017. [Google Scholar] [CrossRef]
- Rafael, R.B.A.; Fernández-Marcos, M.L.; Cocco, S.; Ruello, M.L.; Weindorf, D.C.; Cardelli, V.; Corti, G. Assessment of potential nutrient release from phosphate rock and dolostone for application in acid soils. Pedosphere 2018, 28, 44–58. [Google Scholar] [CrossRef]
- Munir, A.; Adel, G.; Saud, S.A.O.; Khaled, D.A.; Mahmoud, N. Acidulated activation of phosphate rock enhances release, lateral transport and uptake of phosphorus and trace metals upon direct-soil application. Soil Sci. Plant Nutr. 2019, 65, 183–195. [Google Scholar] [CrossRef]
- Powers, S.M.; Chowdhury, R.B.; MacDonald, G.K.; Metson, G.S.; Beusen, A.H.W.; Bouwman, A.F.; Hampton, S.E.; Mayer, B.K.; McCrackin, M.L.; Vaccari, D.A. Global opportunities to increase agricultural independence through phosphorus recycling. Earth’s Future 2019, 7, 370–383. [Google Scholar] [CrossRef]
- UN. Make the SDGs a Reality. Department of Economic and Social Affairs of the United Nations (UN). Available online: https://sdgs.un.org/goals (accessed on 15 October 2022).
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Doody, D.G.; Sylvester-Bradley, R. Achieving Sustainable Phosphorus Use in Food Systems through Circularisation. Sustainability 2018, 10, 1804. [Google Scholar] [CrossRef]
- El Chami, D.; Daccache, A.; El Moujabber, M. How can sustainable agriculture increase climate resilience? A systematic review. Sustainability 2020, 12, 3119. [Google Scholar] [CrossRef]
- El Chami, D. Towards sustainable organic farming systems. Sustainability 2020, 12, 9832. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanisławska-Glubiak, E.; Hoffmann, J.; Górecka, H.; Jóźwiak, W.; Wiśniewska, G. Improvement of the solubility of rock phosphate by co-composting it with organic components. Pol. J. Chem. Technol. 2013, 15, 10–14. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Ceglie, F.G.; Aly, A.; Mihreteab, H.T.; Ciaccia, C.; Tittarelli, F. Phosphorus availability from rock phosphate: Combined effect of green waste composting and sulfur addition. J. Environ. Manag. 2016, 182, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Grant, P.; Biron, P.; Bariac, T.; Cartes, P.; Mora, M.d.L.L.; Rumpel, C. Synergistic and Antagonistic Effects of Poultry Manure and Phosphate Rock on Soil P Availability, Ryegrass Production, and P Uptake. Agronomy 2019, 9, 191. [Google Scholar] [CrossRef]
- Sabah, N.U.; Tahir, M.A.; Sarwar, G.; Luqman, M.; Aziz, A.; Manzoor, M.Z.; Aftab, M. Biosolubilization of phosphate rock using organic amendments: An innovative approach for sustainable maize production in Aridisols—A review. Sarhad J. Agric. 2022, 38, 617–625. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 2, 971. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.; Khan, M.; Bano, A.; Nisa, S.; Hussain, A.; Dawar, K.M.; Munir, A.; Khan, N. Rock Phosphate-Enriched Compost in Combination with Rhizobacteria; A Cost-Effective Source for Better Soil Health and Wheat (Triticum aestivum) Productivity. Agronomy 2020, 10, 1390. [Google Scholar] [CrossRef]
- Barin, M.; Asadzadeh, F.; Hosseini, M.; Hammer, E.C.; Vetukuri, R.R.; Vahedi, R. Optimization of Biofertilizer Formulation for Phosphorus Solubilizing by Pseudomonas fluorescens Ur21 via Response Surface Methodology. Processes 2022, 10, 650. [Google Scholar] [CrossRef]
