Diversity and Structure of Vegetation Rhizosphere Bacterial Community in Various Habitats of Liaohekou Coastal Wetlands
Abstract
:1. Introduction
2. Materials and Methods
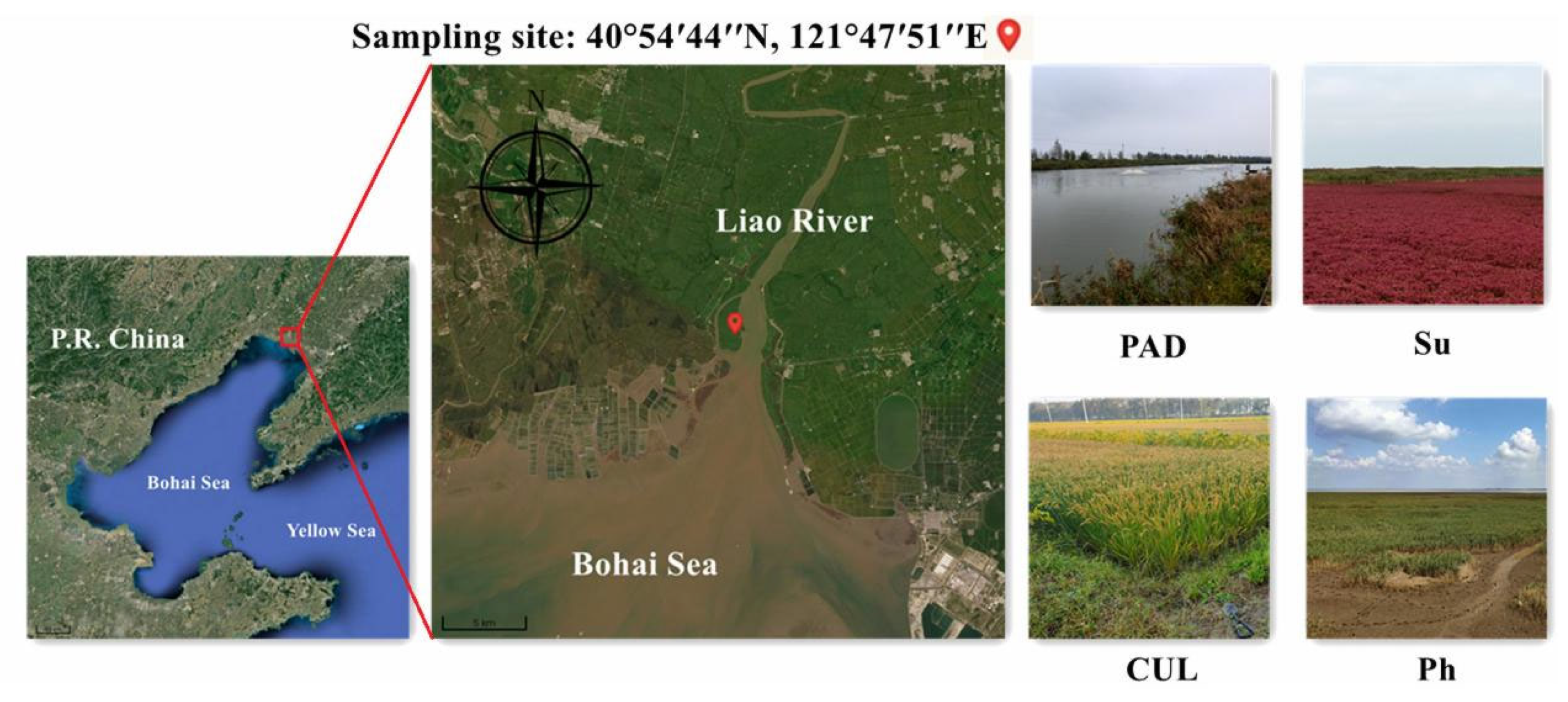
2.1. Study Area
2.2. Soil Samples Collection
2.3. Physicochemical Analysis
2.4. High-Throughput Sequencing
2.5. Data Analysis
3. Results and Discussion
3.1. Soil Physicochemical Properties
3.2. Diversity Indexes and Differences in Bacterial Community
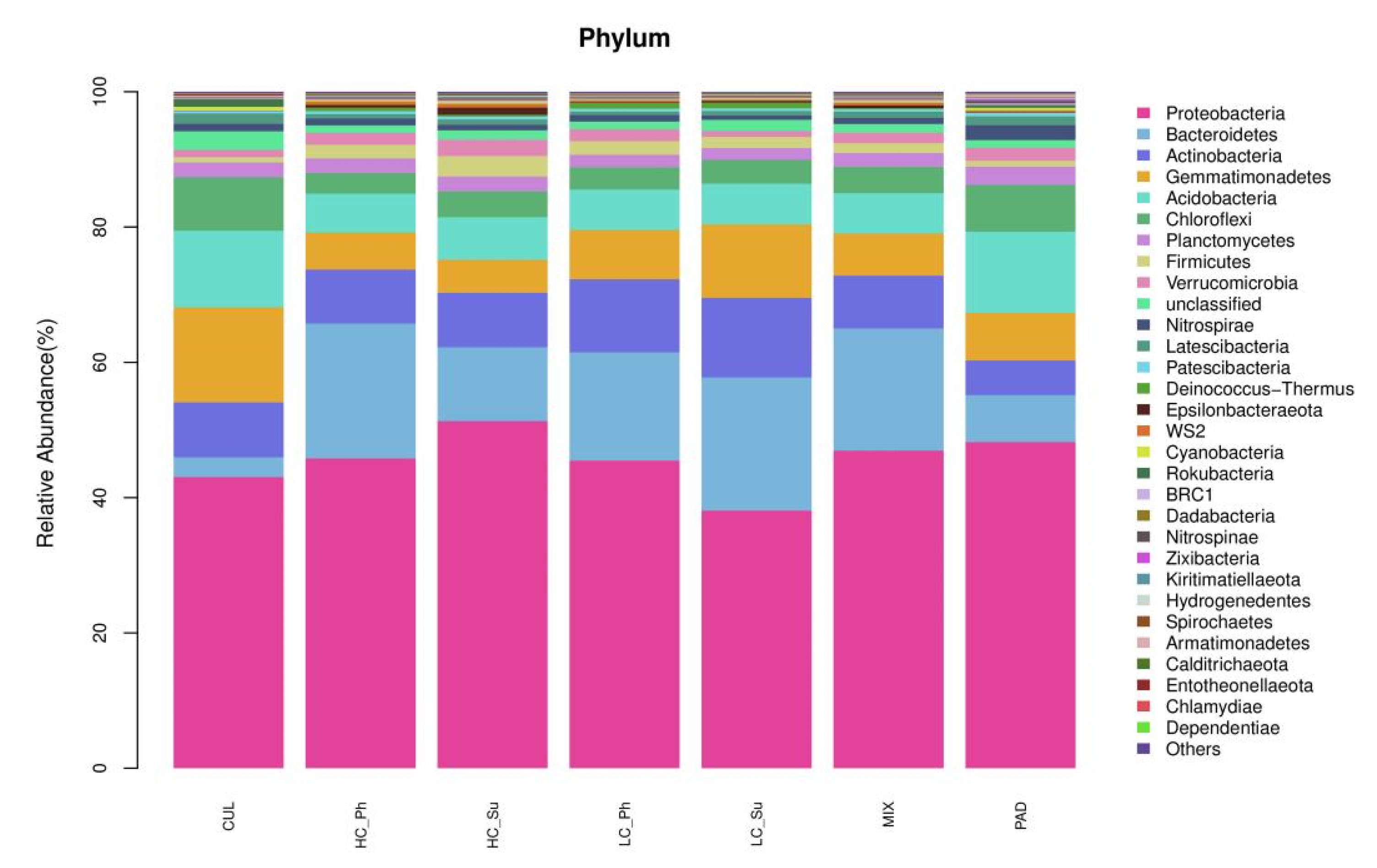
3.3. Composition and Structure of the Bacterial Community
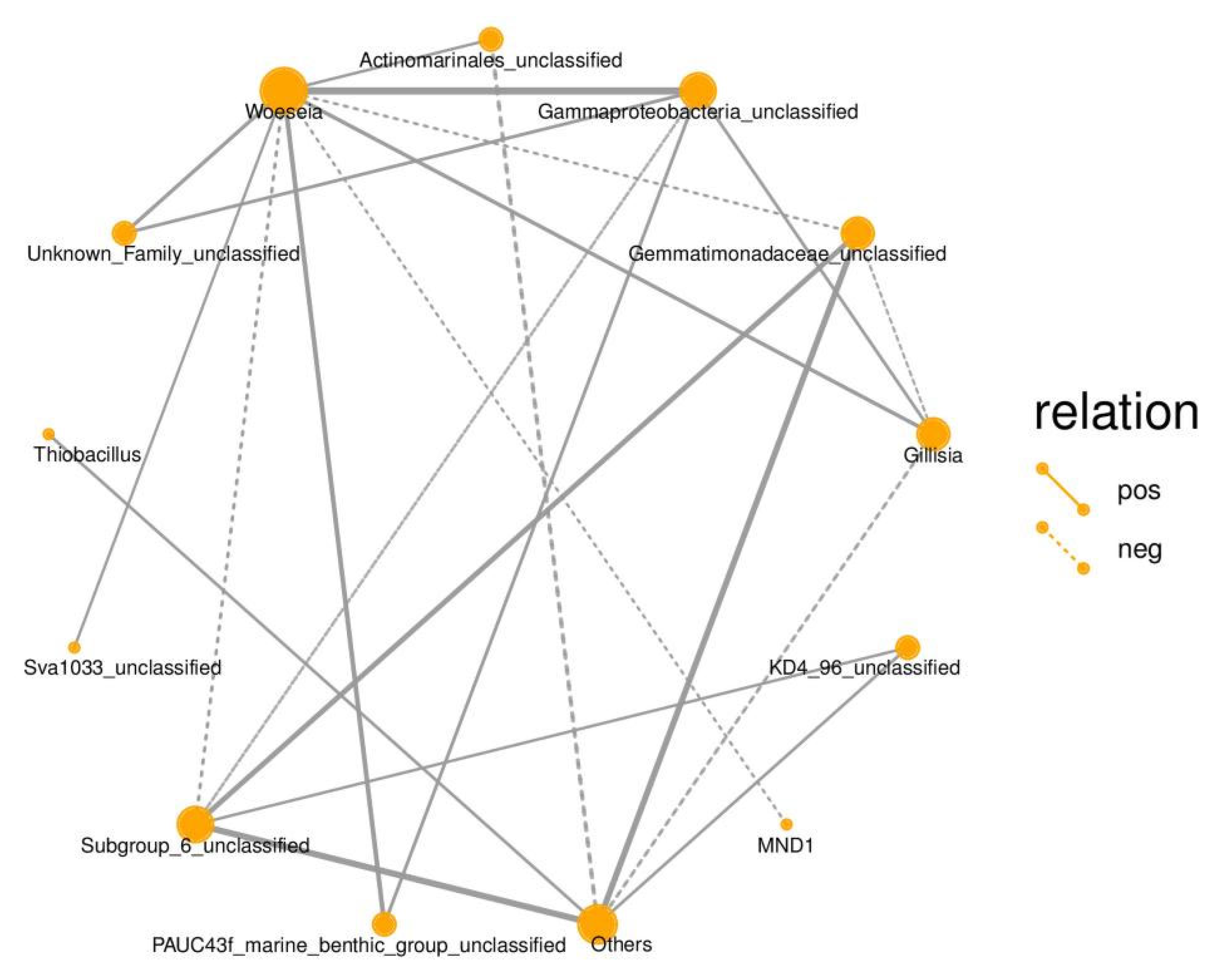
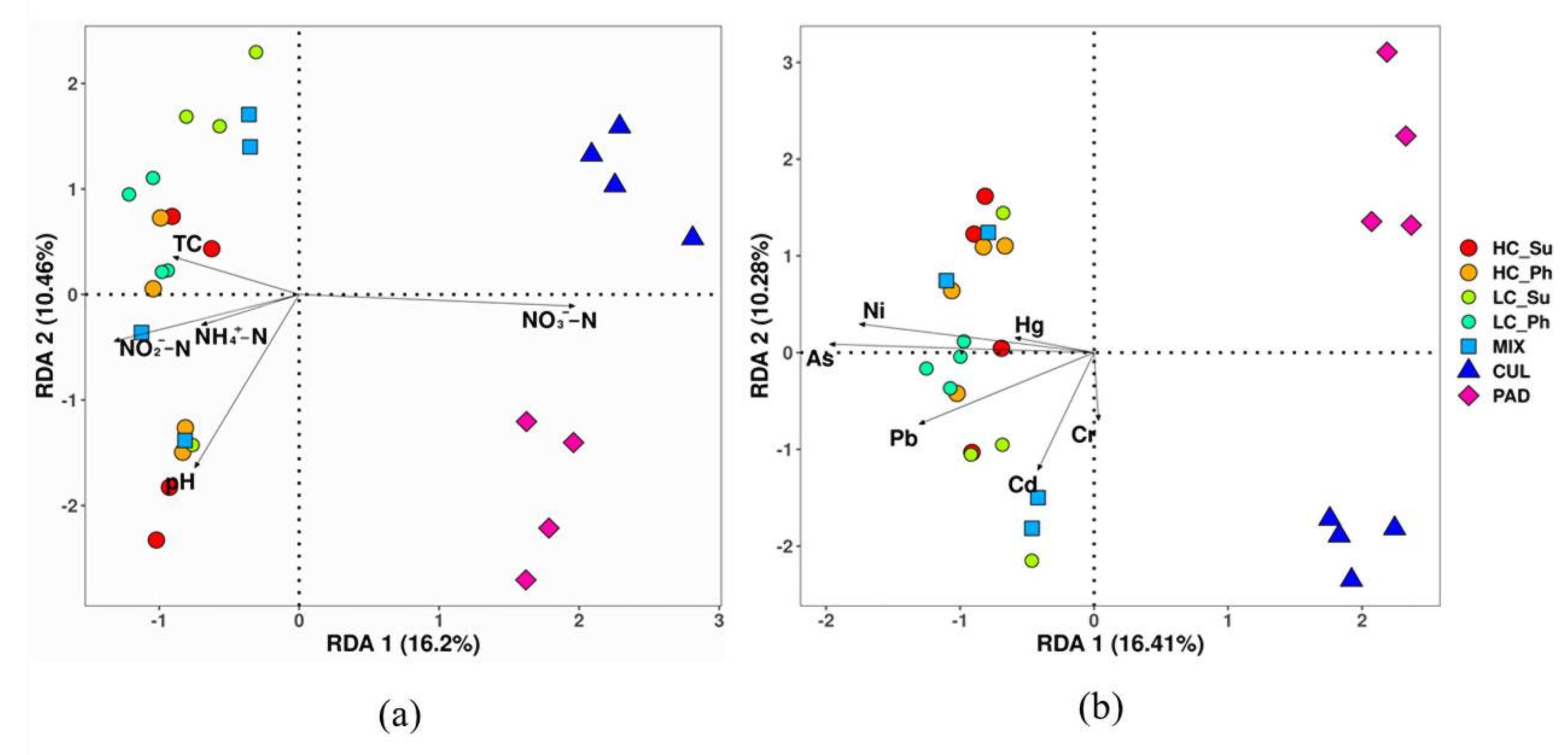
3.4. Correlation Analysis of Environmental Factors
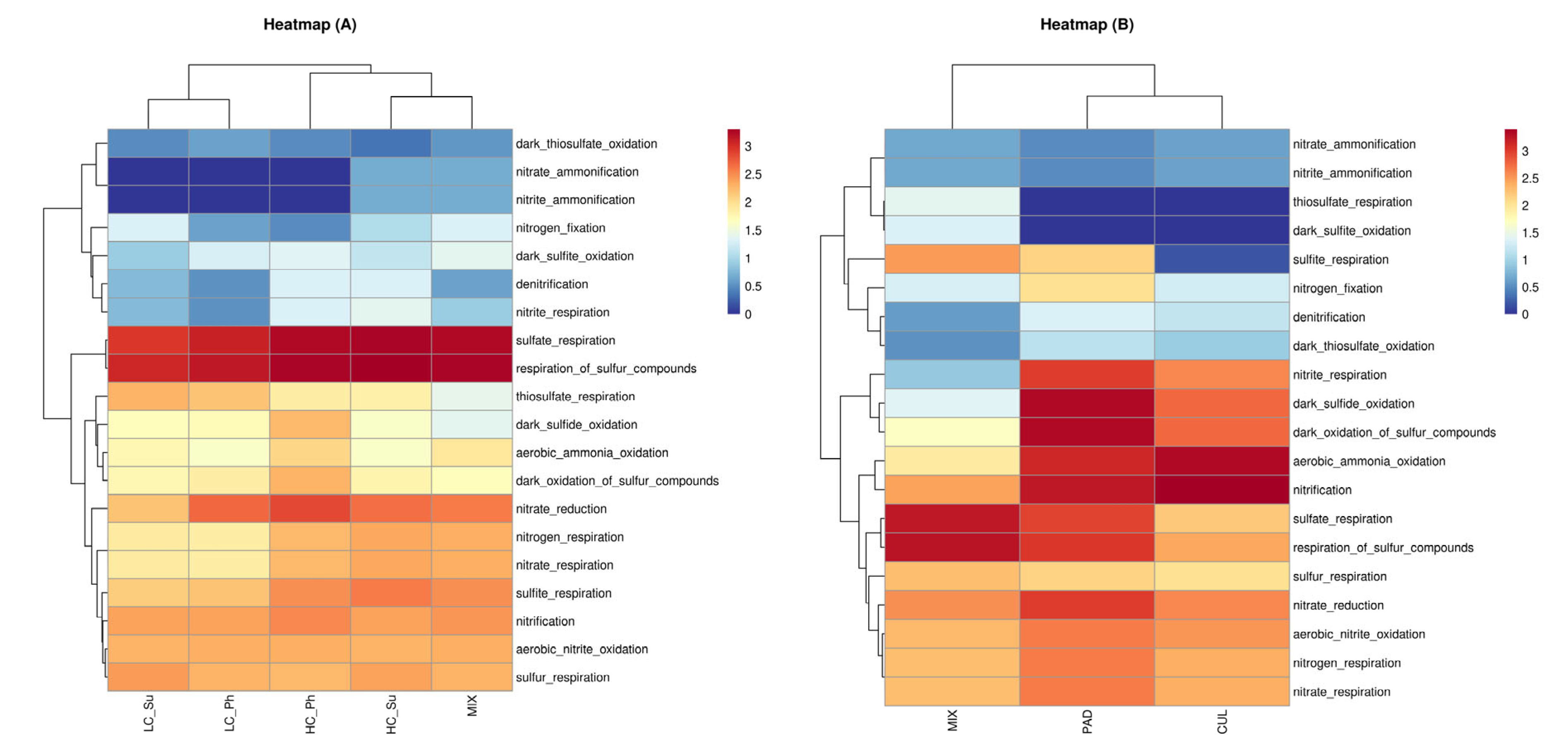
3.5. Metabolic Function Analysis of Bacterial Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Bai, J.; Wang, J.; Zhang, G.; Wang, W.; Wang, X.; Zhang, L.; Wang, Y.; Liu, X.; Cui, B. Different stochastic processes regulate bacterial and fungal community assembly in estuarine wetland soils. Soil Biol. Biochem. 2022, 167, 108586. [Google Scholar] [CrossRef]
- Angeloni, N.L.; Jankowski, K.J.; Tuchman, N.C.; Kelly, J.J. Effects of an invasive cattail species (Typha x glauca) on sediment nitrogen and microbial community composition in a freshwater wetland. FEMS Microbiol. Lett. 2006, 263, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temitayo, T.A.; Olubukola, O.B. Bacterial Diversity and Community Structure in Typical Plant Rhizosphere. Diversity 2019, 11, 179. [Google Scholar]
- Li, E.; de Jonge, R.; Liu, C.; Jiang, H.; Friman, V.P.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Jousset, A. Rapid evolution of bacterial mutualism in the plant rhizosphere. Nat. Commun. 2021, 12, 3829. [Google Scholar] [CrossRef]
