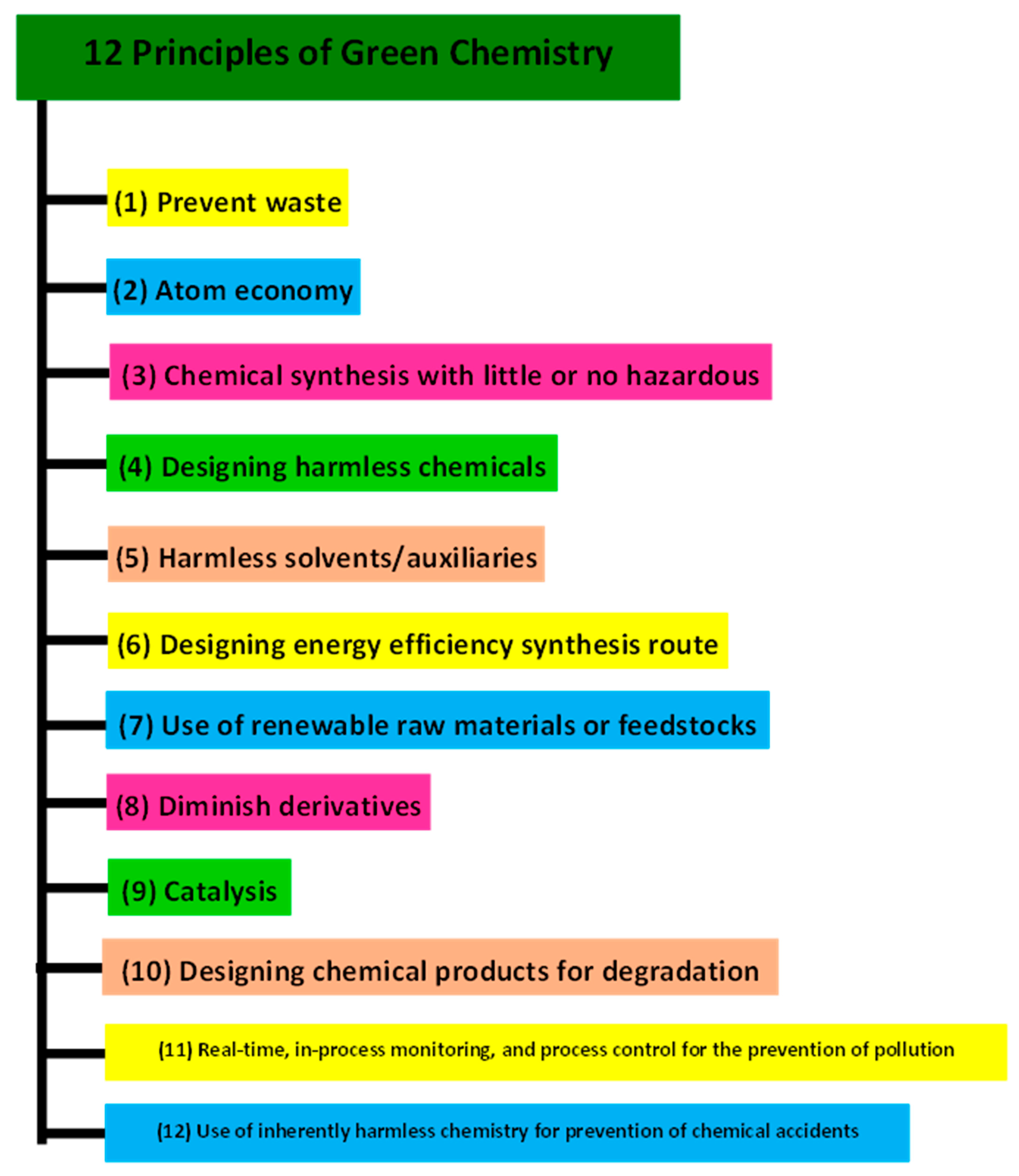
2.2.1. Fabrication of OH-/LDH-/Sulfide-/Oxide-Based Earth-Abundant Electrocatalysts
The fabrication of nanostructured, amorphous transition bimetallic hydroxide on an electrode as the electrocatalyst for OER through a simple and facile electrochemical process using the immersion of a target material in only water could alleviate the usage of any chemical additives. Amorphous, nanostructured Ni
0.71Fe
0.29(OH)
x [
84] exhibited an enhanced activity (η of 296 mV at 10 mA cm
−2), stability (negligible decay at 5 mA cm
−2 for 24 h), and durability (negligible decay at 10 mA cm
−2 after 30,000 cycles of CV) for OER in 0.1 M KOH; it was obtained on a graphite electrode through a simple and facile electrochemical process at 100 V for 10 h, where the target material (NiFe alloy) was immersed in water without any chemical additives and where graphite was used as the cathode and anode. Moreover, 3D honeycomb-like amorphous Ni(OH)
2 nanosheets [
85] exhibited an enhanced activity (η of 344 mV at 10 mA cm
−2) and durability (negligible decay at 30 mA cm
−2 after 5000 cycles of CV) for OER in 0.1 M KOH, and were obtained on a graphite electrode through a simple and facile electrochemical process at 90 V for 75 min, where the target material (Ni) was immersed in water without any chemical additives and where graphite was used as the cathode and anode. In addition, amorphous Co(OH)
2 nanosheets [
86] exhibited an enhanced activity (η of 380 mV at 10 mA cm
−2) and stability (negligible decay at 1 mA cm
−2 for 24 h) for OER in 0.1 M KOH, and were obtained on a graphite electrode through a simple and facile electrochemical process at 50 V for 1 h, where the target material was immersed in water without any chemical additives and where graphite was used as the cathode and anode.
