Effects of Exogenous Bacterial Agents on Material Transformation and Microbial Community Composition during Composting of Tomato Stalks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Activation of Microbial Agents
2.2. Composting Process and Sample Collection
2.3. Determination of Physical and Chemical Indicators
2.3.1. Determination of Moisture Content, pH and EC
2.3.2. Determination of Nutrient Content
2.3.3. Determination of Cellulose Content
2.3.4. Determination of Germination Index
2.4. DNA Extraction, PCR Amplification and High-Throughput Sequencing
2.5. Processing of Sequencing Data and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Changes in Physicochemical Parameters during the Composting Process
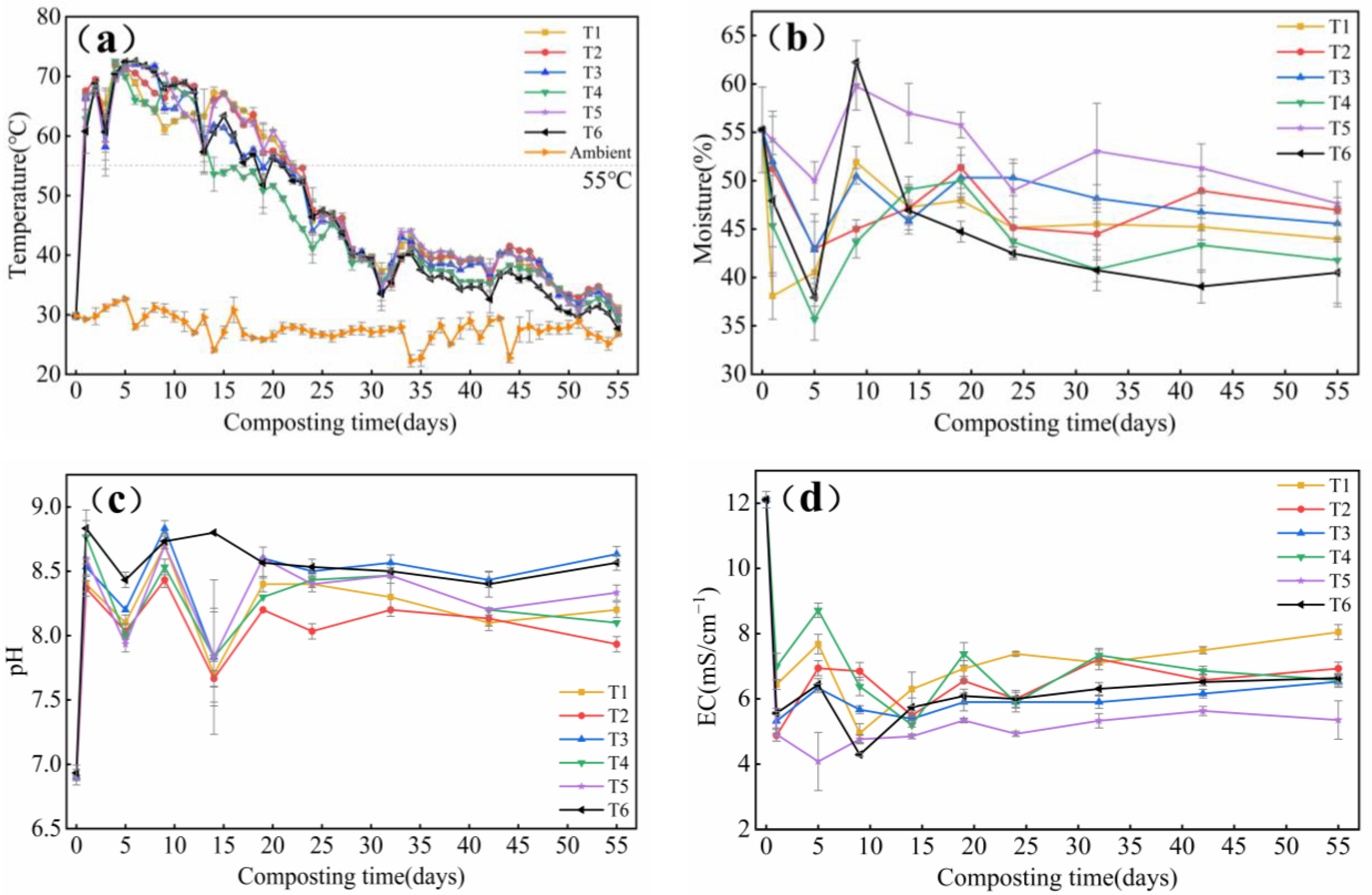
3.1.1. Temperature and Water Content
3.1.2. Changes in pH and EC
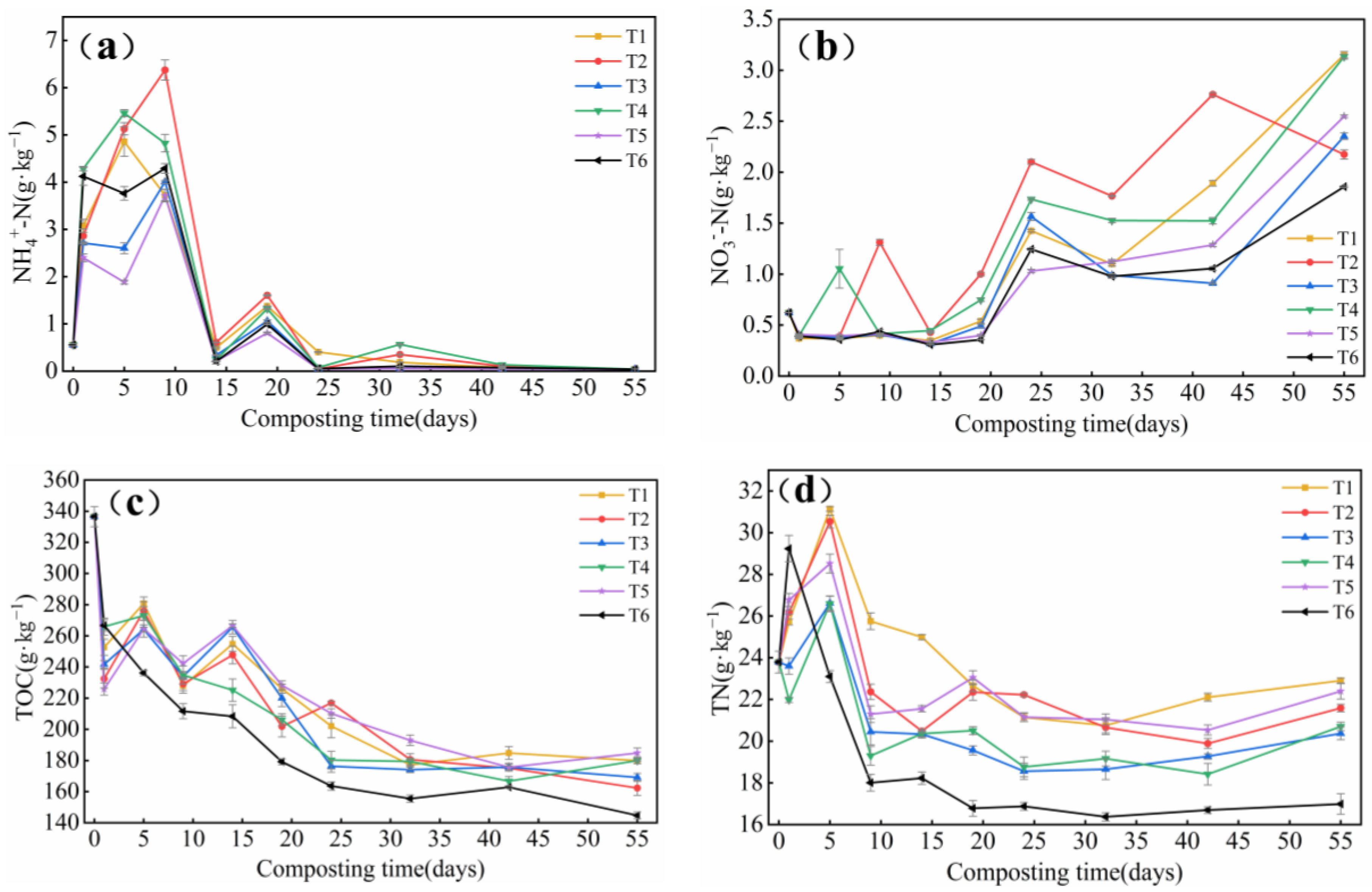
3.1.3. Changes in NH4+-N, NO3−-N, TOC and TN Content
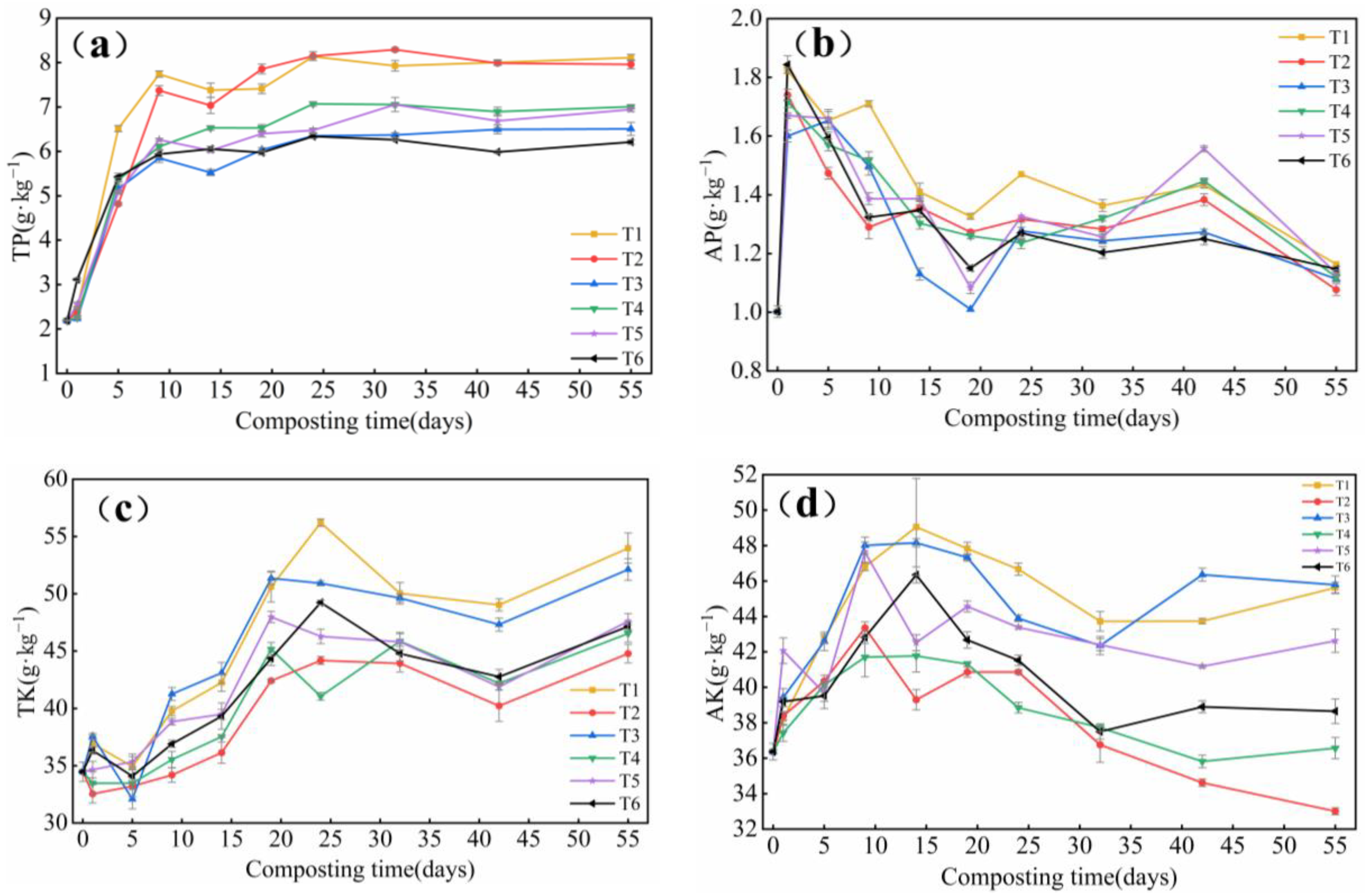
3.1.4. Changes in TP, AP, TK and AK Content
3.1.5. Changes in Germination Index and Cellulose Content
4. Microbial Community Succession in Composting Process