- Eisenhauer, N. Aboveground–Belowground Linkages: Biotic Interactions, Ecosystem Processes and Global Change. Basic Appl. Ecol. 2011, 12, 478–479. [Google Scholar] [CrossRef]
- Kumar, M.; Mannisto, M.; van Elsas, J.; Nissinen, R. Plants impact structure and function of bacterial communities in Arctic soils. Plant Soil 2016, 399, 319–332. [Google Scholar] [CrossRef]
- Fu, Y.; Qi, Y.; Kang, J.; Yu, J.; Ma, G.; Wang, C.; Sunn, Y. Coastal Wetland Vulnerability Under the Influence of Human Activities: A Case Study of the Liaohe Estuary. J. Mar. Environ. Eng. 2020, 10, 181–193. [Google Scholar]
- Ye, H.; Guo, S.; Li, F.; Li, G. Water quality evaluation in Tidal River reaches of Liaohe River Estuary, China using a revised QUAL2K model. Chin. Geogr. Sci. 2013, 23, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Sanjosé, I.; Muñoz-Rodríguez, A.F.; Ruiz, F.; Navarro, F.; Sánchez-Gullón, E.; Nieva, F.J.J.; Polo, A.; Infante, M.D.; Castillo, J.M. Metal effects on germination and seedling development in closely-related halophyte species inhabiting different elevations along the intertidal gradient. Mar. Pollut. Bull. 2022, 175, 113375. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Zhao, Q.; Yu, L. Salinity controls soil microbial community structure and function in coastal estuarine wetlands. Environ. Microbiol. 2020, 23, 1020–1037. [Google Scholar] [CrossRef]
- Yang, J.; Zhan, C.; Li, Y.; Zhou, D.; Yu, Y.; Yu, J. Effect of salinity on soil respiration in relation to dissolved organic carbon and microbial characteristics of a wetland in the Liaohe River estuary, Northeast China. Sci. Total Environ. 2018, 642, 946–953. [Google Scholar] [CrossRef]
- Hou, Y.X.; Wang, Y.; Li, H.Y.; Li, X.X.; Hu, X.J. Accumulation and Distribution of Heavy Metals in Phragmites australis in the Wetland of Liaohe River Estuary. Adv. Mater. Res. 2011, 356–360, 994–997. [Google Scholar] [CrossRef]