Transition bimetallic LDH can be prepared on transition bimetallic foam by a hydrothermal treatment using only deionized water, which could obviate the use of any other chemicals. Then, heteroatom-doped bimetallic phosphide could be obtained by plasma-assisted phosphorization of the above mentioned bimetallic LDH, which could modify the electronic structure and provide a nanostructure, as well as afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and that could enhance the performance for HER and OER. N–NiCoP
x [
73] exhibited an enhanced activity and stability for OER. The N–NiCoP
x was obtained on cleaned NiCo foam by a hydrothermal treatment (where NiCo LDH was obtained only using water without any chemicals), followed by plasma-assisted phosphorization. The N–NiCoP
x exhibited higher HER activity and lower charge-transfer resistance than that of NiCoP
x, while it exhibited higher OER activity than that of NiCoP
x. The N–NiCoP
x is crystalline, which contains Ni
2P, Co
2P, NiCoP, CoN, and Ni
3N phases. It contains Ni, Co, N, P, and O, which are uniformly distributed. Theoretical studies suggest that the Co atom bonded with N in N–NiCoP
x could be the active center for overall water splitting, which could lower the adsorption energy for intermediates; N–NiCoP
x also exhibited a high density of electronic states around the Fermi level. It possesses a nanosheet array morphology. For OER in 1 M KOH, N–NiCoP
x exhibited a η of 298 mV at 10 mA cm
−2, suggesting its considerably high activity, while it exhibited 96.93% retention (delivered ~100 mA cm
−2) for 100 h, suggesting its significantly very high stability; it also exhibited negligible decay at 300 mA cm
−2 after 10,000 cycles of CV, suggesting its robust durability. For HER in 1 M KOH, the N–NiCoP
x exhibited a η of −23 mV at −10 mA cm
−2 (
Table 3), suggesting its substantially high activity, while it exhibited 93.98% retention (delivered ~−100 mA cm
−2) for 100 h (
Table 4), suggesting its considerably very high stability; it also exhibited negligible decay at −300 mA cm
−2 after 10,000 cycles of CV (
Table 5), suggesting its robust durability. For overall water splitting in 1 M KOH, the N–NiCoP
x//N–NiCoP
x exhibited a potential of 1.57 V at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay for 50 h, suggesting its very high stability.
Transition bimetallic LDH can be prepared on transition bimetallic foam by hydrothermal treatment using only deionized water, which could obviate the use of any other chemicals, and bimetallic selenides could be obtained by selenization of the above mentioned bimetallic LDH, while it could modify the electronic structure, provide nanostructure, afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and could enhance the performance for HER and OER. Chen et al. [
75] observed that NiSe
2–CoSe
2 exhibited substantially very high activity and stability for HER and OER. NiSe
2–CoSe
2 was prepared by the following steps (
Figure 2): At first, NiCo LDH was obtained on pre-cleaned NiCo foam by hydrothermal treatment at 200 °C for 24 h, where only 25 mL of deionized water was added to the pre-cleaned NiCo foam, while no other chemicals were used. Finally, the NiSe
2–CoSe
2 was prepared by hydrothermal selenization at 180 °C for 24 h. The NiSe
2–CoSe
2 exhibited higher HER activity and lower charge-transfer resistance than that of NiCo LDH, while it exhibited higher OER activity than that of NiCo LDH. The NiSe
2–CoSe
2 is crystalline, which is composed of NiSe
2, CoSe
2, NiCoSe
2, Co
2O
3, and Ni
2O
3 phases, where the existence of oxides could be due to the partial oxidation of the catalyst. It contains Ni, Co, Se, and O, which are uniformly distributed. The DFT calculations suggest that the Co atoms at the hetero-interface between the NiSe
2 and CoSe
2 phases could act as the active sites for the water electrolysis. It possesses nanosheet array morphology (Thickness of the nanosheet: ~300 nm). For OER in 1 M KOH, the NiSe
2–CoSe
2 exhibited a η of 250 mV at 10 mA cm
−2 (
Table 6), suggesting its very high activity, while it exhibited 95.2% retention (delivered ~100 mA cm
−2) for 100 h, suggesting its significantly very high stability; it also exhibited negligible decay at 150 mA cm
−2 after 10,000 cycles of CV, suggesting its substantially very high durability. For HER in 1 M KOH, the NiSe
2–CoSe
2 exhibited a η of −24 mV at −10 mA cm
−2, suggesting its considerably very high activity, while it exhibited 97.3% retention (delivered ~ −100 mA cm
−2) for 100 h, suggesting its significantly very high stability, and it exhibited negligible decay at −100 mA cm
−2 after 10,000 cycles of CV, suggesting its substantially very high durability. For overall water splitting in 1 M KOH, the NiSe
2–CoSe
2//NiSe
2–CoSe
2 exhibited a potential of 1.63 V at 50 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay for 10 h, suggesting its very high stability.
Exfoliation of bimetallic LDH as the electrocatalyst for OER through an ultrasonic process using only water could alleviate the usage of toxic solvents. The crystalline, exfoliated NiFe
LDH–C single-/few-layer nanosheets [
101] exhibited enhanced activity (η of 220 mV at 10 mA cm
−2) and stability (reasonable stability at 10 mA cm
−2 for 12 h) for OER in 1 M KOH, while the exfoliation was achieved by an ultrasonic process for 15 min in water. In the XPS spectra, the binding energies of Fe 2p
1/2 and Fe 2p
2/3 peaks were shifted for exfoliated NiFe
LDH-C when compared with those of bulk NiFe
LDH-C, suggesting the modified electronic structure, while the exfoliated NiFe
LDH-C exhibited higher OER activity and lower charge-transfer resistance than that of bulk NiFe
LDH-C.