4.1. Alpha Diversity Analysis of Microbial Communities
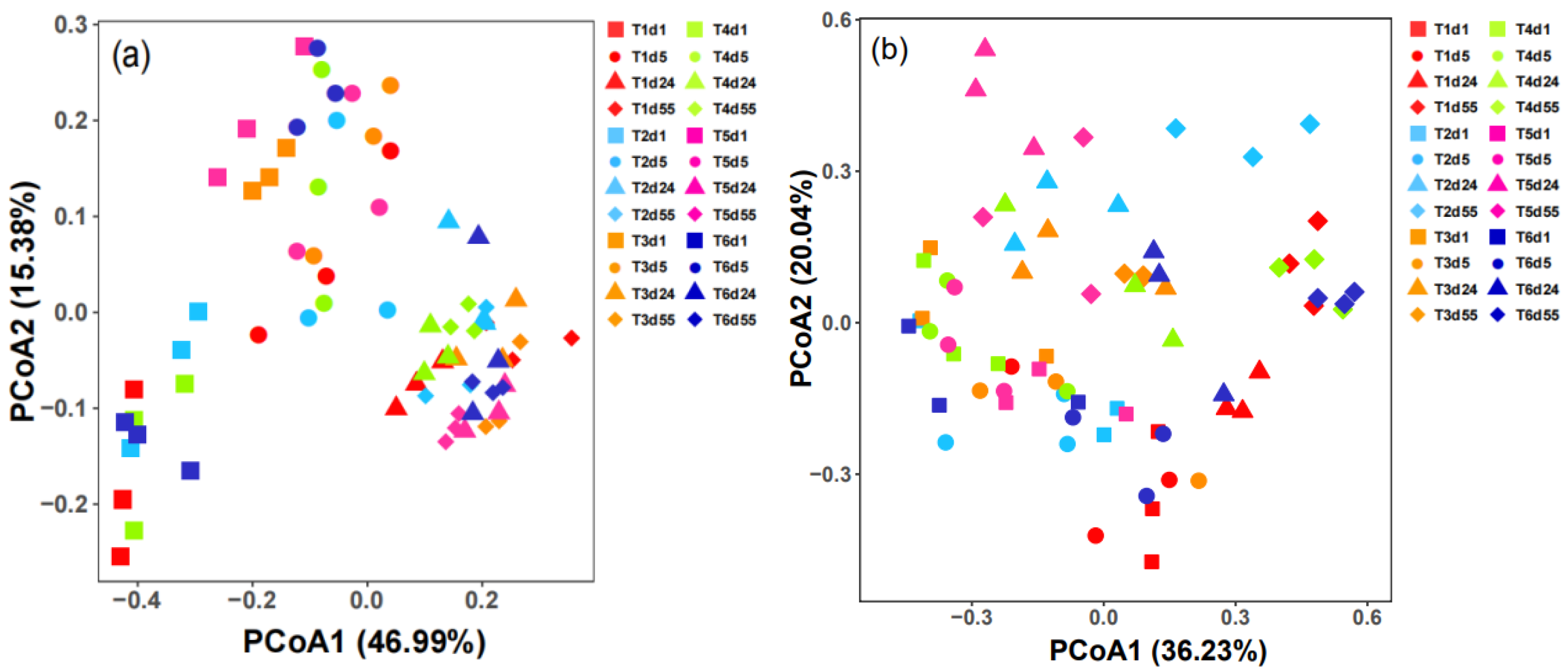
4.2. Beta Diversity of Microbial Communities
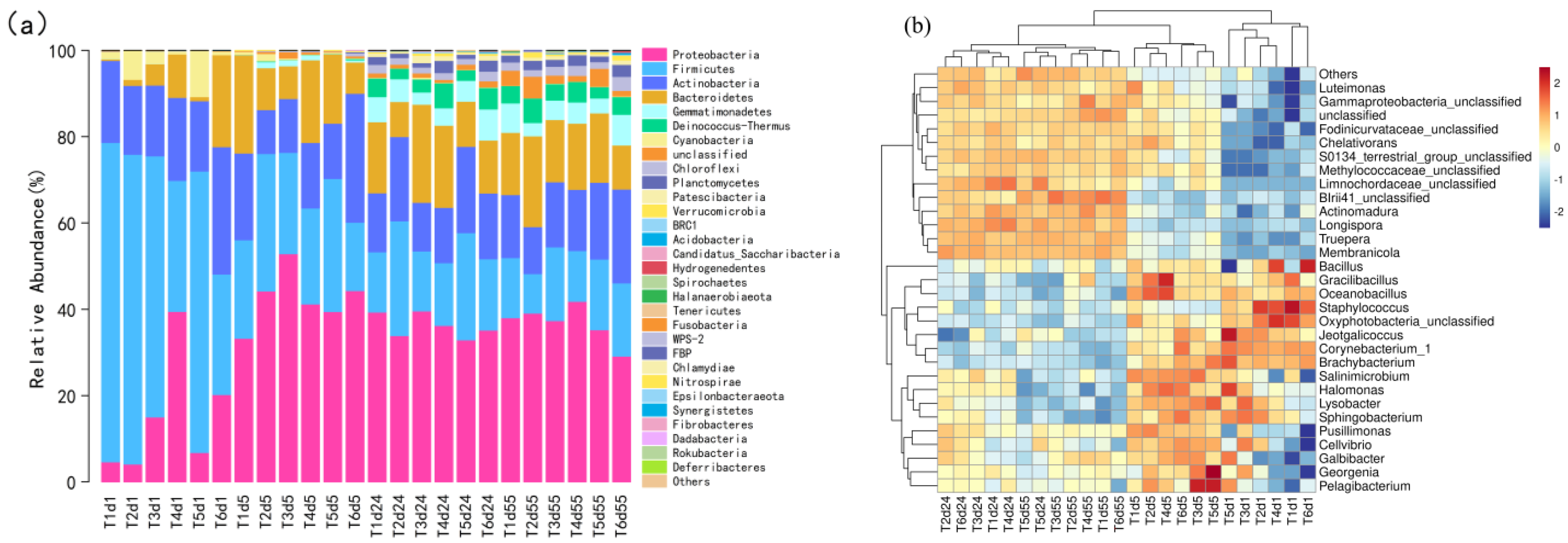
4.3. Changes in Bacterial Community Structure during the Composting Process
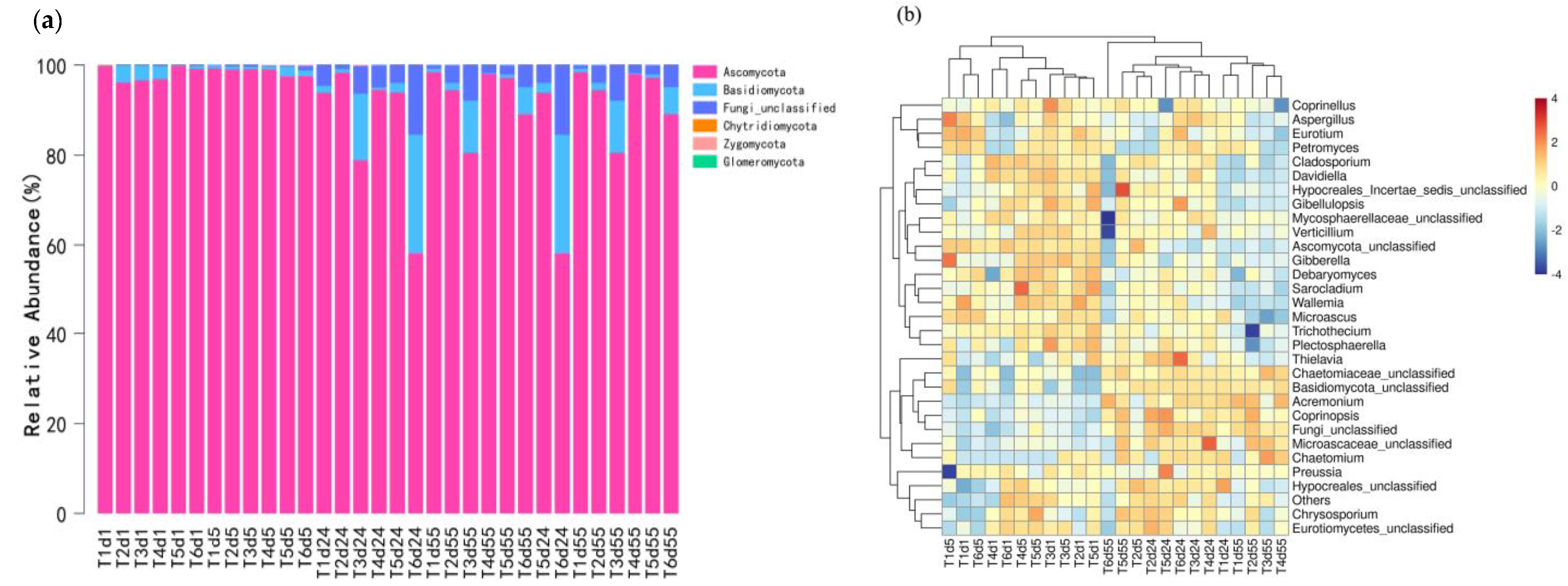
4.4. Changes in the Structure of Fungal Communities during Composting
5. Correlation between Physical and Chemical Indicators and Microorganisms
Correlation of Compost Physicochemical Indicators with Bacteria and Fungi
6. Discussion
6.1. Influence of Inoculants on Physicochemical Parameters of Compost
6.2. Effect of Inoculants on Microbial Community Composition in Composting Process
6.3. Correlation between Physical and Chemical Indicators of Compost and Microorganisms
7. Conclusions
- In this study, it was found that the pile temperature increased rapidly in the 1st d after inoculation with the fungicide, and compared with T6, T1, T2 and T5 could effectively extend the high-temperature period, effectively killing harmful substances and weed seeds in the compost and realizing the resource utilization of tomato stalks.