- Santos, W.O.; Hesterberg, D.; Mattiello, E.M.; Vergütz, L.; Barreto, M.S.; Silva, I.R.; Souza Filho, L.F. Increasing Soluble Phosphate Species by Treatment of Phosphate Rocks with Acidic Waste. J. Environ. Qual. 2016, 45, 1988–1997. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Li, Y.; Hu, C.; He, Z.-Q.; Wen, M.-X.; Gai, G.-S.; Huang, Z.-H.; Yang, Y.-F.; Hao, X.-Y.; Li, X.-Y. Enhanced Phosphorus Release from Phosphate Rock Activated with Lignite by Mechanical Microcrystallization: Effects of Several Typical Grinding Parameters. Sustainability 2019, 11, 1068. [Google Scholar] [CrossRef]
- Teles, A.P.B.; Rodrigues, M.; Pavinato, P.S. Solubility and Efficiency of Rock Phosphate Fertilizers Partially Acidulated with Zeolite and Pillared Clay as Additives. Agronomy 2020, 10, 918. [Google Scholar] [CrossRef]
- Avşar, C. A novel assessment strategy for nanotechnology in agriculture: Evaluation of nanohydroxyapatite as an alternative phosphorus fertiliser. Kem. Ind. 2022, 71, 327–334. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Nesme, T.; Colomb, B.; Hinsinger, P.; Watson, C.A. Soil phosphorus management in organic cropping systems: From current practices to avenues for a more efficient use of P resources. In Organic Farming, Prototype for Sustainable Agricultures; Bellon, S., Penvern, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 23–45. [Google Scholar] [CrossRef]
- Kneese, A.V. The Economics of Natural Resources. Popul. Dev. Rev. 1988, 14, 281–309. [Google Scholar] [CrossRef]
- Pearce, D.W.; Turner, R.K. Economics of Natural Resources and the Environment; Johns Hopkins University Press: Baltimore, MD, USA, 1989; 392p. [Google Scholar]
- EPA. The National Recycling Strategy: Part One of a Series on Building a Circular Economy. Available online: https://www.epa.gov/recyclingstrategy/national-recycling-strategy (accessed on 15 October 2022).
- EC. Circular Economy Action Plan: For a Cleaner and More Competitive Europe. The European Commission (EC). Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 15 October 2022).
- Zhu, J.; Fan, C.; Shi, H.; Shi, L. Efforts for a Circular Economy in China: A Comprehensive Review of Policies. J. Ind. Ecol. 2019, 23, 110–118. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Patsios, S.I.; Kontogiannopoulos, K.N.; Mitrouli, S.; Plakas, K.V.; Karabelas, A.J. Characterisation of agricultural waste co- and by-products. Agrocycle-EU Horizon 2020 2016. [Google Scholar]
- Piscitelli, L.; Colovic, M.; Aly, A.; Hamze, M.; Todorovic, M.; Cantore, V.; Albrizio, R. AdaptiveAgricultural Strategies for FacingWater Deficit in Sweet MaizeProduction: A Case Study of a Semi-Arid Mediterranean Region. Water 2021, 13, 3285. [Google Scholar] [CrossRef]
- UNI CEN/TS 15370-1:2006; Solid Biofuels—Method for the Determination of Ash Melting Behaviour—Part 1: Characteristic Temperatures Method. CEN: Brussels, Belgium, 2006.
- ISO—15958; Fertilizers—Extraction of Water-Soluble Phosphorus. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/obp/ui/#iso:std:iso:5316:ed-1:v1:en (accessed on 3 March 2019).