NiFe
LDH can be prepared by hydrothermal treatment, while Ostwald ripening-driven in situ exfoliation can be achieved by a subsequent hydrothermal treatment, and it could modify the electronic structure and provide nanostructure, which could afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and enhance the performance for OER. Chen et al. [
119] observed that the exfoliated NiFe
LDH exhibited enhanced activity and stability for OER. The exfoliated NiFe
LDH was prepared by the following steps (
Figure 3a–e): At first, Cu
xO was obtained on Cu mesh by heating the Cu mesh at 500 °C for 2 h under air atmosphere. Then, the NiFe
LDH was grown on Cu
xO/Cu mesh by hydrothermal treatment at 120 °C for 15 h using a precursor solution, while Ostwald ripening driven in situ exfoliation was achieved by a subsequent hydrothermal treatment at 160 °C for 8 h (autoclave was unopened) without adding any other reagent or surfactant. The exfoliated NiFe
LDH exhibited higher OER activity and electrochemically active surface areas than that of bulk NiFe
LDH. The exfoliated NiFe
LDH is crystalline. It contains Ni, Fe, and O, which are uniformly distributed. It contains Ni
2+, and Fe
3+. It possesses ultrathin nanosheet morphology (thickness: ~10 nm). For OER in 1 M KOH, the NiFe
LDH exhibited a η of 292 mV at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at 10 mA cm
−2 for >60 h, suggesting its very high stability.
The fabrication of hollow-structured, transition bimetallic sulfide as the electrocatalyst for OER through a facile synthesis route without using surfactant and template at relatively low-temperature could be desirable to achieve efficient water electrolysis. The NiCo
2S
4 [
115] exhibited enhanced activity (η of 400 mV at 10 mA cm
−2), stability (reasonable stability at η of 400 mV for ~11 h), and durability (8 mV decay at 10 mA cm
−2 after 1000 cycles of CV) for OER in 0.1 M KOH. It is polycrystalline, which is composed of cubic NiCo
2S
4 phase. It possesses hollow sphere morphology with high surface area, while it contains mesopores. The NiCo
2S
4 was obtained by a surfactant-free and template-free method using an binary solution comprising of N,N-dimethylformamide and ethylene glycol through a reflux route at low temperature (<200 °C).
Besides, the Co–TiO
2 [
118] exhibited enhanced activity (η of 474 mV at 10 mA cm
−2) for OER in 0.5 M KOH, composed of Co-doped anatase TiO
2 nanoparticles, while it was obtained by the sol–gel method, where gelatin (biodegradable material) was used in the preparation process.
2.2.2. Fabrication of Phosphide-Based Earth-Abundant Electrocatalysts
The fabrication of cobalt phosphide using cobalt (II) complex (trioctylphosphine ligand) through a microwave-assisted method could alleviate toxic PH
3 gas emission, while it could generate metallic character, modify the electronic structure, generate OER-active thin in situ surface layers during OER, and provide ultrathin nanostructure, and could afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and enhance the performance for HER and OER. Jin et al. [
41] observed that Co
2P exhibited enhanced activity for HER and OER. The Co
2P was prepared using cobalt (II) complex (trioctylphosphine ligand) through a microwave-assisted method, which could alleviate toxic PH
3 gas emission. The Co
2P nanowire exhibited higher OER activity than that of CoP nanowire and CoP film. The Co
2P is composed of crystalline cobalt phosphide with the obvious lattice planes of (112) and (020). In the XPS spectra (XPS: X-ray photoelectron spectroscopy), the Co
2P exhibited lower binding energy for Co 2p when compared with that of CoP, suggesting the modified electronic structure, while the Co
2P exhibited a peak at 778.2 eV, which is almost close to the metallic Co (Co
0), suggesting the existence of metallic character in Co
2P (Valence state of Co could be ~+0.3), which could be due to the rapid phosphorization of the microwave-assisted method. Moreover, the density-of-states (DOS) near the Fermi level reveal that the Co
2P exhibited higher intensity of the electrons when compared with that of CoP, suggesting the superior electrical conductivity of Co
2P. It could exhibit the in situ formation of thin Co oxo/hydroxide layers as an active species on the surface of the Co
2P during OER. It possesses ultrathin nanowire morphology. For OER in 1 M KOH, the Co
2P exhibited a η of 260 mV at 10 mA cm
−2, suggesting its very high activity. For HER in 1 M KOH, the Co
2P exhibited a η of −95 mV at −10 mA cm
−2, suggesting its very high activity. For overall water splitting at 10 mA cm
−2 in 1 M KOH, the Co
2P//Co
2P affords cell voltage of 1.44 V, where this activity was higher than that of the activity of electrocatalysts prepared using toxic P source ((Ni
0.33Fe
0.67)
2P//(Ni
0.33Fe
0.67)
2P (1.49 V) [
121] and Co
4Ni
1P//Co
4Ni
1P (1.59 V) [
122]).
The fabrication of transition metal phosphide as the electrocatalyst from a molecular metal phosphide precursor using relatively low-temperature could be desirable to achieve efficient water electrolysis. The crystalline FeP nanoparticles [
74] exhibited enhanced activity and stability for HER and OER. The FeP was obtained by conversion of a molecular iron phosphide precursor at a hot injection condition (low-temperature) in oleylamine (CH
3(CH
2)
7CH=CH(CH
2)
7CH
2NH
2), where the precursor was composed of Fe
2P
3 core having mixed-valence Fe
IIFe
III sites, which was bridged by an asymmetric cyclo-P
(2+1)3− ligand, while the FeP was electrophoretically deposited on Ni foam, which was used as the anode and cathode. For OER in 1 M KOH, the FeP exhibited a η of 227 mV at 10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability at 1.46 V for 15 h, suggesting its very high stability. For HER in 1 M KOH, the FeP exhibited a η of −165 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability for 15 h, suggesting its very high stability. For overall water splitting in 1 M KOH, the FeP//FeP exhibited a potential of 1.59 V at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at 1.6 V for 336 h, suggesting its substantially high stability.