- Zhao, J.X.; Hui, J.; Zhang, J. Microbial diversity and physicochemical properties of rhizosphere microenvironment in saline-alkali soils of the Yellow River delta. Environ. Sci. 2020, 41, 1449–1455. [Google Scholar]
- Zhang, X.; Ji, Z.; Shao, Y.; Guo, C.; Zhou, H.; Liu, L.; Qu, Y. Seasonal variations of soil bacterial communities in Suaeda wetland of Shuangtaizi River estuary, Northeast China. J. Environ. Sci. 2020, 97, 45–53. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhao, Y.; Yao, X.; Wang, J.; Zheng, P.; Xi, C.; Hu, B. Dominance of comammox Nitrospira in soil nitrification. Sci. Total Environ. 2021, 780, 146558. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Wang, Z.; Wu, X.; Lai, C.; Chen, X. Effects of different cropping systems on ammonia nitrogen load in a typical agricultural watershed of South China. J. Contam. Hydrol. 2022, 246, 103963. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; Xiao, W.; Jia, L.; Zhang, X.; Wang, J.; Zhang, Z.; Xie, Y.; Pu, Y.; Liu, S.; et al. Large methane emission from freshwater aquaculture ponds revealed by long-term eddy covariance observation. Agric. For. Meteorol. 2021, 308–309, 108600. [Google Scholar] [CrossRef]
- Strecker, T.; Jesch, A.; Bachmann, D.; Jüds, M.; Karbstein, K.; Ravenek, J.; Roscher, C.; Weigelt, A.; Eisenhauer, N.; Scheu, S. Incorporation of mineral nitrogen into the soil food web as affected by plant community composition. Ecol. Evol. 2021, 11, 4295–4309. [Google Scholar] [CrossRef]