- The degradation rate of TOC in T6 reached 57.0%, which was significantly different from other treatments. The TN content of each treatment showed different degrees of reduction. the NH4+-N content in T1 was reduced by 92.3% compared with the initial one, and the difference was not significant compared with T6. After inoculation, TP content in T1 treatment was significantly higher (p < 0.05) especially in T1, AP content in T1 increased by 16.0%, significantly higher than T2 (p < 0.05), but not significantly different from T6. TK content in T1 increased by 56.6%, significantly higher than the T6 treatment (p < 0.05). The differences were not significant but were significantly higher than the other inoculated groups. The GI at the end of composting was more than 80%, so it can be said that the compost reached the standard of decomposition.
- The results of α and β diversity analysis showed that the bacterial α diversity index was significantly higher than that of fungi in the tomato stalk composting system, and the Chao1 and Shannon indices gradually increased in T1, T2 and T5 in the bacterial community, and the Chao1 and Shannon indices in T3, T4 and T6 were the largest in the cooling stage. The Chao1 index in the fungal community was greatest at 55 d in T1, T2 and T5 treatments, and at 24 d in T3, T4 and T6, while the Shannon index was highest at the cooling stage in each treatment. T1 treatment significantly increased the abundance and diversity of the bacterial and fungal communities and improved the composition of the microbial community. Both bacteria and fungi had significant temporal succession patterns, and bacterial communities were more closely aggregated among samples at different periods of each treatment, while fungal communities were relatively dispersed among them, indicating that inoculation had a greater effect on fungal communities.
- Proteobacteria, Firmicutes, Actinomycetes and Bacteroides are the four most abundant bacterial phyla. T1 significantly increases the abundance of Proteobacteria at the high-temperature stage and decreases the abundance of Staphylococcus. T5 significantly increased the relative abundance of Lysobacter in a high-temperature period. Ascomycetes is the absolute dominant phylum during composting. The abundance of Basidiomycetes in the T2 and T5 cooling stages increased significantly, and T2 and T3 treatments significantly reduced the relative abundance of pathogenic fungi.
- The results of RDA and Spearman correlation analysis showed that Cellulose, AP, T, TP, NH4+-N and NO3−-N were the six physicochemical parameters that significantly affected the structure of bacterial communities, and T and GI significantly affected the two physicochemical parameters of fungal communities.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/ (accessed on 24 June 2022).
- Zhang, X.M.; Zhu, Y.; Li, J.L.; Zhu, P.C.; Liang, B. Exploring dynamics and associations of dominant lignocellulose degraders in tomato stalk composting. J. Environ. Manag. 2021, 294, 113162. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Domestic waste composting facilities: A review of human health risks. Environ Int. 2009, 35, 382–389. [Google Scholar] [CrossRef]
- Taha, I.; Elkafafy, M.S.; Mously, H.E. Potential of utilizing tomato stalk as raw material for particleboards. Ain Shams Eng. J. 2018, 9, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Alavi, N.; Daneshpajou, M.; Shirmardi, M.; Goudarzi, G.; Neisi, A.; Babaei, A.A. Investigating the efficiency of co-composting and vermicomposting of vinasse with the mixture of cow manure wastes, bagasse, and natural zeolite. Waste Manag. 2017, 69, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, M.; Pandit, P.; Khardenavis, A.; Singh, D.; Purohit, H. Study of microbial community plasticity for anaerobic digestion of vegetable waste in anaerobic baffled reactor. Renew. Energy 2017, 101, 59–66. [Google Scholar] [CrossRef]
- Yogev, A.; Raviv, M.; Hadar, Y.; Cohen, R.; Wolf, S.; Gil, L.; Katan, J. Induced resistance as a putative component of compost suppressiveness. Biol. Control 2010, 54, 46–51. [Google Scholar] [CrossRef]
- Cai, Y.Y.; He, Y.H.; He, K.; Gao, H.J.; Ren, M.J.; Qu, G. Degradation mechanism of lignocellulose in dairy cattle manure with the addition of calcium oxide and superphosphate. Environ. Sci. Pollut. Res. 2019, 26, 33683–33693. [Google Scholar] [CrossRef]
- Pan, J.T.; Cai, H.Z.; Zhang, Z.Q.; Liu, H.B.; Mao, H.; Awasthi, M.K.; Wang, Q.; Zhai, L.M. Comparative evaluation of the use of acidic additives on sewage sludge composting quality improvement, nitrogen conservation, and greenhouse gas reduction. Bioresour. Technol. 2018, 270, 467–475. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Zhang, Z.Q.; Wang, Q.; Shen, F.; Li, R.H.; Li, D.S.; Ren, X.N.; Wang, M.J.; Chen, H.Y.; Zhao, J.C. New insight with the effects of biochar amendment on bacterial diversity as indicators of biomarkers support the thermophilic phase during sewage sludge composting. Bioresour. Technol. 2017, 238, 589–601. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Q.; Wei, Y.Q.; Cui, H.Y.; Zhang, X.; Wang, X.; Shan, S.; Wei, Z.M. Effect of actinobacteria agent inoculation methods on cellulose degradation during composting based on redundancy analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Fan, B.Q.; Hu, Q.X.; Yin, Z.W. Effect of inoculation with Penicillium expansum on the microbial community and maturity of compost. Bioresour. Technol. 