The fabrication of transition bimetallic phosphide as the electrocatalyst using triphenylphosphine as the phosphorus source at relatively low temperature could alleviate toxic PH
3 gas emission. The crystalline NiCoP nanoparticles [
92] exhibited enhanced activity for HER and OER. The NiCoP was obtained using triphenylphosphine (PPh
3) as the phosphorus source at relatively low temperature (250 °C), where NaBH
4 was used to facilitate the reaction. For OER in 1 M KOH, the NiCoP exhibited a η of 340 mV at 10 mA cm
−2, suggesting its high activity. For HER in 1 M KOH, the NiCoP exhibited a η of −314 mV at −10 mA cm
−2, suggesting its high activity.
The fabrication of heteroatom-doped metal phosphide as the electrocatalyst through the electrochemical deposition method could alleviate toxic PH
3 gas emission. The nanostructured S-doped NiP (S–NiP) [
70] exhibited enhanced activity and stability for HER and OER, where the S doping could modify the electronic structure, while the nanostructure could facilitate the gas evolution. The S–NiP was obtained on pretreated Cu substrate by pulse electrochemical deposition method. For OER in 1 M KOH, the S–NiP exhibited a η of 229 mV at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at 100 mA cm
−2 for 10 h, suggesting its very high stability, and it exhibited negligible decay at 100 mA cm
−2 after 1000 cycles of CV, suggesting its very high durability. For HER in 1 M KOH, the S–NiP exhibited a η of −55 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at −100 mA cm
−2 for 10 h, suggesting its very high stability, and it exhibited negligible decay at −100 mA cm
−2 after 1000 cycles of CV, suggesting its very high durability. For overall water splitting in 1 M KOH, the S–NiP//S–NiP exhibited a potential of 1.51 V at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at 100 mA cm
−2 for 25 h, suggesting its very high stability.
The fabrication of heteroatom-doped metal phosphide through a thiourea-phosphate-assisted method could alleviate toxic and lethal PH
3 gas formation, while it could modify the electronic structure, generate OER-active in situ surface layers during OER, and provide nanostructure, and that could afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and that could enhance the performance for HER and OER. The nanostructured sulfur-doped Co
2P [
51] exhibited enhanced activity and stability for HER and OER. The S–Co
2P was obtained by a thiourea-phosphate-assisted method, which could alleviate toxic and lethal PH
3 gas formation. For OER in 1 M KOH, the S–Co
2P exhibited a η of 288 mV at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at η of 340 mV for 20 h, suggesting its very high stability. For HER in 1 M KOH, the S–Co
2P exhibited a η of −105 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at η of −150 mV for 20 h, suggesting its very high stability. For overall water splitting in 1 M KOH, the S–Co
2P//S–Co
2P exhibited a potential of ~1.63 V at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay for 20 h, suggesting its very high stability.
Moreover, Anjum et al. [
50] observed that S-CoP exhibited enhanced activity and stability for HER and OER. The sulfur-doped CoP was obtained by a thiourea-phosphate-assisted method, which could alleviate toxic and lethal PH
3 gas formation. It was prepared by pouring transparent aqueous solution of cobalt nitrate hexahydrate to the pre-heated (at 60 °C) transparent aqueous urea-phosphate solution (H
3PO
4 + (NH
2)
2CS → H
2S + (NH
2)CO(NH
2)·H
3PO
4), while the mixture was subjected to hydrothermal treatment at 160 °C for 24 h ((NH
2)CO(NH
2)·H
3PO
4 +
xH
2S + Co
2+→ S-doped Co·urea·phosphate) followed by thermal reduction at 600 °C for 3 h under H
2 atmosphere. The S–CoP exhibited higher HER activity and lower charge-transfer resistance than that of CoP, while it exhibited higher OER activity than that of CoP. The S–CoP is composed of S-doped CoP, and it exhibited the orthorhombic crystal structure of CoP, while the average crystal size is reduced from ~ 32 nm to ~ 20 nm due to the S doping in CoP. It possesses nanoparticle morphology. It could exhibit the in situ formation of a CoO/Co
3O
4 layer as the active species on the surface of the S–CoP during OER. The density of states (DOS) of S–CoP at the Fermi level was higher than that of bare CoP for the (011) and (111) planes, suggesting the existence of more electrons in the conduction band for S–CoP. For OER in 1 M KOH, the S–CoP exhibited a η of 270 mV at 10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability at η of ~350 mV for 20 h, suggesting its very high stability. For HER in 1 M KOH, the S–CoP exhibited a η of −109 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at η of −130 mV for 20 h, suggesting its very high stability. For overall water splitting in 1 M KOH, the S–CoP//S–CoP exhibited a potential of 1.617 V at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at ~1.8 V for 20 h, suggesting its very high stability.
Toxic PH
3 gas could be produced by the decomposition of NaH
2PO
2 during the fabrication of electrocatalysts, while passing toxic PH
3 gas into the cupric sulfate solution could alleviate its emission in environment. The CoP–C [
43] exhibited enhanced activity and stability for HER and OER, while the ultrathin CoP nanosheet is crystalline. The CoP was obtained by precipitation followed by low-temperature phosphorization and passivation under an O
2/N
2 mixture. In the phosphorization process, toxic PH
3 gas could be produced by the decomposition of NaH
2PO
2, where toxic PH
3 gas is passed into the cupric sulfate solution (CuSO
4) to prevents its emission in environment, while the PH
3 gas could be converted into H
3PO
4 and H
2SO
4 (4CuSO
4 + PH
3 + 4H
2O → 4Cu↓ + H
3PO
4 + 4H
2SO
4). For OER in 1 M KOH, the CoP–C exhibited a η of 277 mV at 10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability at 10 mA cm
−2 for 24 h, suggesting its very high stability, and it exhibited negligible decay at 30 mA cm
−2 after 1000 cycles of CV, suggesting its high durability. For HER in 1 M KOH, the CoP-C exhibited a η of −111 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability at −10 mA cm
−2 for 24 h, suggesting its high stability, and it exhibited very slight decay at −10 mA cm
−2 after 1000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, the CoP//CoP exhibited a potential of 1.54 V at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at 10 mA cm
−2 for 24 h, suggesting its very high stability.