- Chanalia, P.; Gandhi, D.; Anjana, B.S.; Singh, J.; Dhanda, S. Antioxidant activity and nutritional value of Citrus limetta and Ananas comosus pomace. J. Food Sci. Nutr. Ther. 2018, 4, 004–007. [Google Scholar] [CrossRef]
- Solé, M.M.; Pons, L.; Conde, M.; Gaidau, C.; Bacardit, A. Characterization of Wet Olive Pomace Waste as Bio-Based Resource for Leather Tanning. Materials 2021, 14, 5790. [Google Scholar] [CrossRef]
- Tumbure, A.; Bretherton, M.; Bishop, P.; Hedley, M. Phosphorus recovery from an igneous phosphate rock using organic acids and pyrolysis condensate. Sci. Afr. 2022, 15, e01098. [Google Scholar] [CrossRef]
- Zukswert, J.; Prescott, C. Relationships among leaf functional traits, litter traits, and mass loss during early phases of leaf litter decomposition in 12 woody plant species. Oecologia 2017, 185, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Cequier, E.; Aguilera, J.; Balcells, M.; Canela-Garayoa, R. Extraction and characterization of lignin from olive pomace: A comparison study among ionic liquid, sulfuric acid, and alkaline treatments. Biomass Convers. Biorefinery 2019, 9, 241–252. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Guizani, C.; Ouederni, A.; Limousy, L. Pyrolysis of Olive Pomace: Degradation Kinetics, Gaseous Analysis and Char Characterization. Waste Biomass Valoris. 2017, 8, 1689–1697. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, X.; Deuss, P.J. The effect of ball milling on birch, pine, reed, walnut shell enzymatic hydrolysis recalcitrance and the structure of the isolated residual enzyme lignin. Ind. Crops Prod. 2021, 167, 113493. [Google Scholar] [CrossRef]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Meyer, A.S.; Raj, A. Bioremediation of lignin derivatives and phenolics in wastewater with lignin modifying enzymes: Status, opportunities and challenges. Sci. Total Environ. 2021, 777, 145988. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The role of microorganisms on biotransformation of brewers’ spent grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef]
- Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers 2021, 13, 1280. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Fang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 2019, 10, 5112. [Google Scholar] [CrossRef]
- Pérez, P.; Barro, R.; Pérez, J.; Fernández, M.J.; Moyano, A.; Ciria, P. Nutrient Release through Litterfall in Short Rotation Poplar Crops in Mediterranean Marginal Land. Forests 2021, 12, 1185. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Mahsefat, M.; Farhangi, M.B.; Khalili Rad, M.; Proietti, P. Short-term impacts of pomace application and Pseudomonas bacteria on soil available phosphorus. Biocatal. Agric. Biotechnol. 2020, 28, 101742. [Google Scholar] [CrossRef]
- Jamal, A.; Khan, A.; Sharif, M.; Jamal, H. Application of Different Organic Acids on Phosphorus Solubility from Rock Phosphate. J. Hortic. Plant Res. 2018, 2, 43–48. [Google Scholar] [CrossRef]
- Huang, L.; Mao, X.; Wang, J.; Chen, X.Y.; Wang, G.; Liao, Z. The effect and mechanism of improved efficiency of physicochemical pro-release treatment for low-grade phosphate rock. J. Soil Sci. Plant Nutr. 2014, 14, 316–331. [Google Scholar] [CrossRef]
- Idrissi, H.; Taha, Y.; Elghali, A.; El Khessaimi, Y.; Aboulayt, A.; Amalik, J.; Hakkou, R.; Benzaazoua, M. Sustainable use of phosphate waste rocks: From characterization to potential applications. Mater. Chem. Phys. 2021, 260, 124119. [Google Scholar] [CrossRef]
- Wu, J.; Hartmann, T.H.; Chen, W.S. Toward sustainable management of phosphorus flows in a changing rural–urban environment: Recent advances, challenges, and opportunities. Curr. Opin. Environ. Sustain. 2019, 40, 81–87. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.O.; Gunes, B. Waste biomass valorization for the production of biofuels and value-added products: A comprehensive review of thermochemical, biological and integrated processes. Process Saf. Environ. Prot. 2022, 159, 323–344. [Google Scholar] [CrossRef]