2011, 102, 11189–11193. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Araya, S.; Mimoto, H. Inoculation of Pichia kudriavzevii RB1 degrades the organic acids present in raw compost material and accelerates composting. Bioresour. Technol. 2013, 144, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.D.; He, X.S.; Wei, Z.M.; Jiang, Y.H.; Li, M.X.; Li, D.; Li, Y.; Dang, Q.L. Effect of inoculation methods on the composting efficiency of municipal solid wastes. Chemosphere 2012, 88, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Garcia, M.D.; Suarez-Estrella, F.F.; Lopez, M.J.; Moreno, J. Influence of microbial inoculation and co-composting material on the evolution of humic-like substances during composting of horticultural wastes. Process Biochem. 2006, 41, 1438–1443. [Google Scholar] [CrossRef]
- Silva, M.E.F.; Lopes, A.R.; Cunha-Queda, A.C.; Nunes, O.C. Comparison of the bacterial composition of two commercial composts with different physicochemical, stability and maturity properties. Waste Manag. 2016, 50, 20–30. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Z.; Shi, W.C.; Li, L.C.; Tian, R.M.; Huang, J.; Lin, R.S.; Wang, B.; Zhou, B. Material conversion, microbial community composition and metabolic functional succession during green soybean hull composting. Bioresour. Technol. 2020, 316, 123823. [Google Scholar] [CrossRef]
- Jia, X.J.; Qin, X.M.; Tian, X.P.; Zhao, Y.; Yang, T.; Huang, J. Inoculating with the microbial agents to start up the aerobic composting of mushroom residue and wood chips at low temperature. J. Environ. Chem. Eng. 2021, 9, 105294. [Google Scholar] [CrossRef]
- Insam, H.; Frankewhittle, I.; Goberna, M. Microbes in Aerobic and Anaerobic Waste Treatment; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–34. [Google Scholar]
- Meng, Q.X.; Yang, W.; Men, M.Q.; Bello, A.; Xu, X.H.; Xu, B.S.; Deng, L.T.; Jiang, X.; Sheng, S.Y.; Wu, X.T.; et al. Microbial Community Succession and Response to Environmental Variables During Cow Manure and Corn Straw Composting. Front. Microbiol. 2019, 10, 529. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.C.; Zeng, G.M.; Chen, Y.N.; Yu, M.; Yu, Z.; Li, H.; Yu, Y.; Huang, H.L. Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Bioresour. Technol. 2011, 102, 2950–2956. [Google Scholar] [CrossRef]
- Zhou, H.X.; Zhao, Y.; Yang, H.Y.; Zhu, L.J.; Cai, B.Y.; Luo, S.; Cao, J.X.; Wei, Z.M. Transformation of organic nitrogen fractions with different molecular weights during different organic wastes composting. Bioresour. Technol. 2018, 262, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F.; Pera, A.; Forte, M.; Bertoldi, M.D. Evaluating Toxicity of Immature Compost; BioCycle: Emmaus, PA, USA, 1981; Volume 22, pp. 54–57. [Google Scholar]
- Karlsson, I.; Friberg, H.; Steinberg, C.; Persson, P. Fungicide effects on fungal community composition in the wheat phyllosphere. PLoS ONE 2014, 9, e111786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.H.; Liu, Y.; Wang, S.Q.; Wang, K.; Zhang, Y. Development of a compound microbial agent beneficial to the composting of Chinese medicinal herbal residues. Bioresour. Technol. 2021, 330, 124948. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.L.; Wang, Y.Q.; Zhang, X.Q.; Lou, Y.J.; Gao, Y.H. Inoculation with a psychrotrophic-thermophilic complex microbial agent accelerates onset and promotes maturity of dairy manure-rice straw composting under cold climate conditions. Bioresour. Technol. 2017, 243, 339. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zhuge, C.X.; Weng, Q.; Hu, B.L. Additional strains acting as key microbes promoted composting process. Chemosphere 2022, 287, 132304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.N.; Chen, G.F.; Sun, H.F.; Zhou, S.; Zou, G.Y. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Ceglie, F.G.; Aly, A.; Mihreteab, H.T.; Ciaccia, C.; Tittarelli, F. Phosphorus availability from rock phosphate: Combined effect of green waste composting and sulfur addition. J. Environ. Manag. 2016, 182, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G. Effect of composting on nutrient loss and nitrogen availability of cattle deep litter. Eur. J. Agron. 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Chi, C.P.; Chu, S.H.; Wang, B.; Zhang, D.; Zhi, Y.E.; Yang, X.J.; Zhou, P. Dynamic bacterial assembly driven by Streptomyces griseorubens JSD-1 inoculants correspond to composting performance in swine manure and rice straw co-composting. Bioresour. Technol. 2020, 313, 123692. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, D.Y.; Li, J.J.; Li, Y.Y.; Li, G.X.; Zang, B.; Li, Y. Effect of spent mushroom substrate as a bulking agent on gaseous emissions and compost quality during pig manure composting. Environ. Sci. Pollut. Res. 2018, 25, 12398–12406. [Google Scholar] [CrossRef]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itavaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- De Gannes, V.; Eudoxie, G.; Hickey, W.J. Insights into fungal communities in composts revealed by 454-pyrosequencing: Implications for human health and safety. Front. Microbiol. 2013, 5, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.W.; Wang, L.H.; Hassan, M.; Xie, B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef] [PubMed]
