Silicon-Rich Biochar Detoxify Multiple Heavy Metals in Wheat by Regulating Oxidative Stress and Subcellular Distribution of Heavy Metal
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils and Amendments
2.2. Experimental Design
2.3. Analysis Methods
2.3.1. Soil Analysis
2.3.2. Plant Element Analysis
2.3.3. Subcellular Fractionation of Heavy Metals
2.3.4. Determination of Plant Photosynthetic Parameters
2.3.5. Plant Antioxidant System
2.4. Statistical Analysis
3. Results
3.1. Growth and Grain Yield
3.2. Heavy Metal Accumulation in Shoots and Grains
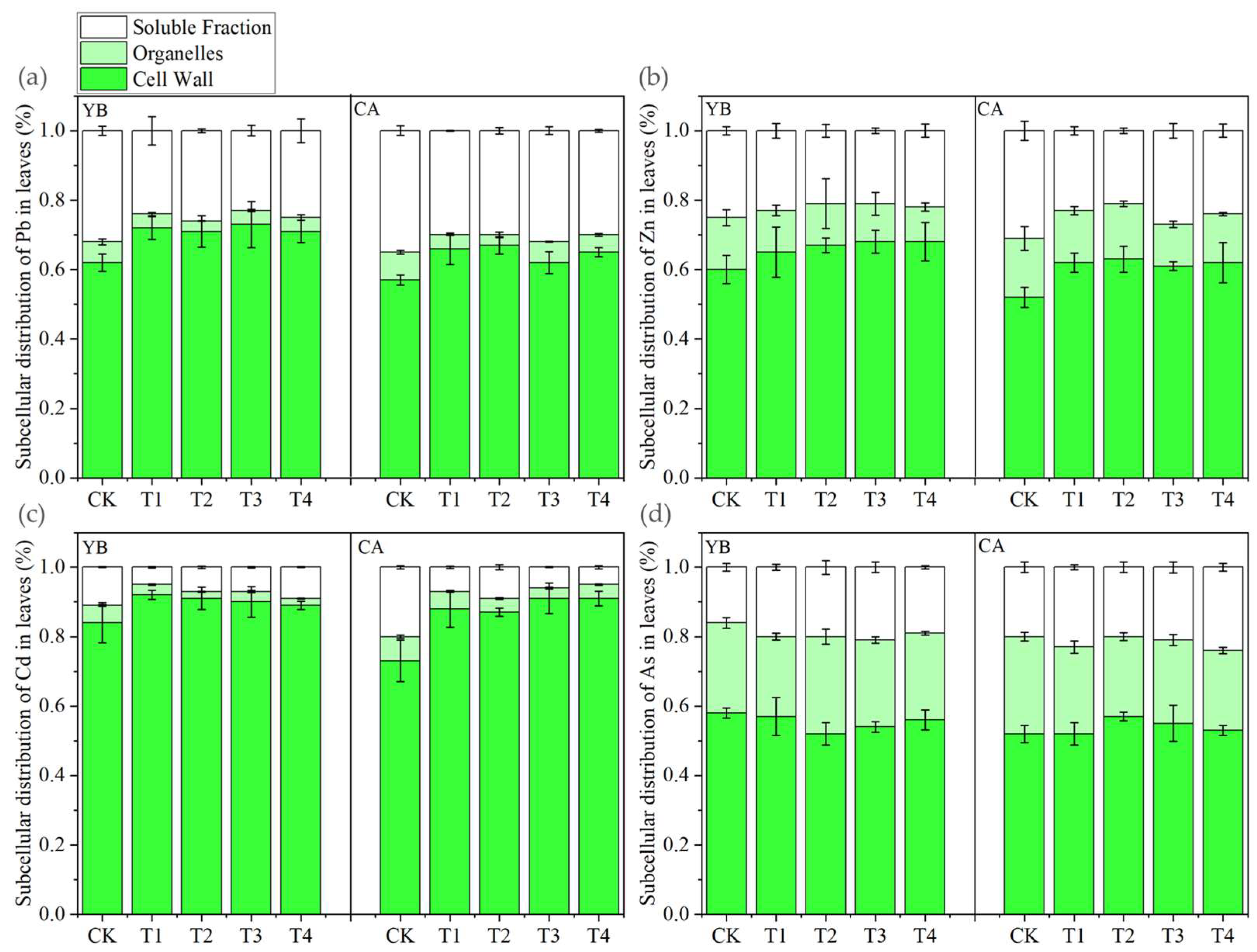
3.3. Subcellular Distribution
3.4. Photosynthetic Parameters
3.5. Soil Heavy Metal Fractionation
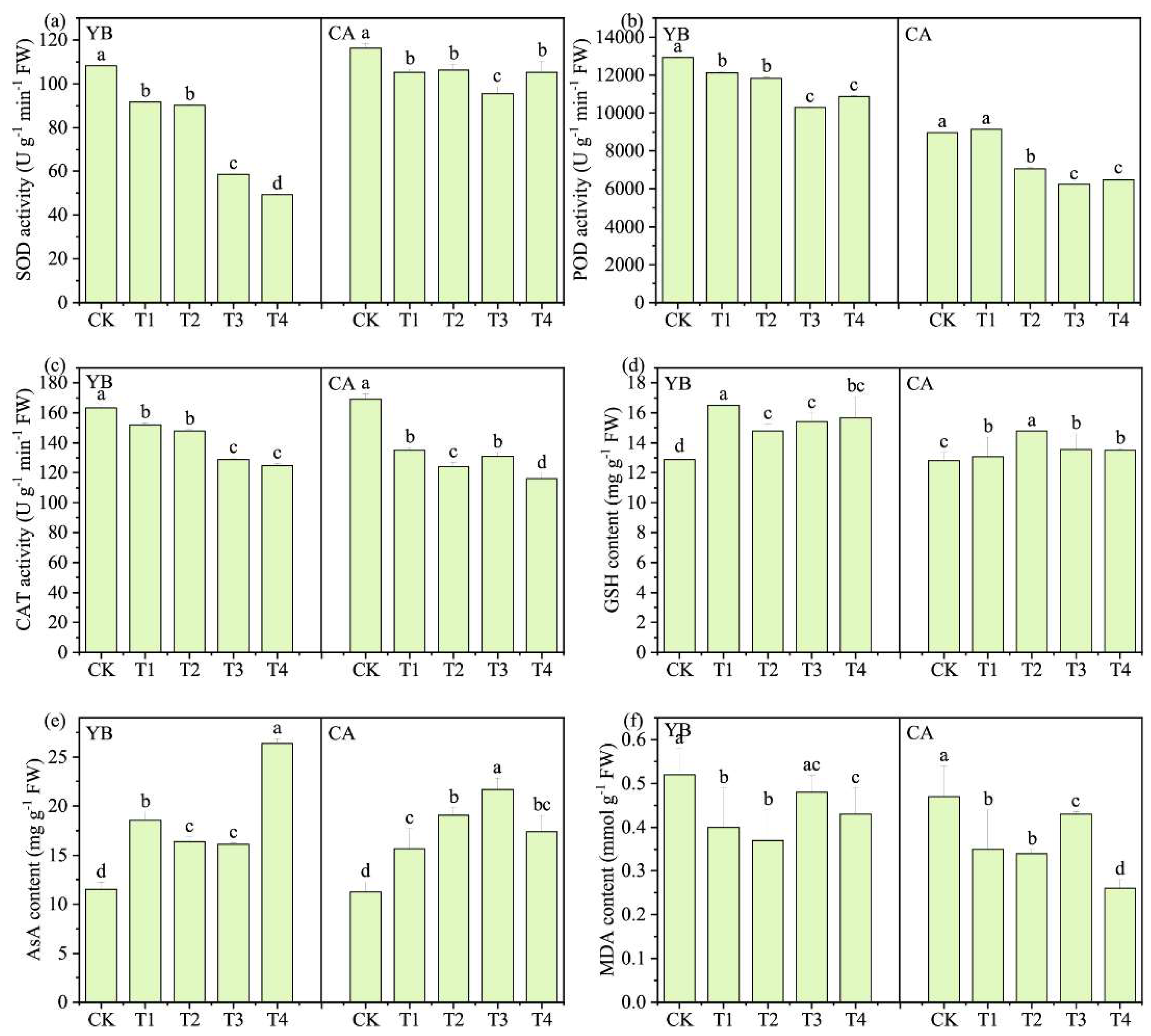
3.6. Antioxidant System
4. Discussion
4.1. Heavy Metal Accumulation
4.2. Plant Growth
4.3. Plant Photosynthesis
4.4. Antioxidant Indicators
4.5. Detoxification Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardena, M.A.A.; Megharaj, M.; Naidu, R. Chapter Three—Exposure, Toxicity, Health Impacts, and Bioavailability of Heavy Metal Mixtures. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 138, pp. 175–234. [Google Scholar]
- Gopalapillai, Y.; Hale, B.A. Internal versus External Dose for Describing Ternary Metal Mixture (Ni, Cu, Cd) Chronic Toxicity to Lemna minor. Environ. Sci. Technol. 2017, 51, 5233–5241. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; He, E.; Qiu, H.; Van Gestel, C.A.M.; Romero-Freire, A.; Zhao, L.; Xu, X.; Cao, X. Interactions of arsenic, copper, and zinc in soil-plant system: Partition, uptake and phytotoxicity. Sci. Total Environ. 2020, 745, 140926. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wu, B.; Wang, C.; Ran, Q. Ecotoxicological effects of metals with different concentrations and types on the morphological and physiological performance of wheat. Ecotoxicol. Environ. Saf. 2019, 167, 345–353. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Shaaban, M.; Hu, H. Efficiency and surface characterization of different plant derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere 2018, 211, 632–639. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Adrees, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Qayyum, M.F.; Nawaz, R. Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar] [CrossRef]