- Chen, X.; Wei, W.; Wang, J.; Li, H.; Sun, J.; Ma, R.; Jiao, N.; Zhang, R. Tide driven microbial dynamics through virus-host interactions in the estuarine ecosystem. Water Res. 2019, 160, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Heo, Y.; Kwon, S.; Yoo, Y.; Kim, D.; Lee, J.; Kwon, B.; Khim, J.; Kim, J. Environmental drivers affecting the bacterial community of intertidal sediments in the Yellow Sea. Sci. Total Environ. 2020, 755, 142726. [Google Scholar] [CrossRef]
- Chang, Y.; Fan, J.; Su, J.; Ming, H.; Zhao, W.; Shi, Y.; Ji, F.; Guo, L.; Zan, S.; Li, B.; et al. Spatial Abundance, Diversity, and Activity of Ammonia-Oxidizing Bacteria in Coastal Sediments of the Liaohe Estuary. Curr. Microbiol. 2017, 74, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Daraz, U.; Sun, Q.; Chen, P.; Wei, X. Denitrifier abundance and community composition linked to denitrification potential in river sediments. Environ. Sci. Pollut. Res. 2021, 28, 51928–51939. [Google Scholar] [CrossRef]
- Bauer, M.; Kube, M.; Teeling, H.; Richter, M.; Lombardot, T.; Allers, E.; Würdemann, C.A.; Quast, C.; Kuhl, H.; Knaust, F.; et al. Whole genome analysis of the marine Bacteroidetes ‘Gramella forsetii’ reveals adaptations to degradation of polymeric organic matter. Environ. Microbiol. 2006, 8, 2201–2213. [Google Scholar] [CrossRef]
- Mawang, C.-I.; Azman, A.-S.; Fuad, A.-S.M.; Ahamad, M. Actinobacteria: An eco-friendly and promising technology for the bioaugmentation of contaminants. Biotechnol. Rep. 2021, 32, e00679. [Google Scholar] [CrossRef]
- Zeng, Y.; Baumbach, J.; Barbosa, E.G.V.; Azevedo, V.; Zhang, C.; Koblížek, M. Metagenomic evidence for the presence of phototrophic Gemmatimonadetes bacteria in diverse environments. Environ. Microbiol. Rep. 2016, 8, 139–149. [Google Scholar] [CrossRef]
- Fan, D.; Han, J.; Chen, Y.; Zhu, Y.; Li, P. Hormetic effects of Cd on alkaline phosphatase in soils across particle–size fractions in a typical coastal wetland. Sci. Total Environ. 2018, 613–614, 792–797. [Google Scholar] [CrossRef]