- de Gannes, V.; Eudoxie, G.; Hickey, W.J. Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresour. Technol. 2013, 133, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Li, L.J.; Pan, X.G.; Shi, Z.; Feng, X.H.; Gong, B.; Li, J.; Wang, L.S. Enhanced Growth and Activities of the Dominant Functional Microbiota of Chicken Manure Composts in the Presence of Maize Straw. Front. Microbiol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Hanajima, D.; Toyoda, S.; Yoshida, N.; Morioka, R.; Osada, T. Microbiology of nitrogen cycle in animal manure compost. Microb. Biotechnol. 2011, 4, 700–709. [Google Scholar] [CrossRef]
- Berg, B.; Hofsten, B.V.; Pettersson, G. Electronmicroscopic observations on the degradation of cellulose fibres by cellvibrio fulvus and sporocytophaga myxococcoides. J. Appl. Bacteriol. 1972, 35, 215–219. [Google Scholar] [CrossRef]
- Holman, D.B.; Hao, X.Y.; Topp, E.; Yang, H.E.; Alexander, T.W. Effect of Co-Composting Cattle Manure with Construction and Demolition Waste on the Archaeal, Bacterial, and Fungal Microbiota, and on Antimicrobial Resistance Determinants. PLoS ONE 2016, 11, e0157539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salo, K.; Domisch, T.; Kouki, J. Forest wildfire and 12 years of post-disturbance succession of saprotrophic macrofungi (Basidiomycota, Ascomycota). For. Ecol. Manag. 2019, 451, 117454. [Google Scholar] [CrossRef]
- Meng, L.X.; Xu, C.X.; Wu, F.L.; Huhe. Microbial co-occurrence networks driven by low-abundance microbial taxa during composting dominate lignocellulose degradation. Sci. Total Environ. 2022, 845, 157197. [Google Scholar] [CrossRef]
- Rainer, J.; De Hoog, G.S. Molecular taxonomy and ecology of Pseudallescheria, Petriella and Scedosporium prolificans (Microascaceae) containing opportunistic agents on humans. Mycol. Res. 2006, 110, 151–160. [Google Scholar] [CrossRef]
- Yun, C.X.; Yan, C.R.; Xue, Y.H.; Xu, Z.Y.; Jin, T.; Liu, Q. Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts. Sustainability 2021, 13, 2925. [Google Scholar] [CrossRef]
- Liu, N.; Hou, T.; Yin, H.; Han, L.; Huang, G. Effects of amoxicillin on nitrogen transformation and bacterial community successionduring aerobic composting. J. Hazard. Mater. 2019, 362, 258–265. [Google Scholar] [CrossRef]
- Jumnoodoo, V.; Mohee, R. Evaluation of FTIR spectroscopy as a maturity index forherbicide-contaminated composts. Environ. Waste Manag. 2011, 9, 89–99. [Google Scholar] [CrossRef]
- Rich, N.; Bharti, A.; Kumar, S. Effect of bulking agents and cow dung as inoculant on vegetable waste compost quality. Bioresour. Technol. 2018, 252, 83–90. [Google Scholar] [CrossRef]
- Zhang, J.P.; Ying, Y.; Yao, X.H. Effects of turning frequency on the nutrients of Camellia oleifera shell co-compost with goat dung and evaluation of co-compost maturity. PLoS ONE 2019, 14, e0222841. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Clark, I.; Sanchez-Monedero, M.A.; Shea, S.; Meier, S.; Qi, F.J.; Kookana, R.S.; Bolan, N. Physical and chemical properties of biochars co-composted with biowastes and incubated with a chicken litter compost. Chemosphere 2016, 142, 14–23. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Yu, H.Y.; Xie, B.T.; Khan, R.Y.; Shen, G.M. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour. Technol. 2019, 271, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Antil, R.S. Evaluation of maturity and stability parameters of composts prepared from agro-industrial wastes. Bioresour. Technol. 2011, 102, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wang, Y.; Feng, X.J.; Zhai, L.M.; Zhu, G.B. Quantitative analyses of ammonia-oxidizing Archaea and bacteria in the sediments of four nitrogen-rich wetlands in China. Appl. Microbiol. Biotechnol. 2011, 90, 779–787. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y. Characterization and composting of poultry litter in forced-aeration piles. Process Biochem. 2002, 37, 869–880. [Google Scholar] [CrossRef]
- Belyaeva, O.N.; Haynes, R.J.; Sturm, E.C. Chemical, physical and microbial properties and microbial diversity in manufactured soils produced from co-composting green waste and biosolids. Waste Manag. 2012, 32, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, X.L.; Wei, T.; Yang, Z.; Jia, Z.K.; Yang, B.P.; Han, Q.F.; Ren, X.L. Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of China. Soil Tillage Res. 2016, 160, 65–72. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Zhao, Y.; Xi, B.D.; Wei, Z.M.; Li, X.; Cao, Z.Y. Changes in phosphorus fractions during organic wastes composting from different sources. Bioresour. Technol. 2015, 189, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Luo, W.H.; Li, Y.; Wang, G.Y.; Li, G.X. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions. Bioresour. Technol. 2018, 250, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Li, H.Y.; Yao, T.; Su, M.; Ran, F.; Han, B.; Li, J.H.; Lan, X.J.; Zhang, Y.C.; Yang, X.M.; et al. Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef]
- Chang, H.Q.; Zhu, X.H.; Wu, J.; Guo, D.Y.; Zhang, L.H.; Feng, Y. Dynamics of microbial diversity during the composting of agricultural straw. J. Integr. Agric. 2021, 20, 1121–1136. [Google Scholar] [CrossRef]