- Li, Z.; Cao, H.; Yuan, Y.; Jiang, H.; Hu, Y.; He, J.; Zhang, Y.; Tu, S. Combined passivators regulate the heavy metal accumulation and antioxidant response of Brassica chinensis grown in multi-metal contaminated soils. Environ. Sci. Pollut. Res. 2021, 28, 49166–49178. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim, M.; Zia-ur-Rehman, M.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 2230–2248. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Nigam, N.; Khare, P.; Yadav, V.; Mishra, D.; Jain, S.; Karak, T.; Panja, S.; Tandon, S. Biochar-mediated sequestration of Pb and Cd leads to enhanced productivity in Mentha arvensis. Ecotoxicol. Environ. Saf. 2019, 172, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Saeed, D.A.; Rizwan, M.; Khan, M.N.; Aziz, O.; Bashir, S.; Ibrahim, M.; Ditta, A.; Akmal, M.; Mumtaz, M.A.; et al. Impact of different amendments on biochemical responses of sesame (Sesamum indicum L.) plants grown in lead-cadmium contaminated soil. Plant Physiol. Biochem. 2018, 132, 345–355. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, Q.; Sun, Y.; Xu, Z. Ecotoxicological effects of typical personal care products on seed germination and seedling development of wheat (Triticum aestivum L.). Chemosphere 2009, 76, 1428–1434. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Kollárová, K.; Kusá, Z.; Vatehová-Vivodová, Z.; Lišková, D. The response of maize protoplasts to cadmium stress mitigated by silicon. Ecotoxicol. Environ. Saf. 2019, 170, 488–494. [Google Scholar] [CrossRef]
- Huang, F.; Wen, X.H.; Cai, Y.X.; Cai, K.Z. Silicon-Mediated Enhancement of Heavy Metal Tolerance in Rice at Different Growth Stages. Int. J. Environ. Res. Public Health 2018, 15, 2193. [Google Scholar] [CrossRef]
- Cao, F.; Dai, H.; Hao, P.F.; Wu, F. Silicon regulates the expression of vacuolar H(+)-pyrophosphatase 1 and decreases cadmium accumulation in rice (Oryza sativa L.). Chemosphere 2020, 240, 124907. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; Zia Ur Rehman, M.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.; Khan, E.; Panthri, M.; Tripathi, R.D.; Gupta, M. Impact of silicon on Indian mustard (Brassica juncea L.) root traits by regulating growth parameters, cellular antioxidants and stress modulators under arsenic stress. Plant Physiol. Biochem. 2016, 104, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, S.; Shi, H.; Zhao, P.; Liu, X.; Li, F.; Deng, T.; Du, R.; Wang, X.; Wang, F. Comparison of foliar silicon and selenium on cadmium absorption, compartmentation, translocation and the antioxidant system in Chinese flowering cabbage. Ecotoxicol. Environ. Saf. 2018, 166, 157–164. [Google Scholar] [CrossRef]