- An, X.; Wang, Z.; Teng, X.; Zhou, R.; Wang, X.; Xu, M.; Lian, B. Rhizosphere bacterial diversity and environmental function prediction of wild salt-tolerant plants in coastal silt soil. Ecol. Indic. 2022, 134, 108503. [Google Scholar] [CrossRef]
- Martin, B.C.; Middleton, J.A.; Skrzypek, G.; Kendrick, G.A.; Cosgrove, J.; Fraser, M.W. Composition of Seagrass Root Associated Bacterial Communities Are Linked to Nutrients and Heavy Metal Concentrations in an Anthropogenically Influenced Estuary. Front. Mar. Sci. 2022, 8, 768864. [Google Scholar] [CrossRef]
- Fernandes, G.L.; Shenoy, B.D.; Damare, S.R. Diversity of Bacterial Community in the Oxygen Minimum Zones of Arabian Sea and Bay of Bengal as Deduced by Illumina Sequencing. Front. Microbiol. 2019, 10, 3153. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, X.; Luan, S.; Zhou, H.; Qu, Y. Diversity and structure of soil bacterial community in intertidal zone of Daliao River estuary, Northeast China. Mar. Pollut. Bull. 2021, 163, 111965. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mo, X.; Kong, W.; Song, Y. Soil bacterial diversity, structure, and function of Suaeda salsa in rhizosphere and non-rhizosphere soils in various habitats in the Yellow River Delta, China. Sci. Total Environ. 2020, 740, 140144. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ma, B.; Yu, J.; Chang, S.X.; Xu, J.; Li, Y.; Wang, G.; Han, G.; Bo, G.; Chu, X. Bacterial community structure and function shift along a successional series of tidal flats in the Yellow River Delta. Sci. Rep. 2016, 6, 36550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chi, Z.; Li, J.; Wu, H.; Yan, B. Bacterial community structure and function in soils from tidal freshwater wetlands in a Chinese delta: Potential impacts of salinity and nutrient. Sci. Total Environ. 