- Zhong, X.Z.; Li, X.X.; Zeng, Y.; Wang, S.P.; Sun, Z.Y.; Tang, Y.Q. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 2020, 306, 123091. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.M.; Xu, X.H.; Qu, J.J.; Zhu, L.P.; Wang, T.T. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J. Microbiol. Biotechnol. 2016, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Liu, Z.P.; Xia, J.S.; Chen, Y.P. Effect of microbial inoculation on physicochemical properties and bacterial community structure of citrus peel composting. Bioresour. Technol. 2019, 291, 121843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Chen, M.T.; Guo, J.Y.; Liu, N.; Yi, W.Y.; Yuan, Z.T.; Zeng, L.F. Study on dynamic changes of microbial community and lignocellulose transformation mechanism during green waste composting. Eng. Life Sci. 2021, 22, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.N.; Gu, J.; Wang, X.J.; Song, W.; Zhang, K.Y.; Sun, W.; Zhang, X.; Zhang, Y.J.; Li, H.C. Effects of Copper Addition on Copper Resistance, Antibiotic Resistance Genes, and intl1 during Swine Manure Composting. Front. Microbiol. 2017, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Song, T.T.; Li, H.N.; Li, B.X.; Yang, J.X.; Sardar, M.F.; Yan, M.M.; Li, L.Y.; Tian, Y.L.; Xue, S.; Zhu, C.X. Distribution of antibiotic-resistant bacteria in aerobic composting of swine manure with different antibiotics. Environ. Sci. Eur. 2021, 33, 91. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, J.Y.; Wang, K.J. Analysis of microbial community structure of food waste compost based on enhanced plug flow process (PFR). China Biogas 2020, 38, 3–9. [Google Scholar]
- Mayende, L.; Wilhelmi, B.S.; Pletschke, B.I. Cellulases (CMCases) and polyphenol oxidases from thermophilic Bacillus spp. isolated from compost. Soil Biol. Biochem. 2006, 38, 2963–2966. [Google Scholar] [CrossRef]
- Salar, R.K. Thermophilic fungi: Taxonomy and biogeography. J. Agric. Technol. 2007, 3, 77–107. [Google Scholar]
- Kriss, A.B.; Paul, P.A.; Xu, X.M.; Nicholson, P.; Doohan, F.M.; Hornok, L.; Rietini, A.; Edwards, S.G.; Madden, L.V. Quantification of the relationship between the environment and Fusarium head blight, Fusarium pathogen density, and mycotoxins in winter wheat in Europe. Eur. J. Plant Pathol. 2012, 133, 975–993. [Google Scholar] [CrossRef]
- Homans, W.J.; Fischer, K. A composting plant as an odour source, compost as an odour killer. Acta Hortic. 1992, 302, 37–44. [Google Scholar] [CrossRef]
- Yang, Y.J.; Awasthi, M.K.; Bao, H.Y.; Bie, J.Y.; Lei, S.; Lv, J.L. Exploring the microbial mechanisms of organic matter transformation during pig manure composting amended with bean dregs and biochar. Bioresour. Technol. 2020, 313, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.Y.; Li, B.Q.; Jin, H.M.; Dong, Y.W. Increased enzyme activities and fungal degraders by Gloeophyllum trabeum inoculation improve lignocellulose degradation efficiency during manure-straw composting. Bioresour. Technol. 2021, 337, 125427. [Google Scholar] [CrossRef] [PubMed]


| Physical and Chemical Factors | Explains% | Contribution% | Pseudo-F | p |
|---|---|---|---|---|
| Cellulose | 51.8 | 59.4 | 23.7 | 0.002 |
| TP | 11.1 | 12.7 | 6.3 | 0.002 |
| AP | 6.8 | 7.8 | 4.5 | 0.002 |
| T | 3.8 | 4.4 | 2.7 | 0.014 |
| NH4+-N | 3 | 3.4 | 2.3 | 0.024 |
| NO3−-N | 2.6 | 2.9 | 2.1 | 0.046 |
| TN | 1.8 | 2 | 1.6 | 0.168 |
| AK | 1.5 | 1.8 | 1.3 | 0.254 |
| pH | 1.4 | 1.6 | 1.2 | 0.34 |
| EC | 1.3 | 1.5 | 1.1 | 0.372 |
| GI | 1 | 1.2 | 0.9 | 0.522 |
| TOC | 0.8 | 0.9 | 0.7 | 0.674 |
| TK | 0.3 | 0.4 | 0.2 | 0.97 |
| Physical and Chemical Factors | Explains% | Contribution% | Pseudo-F | p |
|---|---|---|---|---|
| T | 44.4 | 58.9 | 17.6 | 0.002 |
| GI | 7.3 | 9.7 | 3.2 | 0.018 |
| AK | 5.7 | 7.6 | 2.7 | 0.074 |
| Cellulose | 3.8 | 5 | 1.9 | 0.118 |
| TOC | 3.1 | 4.1 | 1.5 | 0.196 |
| pH | 2.9 | 3.8 | 1.4 | 0.242 |
| AP | 2.2 | 3 | 1.2 | 0.31 |
| NH4+-N | 2 | 2.6 | 1 | 0.33 |
| TP | 1.7 | 2.2 | 0.9 | 0.472 |
| NO3−-N | 0.8 | 1 | 0.4 | 0.8 |
| TN | 0.8 | 1.1 | 0.4 | 0.792 |
| EC | 0.5 | 0.6 | 0.2 | 0.924 |
| TK | 0.3 | 0.4 | 0.1 | 0.962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, G.; Xu, P.; Zhou, S.; Li, Y.; Ma, L.; Yang, Z.; Wu, Y. Effects of Exogenous Bacterial Agents on Material Transformation and Microbial Community Composition during Composting of Tomato Stalks. Sustainability 2022, 14, 16284. https://doi.org/10.3390/su142316284
Li Y, Zhang G, Xu P, Zhou S, Li Y, Ma L, Yang Z, Wu Y. Effects of Exogenous Bacterial Agents on Material Transformation and Microbial Community Composition during Composting of Tomato Stalks. Sustainability. 2022; 14(23):16284. https://doi.org/10.3390/su142316284
Chicago/Turabian StyleLi, Yang, Guanzhi Zhang, Peng Xu, Shun Zhou, Yan Li, Liyuan Ma, Zhenchao Yang, and Yongjun Wu. 2022. "Effects of Exogenous Bacterial Agents on Material Transformation and Microbial Community Composition during Composting of Tomato Stalks" Sustainability 14, no. 23: 16284. https://doi.org/10.3390/su142316284
APA StyleLi, Y., Zhang, G., Xu, P., Zhou, S., Li, Y., Ma, L., Yang, Z., & Wu, Y. (2022). Effects of Exogenous Bacterial Agents on Material Transformation and Microbial Community Composition during Composting of Tomato Stalks. Sustainability, 14(23), 16284. https://doi.org/10.3390/su142316284