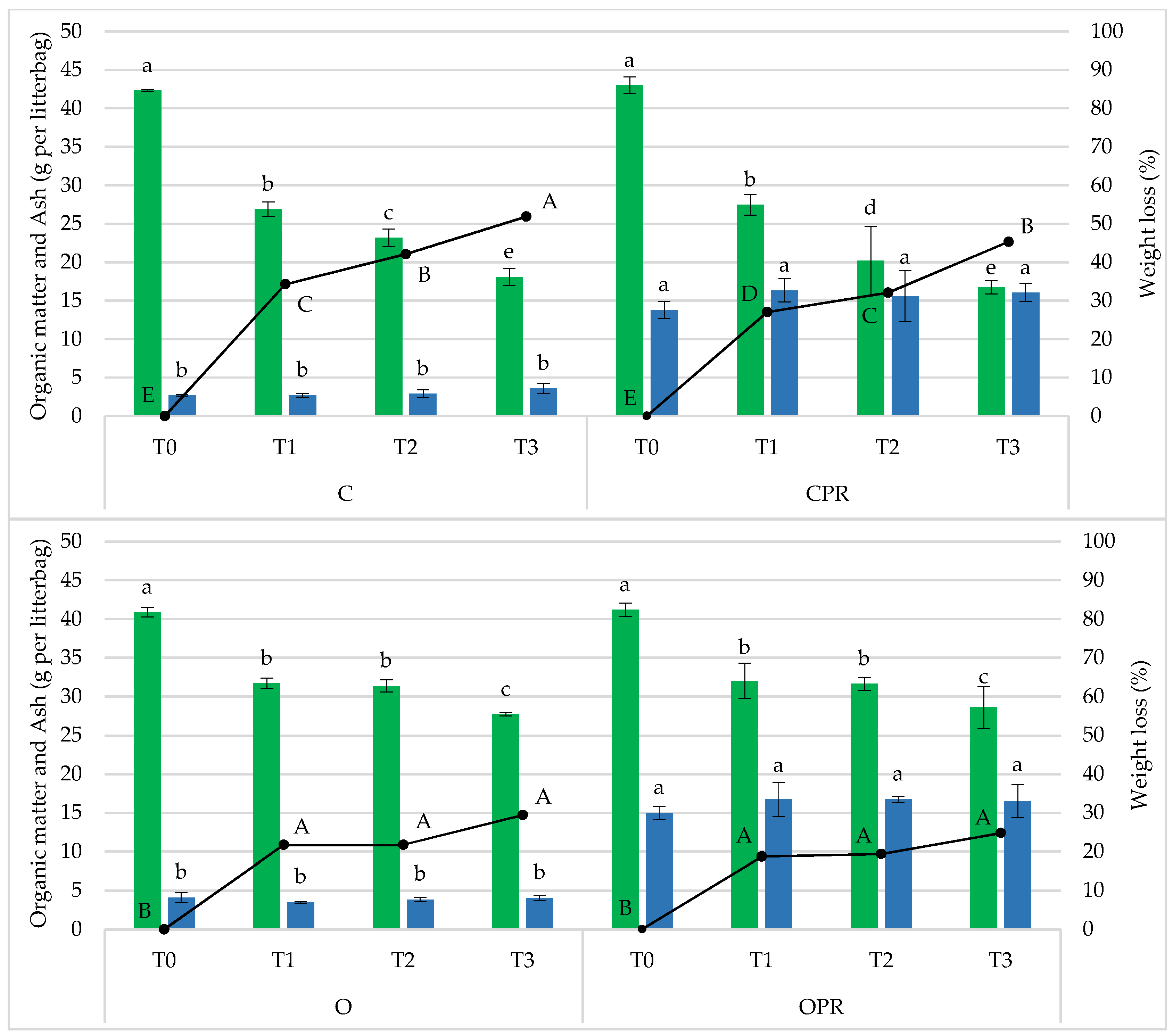


| B | C | O | ||
|---|---|---|---|---|
| pH | H2O | 5.3 ± 0.1 | 3.4 ± 0.1 | 5.5 ± 0.1 |
| Organic Matter | % | 96.0 ± 0.1 | 89.0 ± 0.2 | 94.0 ± 0.2 |
| Ash | % | 4.2 ± 0.1 | 11.2 ± 0.2 | 6.2 ± 0.1 |
| Total P | g/kg | 12.8 ± 0.4 | 64.6 ± 5.7 | 6.3 ± 0.6 |
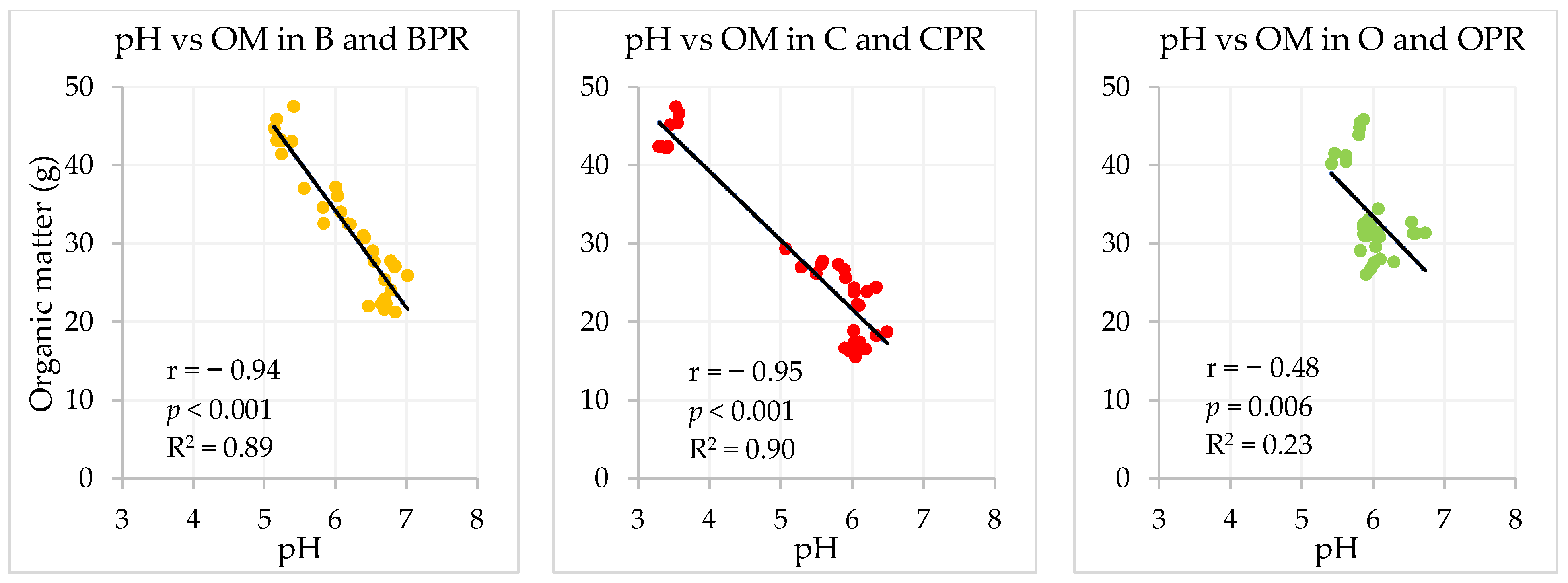
| pH vs. WSP | pH vs. TP | WSP vs. TP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BPR | CPR | OPR | BPR | CPR | OPR | BPR | CPR | OPR | |
| r | −0.85 | −0.99 | −0.58 | −0.86 | −0.97 | −0.58 | 0.88 | 0.98 | 0.97 |
| p | <0.001 | <0.001 | 0.019 | <0.001 | <0.001 | 0.019 | <0.001 | <0.001 | <0.001 |
| R2 | 0.72 | 0.97 | 0.33 | 0.74 | 0.93 | 0.33 | 0.77 | 0.97 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscitelli, L.; Bennani, Z.; El Chami, D.; Mondelli, D. A Circular Economy Model to Improve Phosphate Rock Fertiliser Using Agro-Food By-Products. Sustainability 2022, 14, 16228. https://doi.org/10.3390/su142316228
Piscitelli L, Bennani Z, El Chami D, Mondelli D. A Circular Economy Model to Improve Phosphate Rock Fertiliser Using Agro-Food By-Products. Sustainability. 2022; 14(23):16228. https://doi.org/10.3390/su142316228
Chicago/Turabian StylePiscitelli, Lea, Zineb Bennani, Daniel El Chami, and Donato Mondelli. 2022. "A Circular Economy Model to Improve Phosphate Rock Fertiliser Using Agro-Food By-Products" Sustainability 14, no. 23: 16228. https://doi.org/10.3390/su142316228
APA StylePiscitelli, L., Bennani, Z., El Chami, D., & Mondelli, D. (2022). A Circular Economy Model to Improve Phosphate Rock Fertiliser Using Agro-Food By-Products. Sustainability, 14(23), 16228. https://doi.org/10.3390/su142316228