- Fatemi, H.; Esmaiel Pour, B.; Rizwan, M. Isolation and characterization of lead (Pb) resistant microbes and their combined use with silicon nanoparticles improved the growth, photosynthesis and antioxidant capacity of coriander (Coriandrum sativum L.) under Pb stress. Env. Pollut 2020, 266, 114982. [Google Scholar] [CrossRef]
- Howladar, S.M.; Al-Robai, S.A.; Al-Zahrani, F.S.; Howladar, M.M.; Aldhebiani, A.Y. Silicon and its application method effects on modulation of cadmium stress responses in Triticum aestivum (L.) through improving the antioxidative defense system and polyamine gene expression. Ecotoxicol. Environ. Saf. 2018, 159, 143–152. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Chaurand, P.; Meunier, J.D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Deng, X.; Gong, D.; Du, S.; Wang, S.; Zhang, Z. Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard Mater. 2020, 395, 122679. [Google Scholar] [CrossRef]
- Wallheimer, B.; Brian, Y. Rice Hulls a Sustainable Drainage Option for Greenhouse Growers; Purdue University: West Lafayette, IN, USA, 2010. [Google Scholar]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A Review of Silicon in Soils and Plants and Its Role in US Agriculture: History and Future Perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Leksungnoen, P.; Wisawapipat, W.; Ketrot, D.; Aramrak, S.; Nookabkaew, S.; Rangkadilok, N.; Satayavivad, J. Biochar and ash derived from silicon-rich rice husk decrease inorganic arsenic species in rice grain. Sci. Total Environ. 2019, 684, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Thind, H.S.; Yadvinder, S.; Bijay, S.; Varinderpal, S.; Sharma, S.; Vashistha, M.; Singh, G. Land application of rice husk ash, bagasse ash and coal fly ash: Effects on crop productivity and nutrient uptake in rice–wheat system on an alkaline loamy sand. Field Crops Res. 2012, 135, 137–144. [Google Scholar] [CrossRef]
- Jeer, M.; Suman, K.; Maheswari, T.U.; Voleti, S.; Padmakumari, A. Rice husk ash and imidazole application enhances silicon availability to rice plants and reduces yellow stem borer damage. Field Crop. Res. 2018, 224, 60–66. [Google Scholar] [CrossRef]