2019, 696, 134029. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Huang, J.; Wang, H.; Tang, D. Effects of soil heavy metal pollution on microbial activities and community diversity in different land use types in mining areas. Environ. Sci. Pollut. Res. 2020, 27, 20215–20226. [Google Scholar] [CrossRef]
- Yang, G.; Miao, C.; Xia, J.; Liang, M.; Pei, Z. Plant diversity reduces the effect of multiple heavy metal pollution on soil enzyme activities and microbial community structure. Front. Environ. Sci. Eng. 2012, 6, 213–223. [Google Scholar]
- Yuan, C.; Qiao, J.; Li, F.; Zhang, X.; Du, Y.; Hu, M.; Sun, W. Community dynamics of As(V)-reducing and As(III)-oxidizing genes during a wet–dry cycle in paddy soil amended with organic matter, gypsum, or iron oxide. J. Hazard. Mater. 2020, 393, 122485. [Google Scholar] [CrossRef]
- Munazzam, J.S.; Shafaqat, A.; Ghulam, S.; Muhammad, S.; Muhammad, R.; Mahmoud, F.S.; Muhammad, A. Comparing the performance of four macrophytes in bacterial assisted floating treatment wetlands for the removal of trace metals (Fe, Mn, Ni, Pb, and Cr) from polluted river water. Chemosphere 2020, 243, 125353. [Google Scholar]
- Etteieb, S.; Zolfaghari, M.; Magdouli, S.; Brar, K.K.; Brar, S.K. Performance of constructed wetland for selenium, nutrient and heavy metals removal from mine effluents. Chemosphere 2021, 281, 130921. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Yuan, H.; Badgley, B.D.; He, Z. Mainstream upflow nitritation-anammox system with hybrid anaerobic pretreatment: Long-term performance and microbial community dynamics. Water Res. 2017, 125, 298–308. [Google Scholar] [CrossRef]