Plasma treatment can be considered as a useful technology to improve the surface properties of the materials [
123,
124]. The fabrication of heteroatom-doped bimetallic phosphide as the electrocatalyst using plasma-assisted low-temperature phosphorization of transition bimetallic hydroxide could alleviate the usage of heavy metal ions. The N–NiCoP [
72] exhibited enhanced activity and stability for HER and OER. The N–NiCoP was obtained by hydrothermal treatment followed by plasma-assisted low-temperature phosphorization. The N–NiCoP exhibited higher HER activity and lower charge-transfer resistance than that of NiCoP, while it exhibited higher OER activity than that of NiCoP. The N–NiCoP is crystalline, which contains CoP, Ni
2P, Co
2NiP
4, Ni
3N, and CoN phases. It contains Co, Ni, P, and N, which are uniformly distributed. It possesses polyhedron morphology. It exhibited high density of electronic states around the Fermi level. For OER in 1 M KOH, the N–NiCoP exhibited a η of 225 mV at 10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay for 100 h, suggesting its substantially very high stability, and it exhibited negligible decay at 50 mA cm
−2 after 10,000 cycles of CV, suggesting its significantly very high durability. For HER in 1 M KOH, the N–NiCoP exhibited a η of −78 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay for 100 h, suggesting its considerably very high stability, and it exhibited negligible decay at −100 mA cm
−2 after 10,000 cycles of CV, suggesting its robust durability. For overall water splitting in 1 M KOH, the N–NiCoP//N–NiCoP exhibited a potential of 1.56 V at 10 mA cm
−2, suggesting its very high activity.
The fabrication of transition metal phosphide confined in carbon matrix by pyrolysis of the transition metal phosphonate complex could alleviate toxic PH
3 gas emission, while it could modify the electronic structure, generate OER-active thin in situ surface layers during OER, and provide nanostructure, and that could afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and that could enhance the performance for HER and OER. Wu et al. [
44] observed that CoP@PC exhibited enhanced activity and stability for OER and HER. The CoP@PC was obtained by pyrolysis of the cobalt–phosphonate complex under H
2/Ar atmosphere, where the complex was prepared by refluxing at 140 °C for 5 h, where poisonous PH
3 gas releasing P sources such as NaH
2PO
2 and NH
4H
2PO
2 are not used. The CoP@PC is composed of cobalt phosphides (crystalline orthorhombic CoP with small amount of Co
2P), which are confined in porous P-doped carbon materials, where the CoP/Co
2P nanoparticles are almost homogenously dispersed along the carbonized fibers, while the CoP/Co
2P nanoparticles are enwrapped by thin layer of carbon shell. It contains Co, P, and C. It exhibited irregular lattice pattern, suggesting the existence of carbon matrix with low graphitization degree, while it exhibited I
D/I
G ratio of 0.96. It inherits the 1D nanofiber morphology of cobalt–phosphonate complex. It exhibited high specific surface area of 88.4 m
2 g
−1. In the XPS spectra, the binding energy of the P 2p
3/2 peak (130.0 eV) for the CoP@PC is lower than that of P element, while the binding energy of the Co 2p
3/2 peak (779.1 eV) for the CoP@PC is higher than that of metal Co, suggesting the modified electronic structure, which could be due to the transfer of partial electrons from Co to P. It could exhibit the in situ formation of Co oxides/hydroxides layer as the active species on the surface of the CoP@PC during OER. For OER in 1 M KOH, the CoP@PC exhibited a η of 280 mV at 10 mA cm
−2, suggesting its high activity, while it exhibited 95.8% retention at 1.51 V for 20 h, suggesting its very high stability. For HER in 1 M KOH, the CoP@PC exhibited a η of −76 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay for 20 h, suggesting its very high stability. For overall water splitting in 1 M KOH, the CoP@PC//CoP@PC exhibited a potential of 1.6 V at 10 mA cm
−2 suggesting its very high activity, while it exhibited negligible decay at 1.65 V for 60 h, suggesting its very high stability.
Phytic acid can be extracted from plant materials [
125,
126], while fabrication of transition bimetallic phosphide as the electrocatalyst for OER using phytic acid as a green organophosphorus source could alleviate toxic PH
3 gas emission. The nanostructured Ni
0.65Fe
0.35P [
48] exhibited enhanced activity (η of 270 mV at 10 mA cm
−2) and stability (Reasonable stability at 20 mA cm
−2 for >6 h) for OER in 1 M KOH, while it was obtained through phosphorization using phytic acid as the green organophosphorus source. However, melamine should be cautiously handled in the synthesis process. The melamine is toxic, while high content of melamine in milk or food can cause adverse effects such as urinary and kidney problems, or even death [
127,
128]. On the other hand, melamine is a nitrogen-rich compound, which can be used as a fertilizer in soil [
128], whereas the plants could absorb the melamine beyond the recommended level [
129]. Moreover, melamine has been used for the manufacture of tableware [
130], whereas oxidative stress and the risk of early damage to kidneys in humans can be increased due to the low-dose exposure of melamine to the workers from melamine tableware factories [
131]. The FeP@NPC [
49] exhibited enhanced activity (η of −214 mV at −10 mA cm
−2) for HER in 1 M KOH, while it is composed of FeP nanoparticles, which are encapsulated in N, P codoped carbon. The FeP@NPC was obtained by the following steps using phytic acid as one of the nontoxic and environmentally friendly precursor: At first, a homogeneous powder was obtained by drying a solution A at 60 °C for overnight, where the solution A was obtained by dissolving FeCl
3·6H
2O and phytic acid in deionized water under stirring followed by adding melamine. Finally, FeP@NPC was prepared by annealing the powder at 900 °C for 2 h under N
2 atmosphere.