- Singh Karam, D.; Nagabovanalli, P.; Sundara Rajoo, K.; Fauziah Ishak, C.; Abdu, A.; Rosli, Z.; Melissa Muharam, F.; Zulperi, D. An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J. Saudi Soc. Agric. Sci. 2022, 21, 149–159. [Google Scholar] [CrossRef]
- Singh, R.; Srivastava, P.; Singh, P.; Sharma, A.K.; Singh, H.; Raghubanshi, A.S. Impact of rice-husk ash on the soil biophysical and agronomic parameters of wheat crop under a dry tropical ecosystem. Ecol. Indic. 2019, 105, 505–515. [Google Scholar] [CrossRef]
- Qu, J.; Li, B.; Wei, T.; Li, C.; Liu, B. Effects of rice-husk ash on soil consistency and compactibility. catena 2014, 122, 54–60. [Google Scholar] [CrossRef]
- Kiran, B.R.; Prasad, M. Biochar and rice husk ash assisted phytoremediation potentials of Ricinus communis L. for lead-spiked soils. Ecotoxicol. Environ. Saf. 2019, 183, 109574. [Google Scholar] [CrossRef]
- Huang, H.; Rizwan, M.; Li, M.; Song, F.; Zhou, S.; He, X.; Ding, R.; Dai, Z.; Yuan, Y.; Cao, M.; et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255, 113146. [Google Scholar] [CrossRef]
- Shen, Z.; Hou, D.; Jin, F.; Shi, J.; Fan, X.; Tsang, D.C.W.; Alessi, D.S. Effect of production temperature on lead removal mechanisms by rice straw biochars. Sci. Total Environ. 2019, 655, 751–758. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Yao, W.; Han, C.; Sun, S.; Zhao, C. C, N, P, K stoichiometric characteristics of the “leaf-root-litter-soil” system in dryland plantations. Ecol. Indic. 2022, 143, 109371. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil. Calif. Agric. Exp. Stn. Bull. 1937, 347. [Google Scholar]
- Jiang, J.; Xu, R.-K.; Jiang, T.-Y.; Li, Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mater. 2012, 229, 145–150. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Li, M.; Yuan, L. Indications of Selenium Protection against Cadmium and Lead Toxicity in Oilseed Rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1875. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.T.; Liu, T.X.; Wang, X.Q.; Li, F.B.; Lv, Y.H.; Cui, J.H.; Zeng, X.D.; Yuan, Y.Z.; Liu, C.P. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Weigel, H.J.; Jger, H.J. Subcellular Distribution and Chemical Form of Cadmium in Bean Plants. Plant Physiol. 1980, 65, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yang, S.; Han, D.; Zhou, Z.; Li, X.; Liu, Y.; Zhang, B. Silicon alleviates cadmium toxicity in wheat seedlings (Triticum aestivum L.) by reducing cadmium ion uptake and enhancing antioxidative capacity. Environ. Sci. Pollut. Res. 2018, 25, 7638–7646. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–297. [Google Scholar]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Teng, F.; Zhang, Y.; Wang, D.; Shen, M.; Hu, D. Iron-modified rice husk hydrochar and its immobilization effect for Pb and Sb in contaminated soil. J. Hazard. Mater. 2020, 398, 122977. [Google Scholar] [CrossRef]
- Qasim, M.; Bashir, A.; Tanvir, M.; Anees, M.M. Effect of Rice Husk Ash on Soil Stabilization. Bull. Energy Econ. 2015, 3, 10–17. [Google Scholar]
- Garg, N.; Kashyap, L. Joint effects of Si and mycorrhiza on the antioxidant metabolism of two pigeonpea genotypes under As (III) and (V) stress. Env. Sci. Pollut Res. Int. 2019, 26, 7821–7839. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol Environ. Saf 2020, 188, 109885. [Google Scholar] [CrossRef] [PubMed]
- An, Y.-J.; Kim, Y.-M.; Kwon, T.-I.; Jeong, S.-W. Combined effect of copper, cadmium, and lead upon Cucumis sativus growth and bioaccumulation. Sci. Total Environ. 2004, 326, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.-M.; Carvalho, A.; Pascoal, C.; Rodrigues, F.; Cássio, F. Responses of antioxidant defenses to Cu and Zn stress in two aquatic fungi. Sci. Total Environ. 2007, 377, 233–243. [Google Scholar] [CrossRef]
- Saffari, M. Chemical Stabilization of Some Heavy Metals in an Artificially Multi-Elements Contaminated Soil, Using Rice Husk Biochar and Coal Fly Ash. Pollution 2018, 4, 547–562. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Dawodu, F.A. Potential of a low-cost bentonite for heavy metal abstraction from binary component system. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105–106, 200–206. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zeng, G.-M.; Niu, Q.-Y.; Liu, Y.; Zhou, L.; Jiang, L.-H.; Tan, X.-f.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M.; et al. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-n.; Tsang, Y.F.; Wang, H.; Sun, Y.; Song, Y.; Pan, X.; Luo, S. Effective stabilization of arsenic in contaminated soils with biogenic manganese oxide (BMO) materials. Environ. Pollut. 2020, 258, 113481. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Tiwari, S.; Gupta, V.K.; Singh, J.S. The effect of rice husk biochar on soil nutrient status, microbial biomass and paddy productivity of nutrient poor agriculture soils. Catena 2018, 171, 485–493. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Li, K.; Wu, M.; Zhang, R.; Zhang, L.; Chen, G. Photosynthetic responses of Oryza sativa L. seedlings to cadmium stress: Physiological, biochemical and ultrastructural analyses. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2014, 27, 389–401. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Q.; Wang, X.; Wei, M.; Yang, F.; Xu, H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci. Hortic. 2010, 123, 521–530. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Wang, H.; Li, R.; Pan, F.; Wu, J.; Feng, Y.; Ying, Y.; Liu, Q. Effect of silicate supplementation on the alleviation of arsenite toxicity in 93-11 (Oryza sativa L. indica). Environ. Sci. Pollut. Res. 2013, 20, 8579–8589. [Google Scholar] [CrossRef]
- Sanglard, L.M.V.P.; Martins, S.C.V.; Detmann, K.C.; Silva, P.E.M.; Lavinsky, A.O.; Silva, M.M.; Detmann, E.; Araújo, W.L.; DaMatta, F.M. Silicon nutrition alleviates the negative impacts of arsenic on the photosynthetic apparatus of rice leaves: An analysis of the key limitations of photosynthesis. Physiol. Plant. 2014, 152, 355. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Kanwal, S.; Ali, Q.; Ibrahim, M.; Gill, R.A.; Khan, M.D. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 2015, 22, 10601–10609. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, Z.; Sun, Y.; Wang, Y.; Bi, C.; Zhou, L.; Zheng, X. Impacts of biochar and silicate fertilizer on arsenic accumulation in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 189, 109928. [Google Scholar] [CrossRef]
- Saleh, S.; Mohammadnejad, S.; Khorgooei, H.; Otadi, M. Photooxidation/adsorption of arsenic (III) in aqueous solution over bentonite/ chitosan/TiO2 heterostructured catalyst. Chemosphere 2021, 280, 130583. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of Silicon in Mitigation of Heavy Metal Stresses in Crop Plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]




| Soils | pH | Ntotal | Ptotal | Organic Matter | Kavailable | Pavailable | Navailable | b As | b Cd | b Pb | b Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||||||
| a YB | 6.09 | 0.86 | 0.55 | 12.1 | 112.0 | 26.2 | 57.9 | 2.52 | 5.99 | 152.6 | 234.2 |
| a CA | 7.14 | 2.21 | 0.89 | 10.2 | 56.5 | 41.9 | 41.2 | 5.23 | 6.79 | 155.4 | 300.5 |
| Parameters | RHB | Bentonite |
|---|---|---|
| pH | 10.5 ± 0.2 | 9.06 ± 0.3 |
| Grain size (mm) | 0.15 | 0.15 |
| Total N (g kg−1) | 6.8 ± 0.4 | – |
| Total P (g kg−1) | 3.5 ± 0.1 | – |