- Sansupa, C.; Wahdan, S.F.M.; Hossen, S.; Disayathanoowat, T.; Wubet, T.; Purahong, W. Can We Use Functional Annotation of Prokaryotic Taxa (FAPROTAX) to Assign the Ecological Functions of Soil Bacteria? Appl. Sci. 2021, 11, 688. [Google Scholar] [CrossRef]
- Wu, G. Hydrogen sulfide-producing kinetics of Shewanella oneidensis in sulfite and thiosulfate respiration. Process Biochem. 2020, 93, 21–27. [Google Scholar]
- Li, J.; Chen, Q.; Li, Q.; Zhao, C.; Feng, Y. Influence of plants and environmental variables on the diversity of soil microbial communities in the Yellow River Delta Wetland, China. Chemosphere 2021, 274, 129967. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Wan, W.; Luo, X.; Zheng, L.; He, G.; Huang, D.; Chen, W.; Huang, Q. High Salinity Inhibits Soil Bacterial Community Mediating Nitrogen Cycling. Appl. Environ. Microbiol. 2021, 87, e0136621. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, X.-F.; Yu, Y.-Q.; Liu, X.; Zeng, R.J.-X.; Zhou, S.-G.; He, Z. Light-driven nitrous oxide production via autotrophic denitrification by self-photosensitized Thiobacillus denitrificans. Environ. Int. 2019, 127, 353–360. [Google Scholar] [CrossRef]
- Waqas, M.A.; Li, Y.E.; Ashraf, M.N.; Ahmed, W.; Wang, B.; Sardar, M.F.; Ma, P.; Li, R.; Wan, Y.; Kuzyakov, Y. Long-term warming and elevated CO2 increase ammonia-oxidizing microbial communities and accelerate nitrification in paddy soil. Appl. Soil Ecol. 2021, 166, 104063. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Wang, B.; Li, X.; Zhang, Q. Synergistic Partial-Denitrification, Anammox, and in-situ Fermentation (SPDAF) Process for Advanced Nitrogen Removal from Domestic and Nitrate-Containing Wastewater. Environ. Sci. Technol. 2020, 54, 3702–3713. [Google Scholar] [CrossRef]