The fabrication of transition metallic phosphide as the electrocatalyst using triphenylphosphine as the phosphorus source through solid-state pyrolysis process could alleviate toxic PH
3 gas emission and eliminate toxic solvent usage. The Co
2P-CNT [
52] exhibited enhanced activity and stability for HER and OER, while it is composed of Co
2P nanoparticles, which are encapsulated in N, P codoped carbon nanotubes. The Co
2P-CNT was obtained through solid-state pyrolysis process (solvent-free and one-pot synthesis) of cobalt acetylacetonate, triphenylphosphine, and melamine under N
2 atmosphere at 900 °C for 2 h. For OER in 1 M KOH, the Co
2P-CNT exhibited a η of 292 mV at 10 mA cm
−2, suggesting its high activity, while it exhibited reasonable stability for >13 h, suggesting its high stability, and it exhibited negligible decay at 10 mA cm
−2 after 2000 cycles of CV, suggesting its high durability. For HER in 1 M KOH, the Co
2P-CNT exhibited a η of −132 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability for >13 h, suggesting its high stability, and it exhibited negligible decay at −10 mA cm
−2 after 2000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, the Co
2P-CNT//Co
2P-CNT exhibited a potential of 1.53 V at 10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability for >13 h, suggesting its high stability. Moreover, the Co
2P-NP-CNT [
109] exhibited enhanced activity (η of 370 mV at 10 mA cm
−2), stability (86.9% retention at η of 370 mV for 10 h), and durability (negligible decay at 10 mA cm
−2 after 1000 cycles of CV) for OER in 1 M KOH, while it is composed of Co
2P nanoparticles, which are encapsulated in N, P codoped carbon nanotubes. The Co
2P-NP-CNT was obtained through solid-state pyrolysis process (solvent-free and one-pot synthesis) of Co(NO
3)
2·6H
2O, triphenylphosphine and melamine at 850 °C under N
2 for 1 h without involving toxic phosphine gas (PH
3) formation.
The fabrication of transition metallic phosphide and alloy encapsulated in heteroatom-doped nanoporous carbon as the electrocatalyst for OER using triphenylphosphine sulfide as the P and S source through solid-state pyrolysis process could alleviate toxic PH
3 gas emission and eliminate toxic solvent usage. The NPSC-Co
2Fe
1 [
111] exhibited enhanced activity (η of ~370 mV at 10 mA cm
−2) for OER in 0.1 M KOH, while it is composed of CoFe and Co
2P nanoparticles, which are encapsulated in multi-doped nanoporous carbon. The NPSC-Co
2Fe
1 was obtained through solid-state pyrolysis process (solvent-free and one-pot synthesis) of Co(NO
3)
2·6H
2O, Fe(NO
3)
3·9H
2O, triphenylphosphine sulfide (Ph
3PS) and melamine under N
2 atmosphere at 850 °C for 1 h.
H
3PO
4 can be used as an etching agent for metal-organic framework (MOF), while it could also act as a phosphorus source, where the metallic phosphide embedded in P-doped carbon as the electrocatalyst for OER can be fabricated by selective-etching of MOF using H
3PO
4 followed by carbonization under inert atmosphere, and that could obviate the usage of toxic phosphine gas forming chemicals. The Co
2P–Co@PN–C [
100] exhibited enhanced activity (η of 311 mV at 10 mA cm
−2), and stability (negligible decay at η of 311 mV for 10 h) for OER in 1 M KOH, while it is composed of Co
2P and Co, which are embedded in P, N codoped carbon. It possesses rhombic dodecahedral morphology. It contains mesopores. The Co
2P–Co@PN–C was obtained by the following steps: At first, ZIF-67 was obtained by co-precipitation method. Finally, Co
2P–Co@PN–C was prepared by selective-etching of ZIF-67 using H
3PO
4 followed by carbonization at 800 °C for 2 h under N
2 atmosphere, where H
3PO
4 could be used as an etching agent for ZIF-67, while it could also act as a phosphorus source, and that could obviate the usage of toxic phosphine gas forming chemicals.
2.2.3. Fabrication of Carbon-Containing Earth-Abundant Electrocatalysts
Polydopamine is a biomaterial [
132,
133], while electrocatalysts can be fabricated using polydopamine as a biopolymer-mediated green synthesis [
34]. The N, S codoped carbon [
120] with high surface area (488.01 m
2 g
−1) exhibited enhanced activity (η of ~545 mV at 10 mA cm
−2) for OER in 0.1 M KOH. The NS-C was prepared by the following steps using polydopamine as a biopolymer-mediated green synthesis: It was obtained by self-polymerization followed by annealing at 900 °C for about 2 h under N
2 atmosphere.
Besides, the Mo
2C–MoP–NC [
88] exhibited substantially very high activity (η of −134 mV at −10 mA cm
−2), and stability (reasonable stability for 120 h) for HER in 1 M KOH, while it is composed of molybdenum carbide-phosphide nanodots, which are encapsulated in N-doped carbon shell. The Mo
2C–MoP–NC was obtained by drop-casting the as-prepared product on carbon fiber paper followed by carbonization at 800 °C for 3 h under Ar atmosphere, where polydopamine was used as the precursor, where the polydopamine could facilitate the Mo ions chelation without the use of toxic chelating agents.