| Total K (g kg−1) | 14.2 ± 2.5 | – |
| Total Si (g kg−1) | 221 ± 10.2 | 145 ± 5.7 |
| Total Cd (mg kg−1) | – | 1.11 ± 0.4 |
| Total Zn (mg kg−1) | 33.7 ± 2.9 | 67.1 ± 3.3 |
| Total Pb (mg kg−1) | 3.01 ± 0.2 | 25.6 ± 0.9 |
| Total As (mg kg−1) | – | 0.33 ± 0.01 |
| Treatment | Seedling Stage | Maturity Stage | ||
|---|---|---|---|---|
| Shoot Length (cm/Plant) | Shoot Dry Weight (g/Plant) | Shoot Dry Weight (g/Plant) | Grain Dry Weight (g/Pot) | |
| YB soil | ||||
| Control | 61.22 ± 0.81 a | 0.52 ± 0.02 a | 1.12 ± 0.05 a | 3.98 ± 0.51 a |
| T1 | 62.71 ± 1.19 a | 0.54 ± 0.05 a | 1.24 ± 0.12 a | 4.32 ± 0.32 a |
| T2 | 64.48 ± 1.08 a | 0.62 ± 0.01 a | 1.22 ± 0.04 a | 4.41 ± 0.21 a |
| T3 | 65.52 ± 2.77 a | 0.63 ± 0.06 a | 1.26 ± 0.08 a | 4.62 ± 0.49 a |
| T4 | 68.84 ± 3.12 a | 0.66 ± 0.03 a | 1.31 ± 0.21 a | 5.05 ± 0.32 a |
| CA soil | ||||
| Control | 57.12 ± 2.10 a | 0.45 ± 0.02 a | 0.92 ± 0.09 a | 4.15 ± 0.31 a |
| T1 | 56.21 ± 1.92 a | 0.44 ± 0.03 a | 0.98 ± 0.14 a | 4.21 ± 0.56 a |
| T2 | 57.33 ± 1.13 a | 0.49 ± 0.05 a | 0.96 ± 0.11 a | 4.28 ± 0.33 a |
| T3 | 58.21 ± 4.71 a | 0.49 ± 0.02 a | 1.02 ± 0.21 a | 4.31 ± 0.47 a |
| T4 | 58.39 ± 5.12 a | 0.52 ± 0.08 a | 1.05 ± 0.04 a | 4.58 ± 0.27 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yuan, Y.; Xiang, L.; Su, Q.; Liu, Z.; Wu, W.; Huang, Y.; Tu, S. Silicon-Rich Biochar Detoxify Multiple Heavy Metals in Wheat by Regulating Oxidative Stress and Subcellular Distribution of Heavy Metal. Sustainability 2022, 14, 16417. https://doi.org/10.3390/su142416417
Li Z, Yuan Y, Xiang L, Su Q, Liu Z, Wu W, Huang Y, Tu S. Silicon-Rich Biochar Detoxify Multiple Heavy Metals in Wheat by Regulating Oxidative Stress and Subcellular Distribution of Heavy Metal. Sustainability. 2022; 14(24):16417. https://doi.org/10.3390/su142416417
Chicago/Turabian StyleLi, Zheyong, Yajun Yuan, Luojing Xiang, Qu Su, Zhenyan Liu, Wenguang Wu, Yihao Huang, and Shuxin Tu. 2022. "Silicon-Rich Biochar Detoxify Multiple Heavy Metals in Wheat by Regulating Oxidative Stress and Subcellular Distribution of Heavy Metal" Sustainability 14, no. 24: 16417. https://doi.org/10.3390/su142416417
APA StyleLi, Z., Yuan, Y., Xiang, L., Su, Q., Liu, Z., Wu, W., Huang, Y., & Tu, S. (2022). Silicon-Rich Biochar Detoxify Multiple Heavy Metals in Wheat by Regulating Oxidative Stress and Subcellular Distribution of Heavy Metal. Sustainability, 14(24), 16417. https://doi.org/10.3390/su142416417