- Xie, X.; Yuan, K.; Yao, Y.; Sun, J.; Lin, L.; Huang, Y.; Lin, G.; Luan, T.; Chen, B. Identification of suspended particulate matters as the hotspot of polycyclic aromatic hydrocarbon degradation-related bacteria and genes in the Pearl River Estuary using metagenomic approaches. Chemosphere 2022, 286, 131668. [Google Scholar] [CrossRef]

| Soil Properties | HC_Ph | HC_Su | LC_Ph | LC_Su | MIX | PAD | CUL |
|---|---|---|---|---|---|---|---|
| pH | 8.86 ± 0.28 a | 8.70 ± 0.39 ab | 8.20 ± 0.12 c | 8.39 ± 0.15 bc | 8.37 ± 0.40 bc | 8.48 ± 0.06 abc | 8.10 ± 0.23 c |
| TC (g·kg−1) | 11.61 ± 1.59 a | 12.09 ± 2.30 a | 10.47 ± 0.33 a | 9.75 ± 1.21 ab | 11.94 ± 2.16 a | 7.57 ± 0.29 b | 10.73 ± 2.10 a |
| NO3−-N (mg·kg−1) | 12.80 ± 1.15 c | 13.17 ± 0.27 c | 11.86 ± 0.34 c | 12.52 ± 3.13 c | 12.98 ± 0.91 c | 19.13 ± 1.51 b | 25.36 ± 1.58 a |
| NO2−-N (mg·kg−1) | 2.02 ± 0.95 abc | 2.48 ± 0.60 ab | 2.45 ± 1.13 ab | 1.52 ± 0.15 bc | 2.86 ± 1.01 a | 1.21 ± 0.11 c | 0.90 ± 0.13 c |
| NH4+-N (mg·kg−1) | 32.02 ± 10.70 | 29.40 ± 5.34 | 29.56 ± 8.48 | 20.65 ± 3.06 | 32.51 ± 3.46 | 19.87 ± 0.46 | 27.34 ± 9.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guo, Z.; Zhang, P.; Du, J.; Gao, P.; Zhang, Z. Diversity and Structure of Vegetation Rhizosphere Bacterial Community in Various Habitats of Liaohekou Coastal Wetlands. Sustainability 2022, 14, 16396. https://doi.org/10.3390/su142416396
Liu Y, Guo Z, Zhang P, Du J, Gao P, Zhang Z. Diversity and Structure of Vegetation Rhizosphere Bacterial Community in Various Habitats of Liaohekou Coastal Wetlands. Sustainability. 2022; 14(24):16396. https://doi.org/10.3390/su142416396
Chicago/Turabian StyleLiu, Yinchu, Zhen Guo, Peidong Zhang, Jun Du, Ping Gao, and Zhiwei Zhang. 2022. "Diversity and Structure of Vegetation Rhizosphere Bacterial Community in Various Habitats of Liaohekou Coastal Wetlands" Sustainability 14, no. 24: 16396. https://doi.org/10.3390/su142416396