Fe is one of the highly earth-abundant element, while Prussian blue (Iron (III) ferrocyanide (Fe
III4[Fe
II(CN)
6]
3) is an iron based dark blue pigment. It is used in some medicines. It is used for the preparation of laundry bluing. Thus, fabrication of nanoalloys encapsulated heteroatom-doped graphene as the electrocatalyst for HER through a facile synthesis route using Prussian blue could be desirable to achieve efficient water electrolysis. The Co
xFe
1−x@N–graphene [
91] exhibited enhanced activity (η of −272 mV at −10 mA cm
−2) and durability (negligible decay at −50 mA cm
−2 after 1000 cycles of CV) for HER in 1 M KOH, while it is composed of CoFe nanoalloys, which are encapsulated in N-doped graphene layers. The Co
xFe
1−x@N–graphene was obtained by pyrolysis of Prussian blue and cobalt nitrate hexahydrate at 750 °C for 2 h.
The fabrication of transition metal carbide encapsulated heteroatom-doped graphitic nanostructures as the electrocatalyst for OER by pyrolysis of Prussian blue without using any inert gas flow could be desirable to achieve efficient water electrolysis. The Fe–Fe
3C–NC [
134] exhibited enhanced activity and stability (slight decay at 10 mA cm
−2 after 1000 cycles of CV) for OER in 0.1 M KOH, while it is composed of Fe-Fe
3C-NC nanoparticles, which are encapsulated by bambo-like N-doped graphitic nanotubes and N-doped graphitic layers. The Fe-Fe
3C-NC was obtained by pyrolysis of Prussian blue at 750 °C for 2 h without using any inert gas flow.
Imidazole (C
3N
2H
4) is a biomaterial [
135,
136], which is incorporated into various important biological compounds including amino acid histidine, while small quantity of boric acid (H
3BO
4) is used in some medicine [
137,
138] and agriculture, whereas large quantity of boric acid could be hazardous to environment and human health [
139,
140]. Thus, fabrication of B, N co-doped metallic carbide nanoparticles embedded in a B, N co-doped carbon using imidazole as the source of C and N, and small quantity of boric acid as the source of B could be desirable to achieve efficient water electrolysis. The BN–Mo
2C@BCN [
76] exhibited enhanced activity and stability for HER and OER in 1 M KOH, while it is composed of B, N co-doped molybdenum carbide nanoparticles, which are embedded in a B, N co-doped carbon. The BN–Mo
2C@BCN was synthesized by annealing the as-prepared Mo–imidazole complex with boric acid (H
3BO
4) at 900 °C for 4 h under Ar atmosphere, where imidazole was used as the precursor. For OER in 1 M KOH, the BN–Mo
2C@BCN exhibited a η of ~360 mV at 100 mA cm
−2, suggesting its high activity, while it exhibited reasonable stability for 20 h, suggesting its high stability. For HER in 1 M KOH, the BN–Mo
2C@BCN exhibited a η of ~−100 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability for 20 h, suggesting its high stability. For overall water splitting in 1 M KOH, the BN–Mo
2C@BCN//BN–Mo
2C@BCN exhibited a potential of 1.84 V at 100 mA cm
−2, suggesting its very high activity, while it exhibited reasonable stability for 20 h, suggesting its high stability.
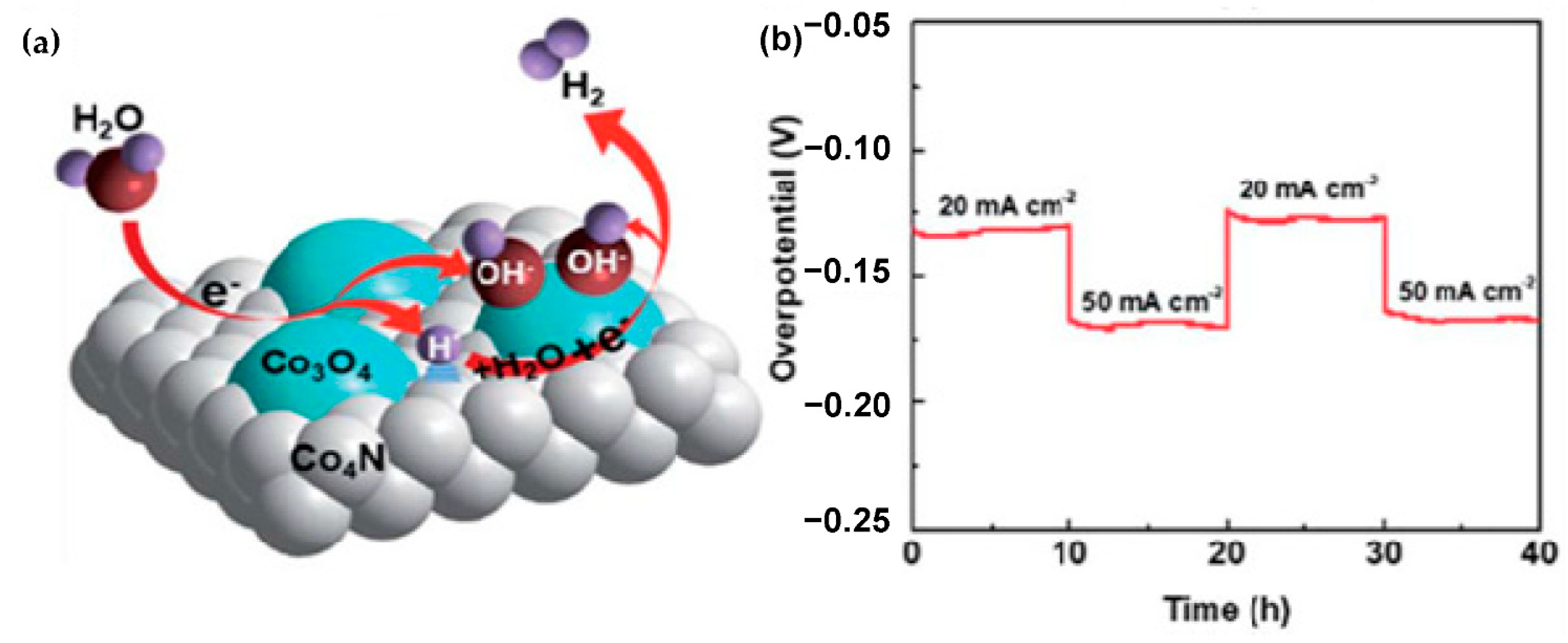
Microwave hydrogen plasma treatment could convert the hydrophobic surface of the carbon cloth into hydrophilic surface, which could alleviate the usage of toxic oxidizing agents such as nitric acid and hydrogen peroxide, while the hydrophilic surface of the carbon cloth could facilitate the fabrication of electrocatalyst, and it could modify the electronic structure, and provide porous nanostructure, and that could afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and that could enhance the performance for HER. Liu et al. [
87] observed that the Co
3O
4–Co
4N–HCC (HCC: Hydrophilic carbon cloth) exhibited enhanced activity and stability for HER. The Co
3O
4–Co
4N–HCC was prepared by the following steps: At first, hydrophilic carbon cloth was obtained by microwave hydrogen plasma treatment for 20 min (without heating and without using any toxic chemicals). Then, α-Co(OH)
2-HCC was obtained by pulse electrodeposition method. Finally, Co
3O
4–Co
4N–HCC was prepared by microwave nitrogen plasma treatment at 300 °C for 15 min. The Co
3O
4–Co
4N–HCC exhibited higher HER activity and lower charge-transfer resistance than that of Co
3O
4–C and Co
4N–C. The Co
3O
4–Co
4N–HCC is composed of crystalline Co
3O
4–Co
4N heterostructures on hydrophilic carbon cloth, where the Co
4N could be enriched with defects. The Co
3O
4 and Co
4N crystallites exhibit an epitaxial relationship of Co
3O
4 (331)//Co
4N (111) having a small-angle grain boundary of ~5.8°, which could enhance the charge-transfer process. In the XPS spectra, the binding energy of the peak attributed to Co−O for Co
3O
4–Co
4N–HCC is higher than that of Co
3O
4, while the binding energy of the peak attributed to Co−N for Co
3O
4–Co
4N–HCC is lower than that of Co
4N, suggesting the modified electronic structure, which could be due to the transfer of partial electrons from Co
3O
4 to Co
4N, and that could lead to synergistic effect, where the desorption of H
ad and the release of OH
− could be occurred during the HER (
Figure 4a), and that could enhance the intrinsic activity for HER. It contains Co, N and O, which are uniformly distributed. It possesses porous nanosheet morphology. For HER in 1 M KOH, the Co
3O
4–Co
4N–HCC exhibited a η of −90 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay for 40 h (
Figure 4b), suggesting its very high stability.
Mo-based electrocatalysts are considered as highly HER active catalysts [
16,
141]. Thus, fabrication of mesoporous carbon spheres with uniform distribution of Mo-based heterostructures through facile metal–organic coordination precursor-assisted synthesis could be desirable to achieve efficient water electrolysis, while it could modify the electronic structure, and provide porous nanostructure, and that could afford abundant active sites, enhance the conductivity, facilitate the gas-evolution behavior, provide optimal adsorption energy with intermediates, and that could enhance the performance for HER. Li et al. [
89] observed that the Mo
2C–Mo
2N–C exhibited enhanced activity and stability for HER. The Mo
2C–Mo
2N–C was prepared through facile metal–organic coordination precursor-assisted synthesis by the following steps: It was obtained by carbonization of the as-prepared metal–organic precipitate at 850 °C for 2 h under Ar atmosphere followed by etching of the silica templates. The Mo
2C–Mo
2N–C exhibited higher HER activity than that of Mo
2C and MoN. The Mo
2C–Mo
2N–C possesses homogenous spherical morphology with a mesoporous structure having thin carbon layers on its surface. It possesses high surface area of 496 m
2 g
−1. It possesses abundant nanoparticles, which are uniformly distributed, where the nanoparticles could be Mo-based nanocrystallites. It is composed of crystalline Mo
2C and Mo
2N nanoparticles, which are uniformly distributed over carbon matrix. It contains Mo
2C/Mo
2N heterojunctions. It contains C, N, and Mo, where the Mo and N are uniformly distributed within the carbon layers. It contains 4.89 atomic percent of N. It could contain pyrrolic N, pyridinic N, graphitic N, N–Mo, and oxidized N. For HER in 1 M KOH, the Mo
2C–Mo
2N–C exhibited a η of −145 mV at −10 mA cm
−2, suggesting its very high activity, while it exhibited negligible decay at >16 h, suggesting its high stability.
The fabrication of transition metal oxide/metal nanoparticles decorated heteroatom-doped graphene as the electrocatalyst for HER through a facile synthesis route could be desirable to achieve efficient water electrolysis. The Fe
2O
3–Co–N–graphene [
93] exhibited enhanced activity (η of ~−409 mV at −10 mA cm
−2) and stability (negligible decay for 11 h) for HER in 1 M NaOH, while it is composed of Fe
2O
3 and Co nanoparticles, which are decorated on N-doped graphene. The Fe
2O
3–Co–N–graphene was obtained by the loading of Co
2+ and iron (II) phthalocyanine on the graphene oxide followed by one-step calcination process, where dopamine was used as the precursor. In addition, the formation of Fe
2O
3 and Co nanoparticles, doping of N, and reduction of graphene oxide were occurred in the one-step calcination process.