Hydrolyzed Fish Collagen Serum from By-Product of Food Industry: Cosmetic Product Formulation and Facial Skin Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of HC Powder of Asian Seabass Skin and Characterization
2.3. Preparation of HC Serum
2.4. Characterization of Physical Properties of HC Serum
2.5. Antioxidant Activity of HC Serum by DPPH Radical Scavenging Assay
2.6. Stability Study of HC Serum
2.7. Microbiological Testing of HC Serum
2.8. Heavy Metals Testing of HC Serum
2.9. Facial Skin Evaluation of HC Serum
2.9.1. Skin Irritation Test
2.9.2. Skin Testing by Visia Skin Analysis
2.9.3. Satisfaction Assessment Questionnaire
2.10. Data Analysis
3. Results
3.1. HC Powder of Asian Seabass Skin and Physicochemical Properties
3.2. HC Serum and Physicochemical Properties
3.3. Stability of HC Serum
3.4. Microbiological Amount in HC Serum
3.5. Heavy Metals Amount in HC Serum
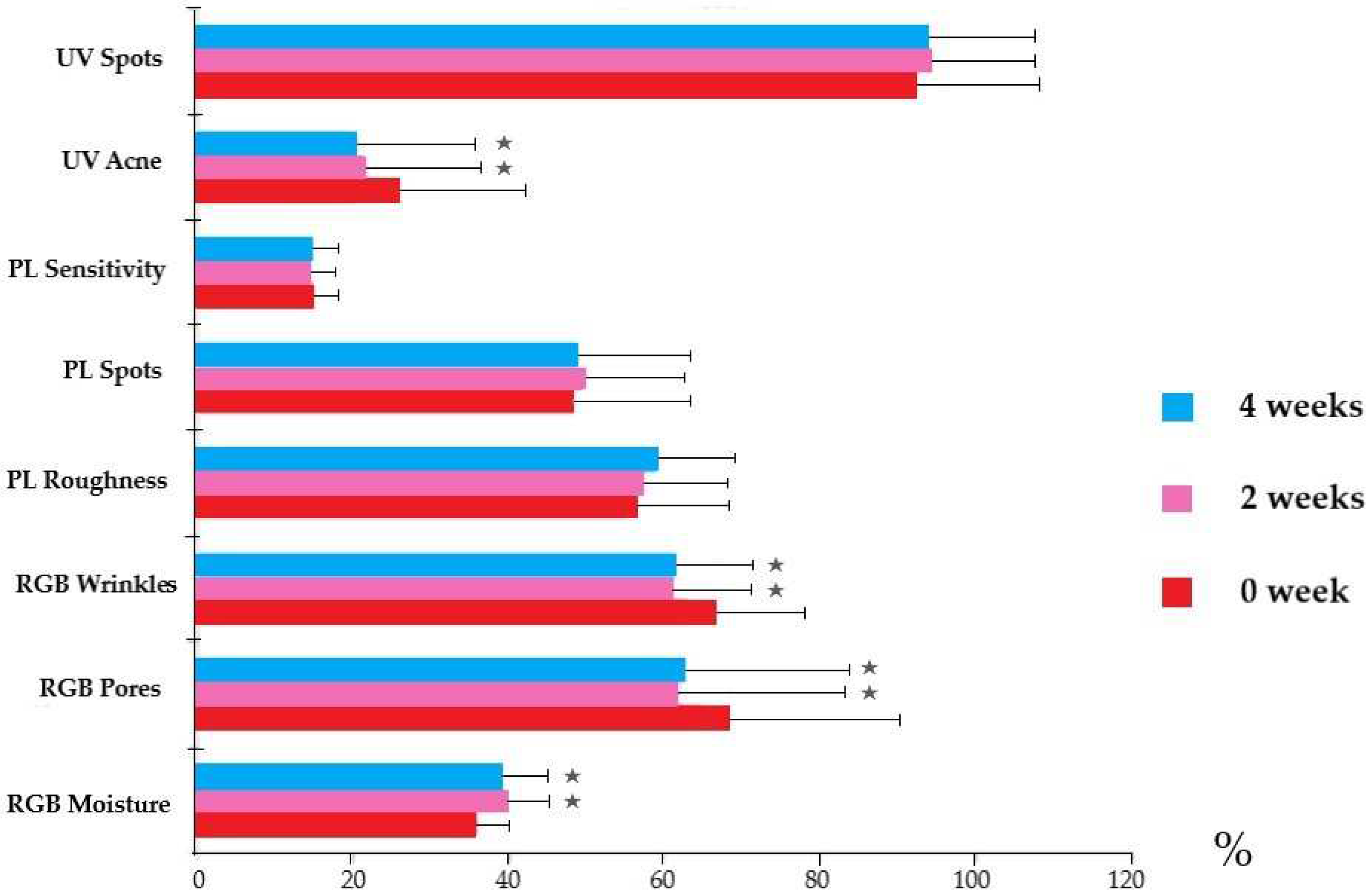
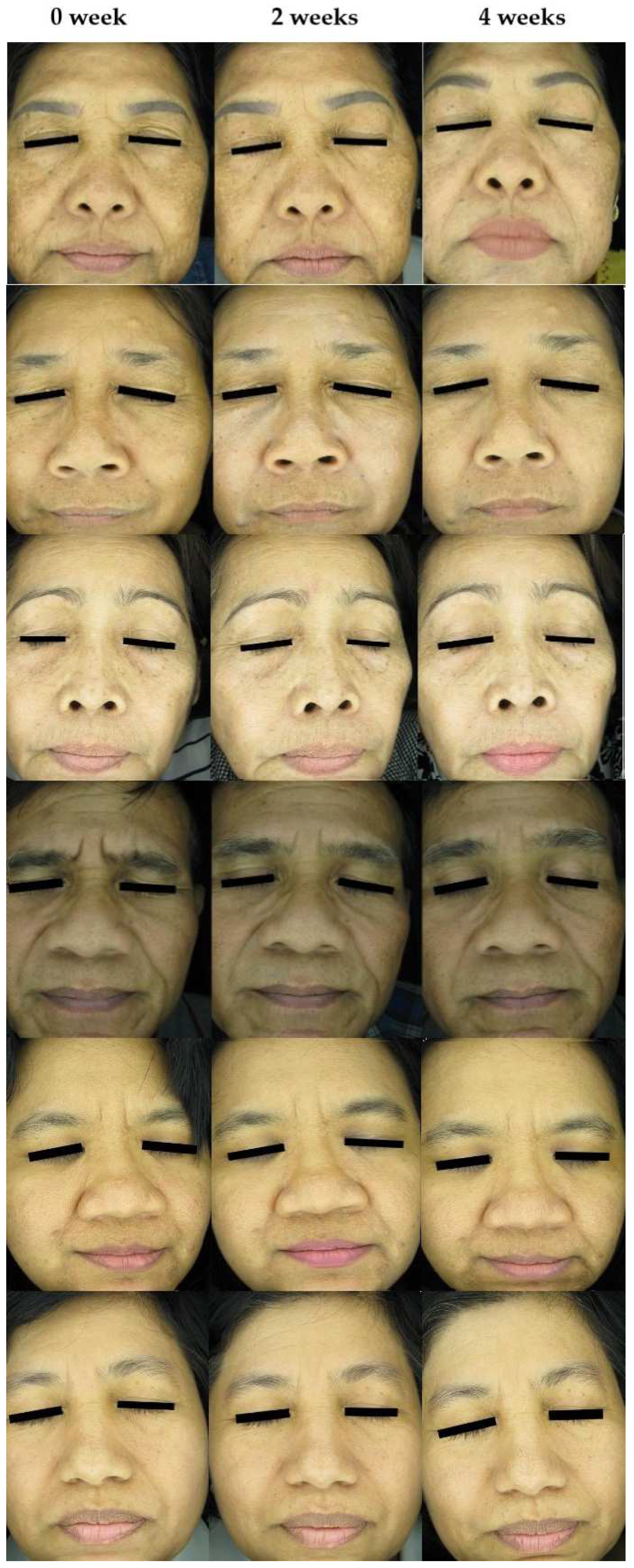
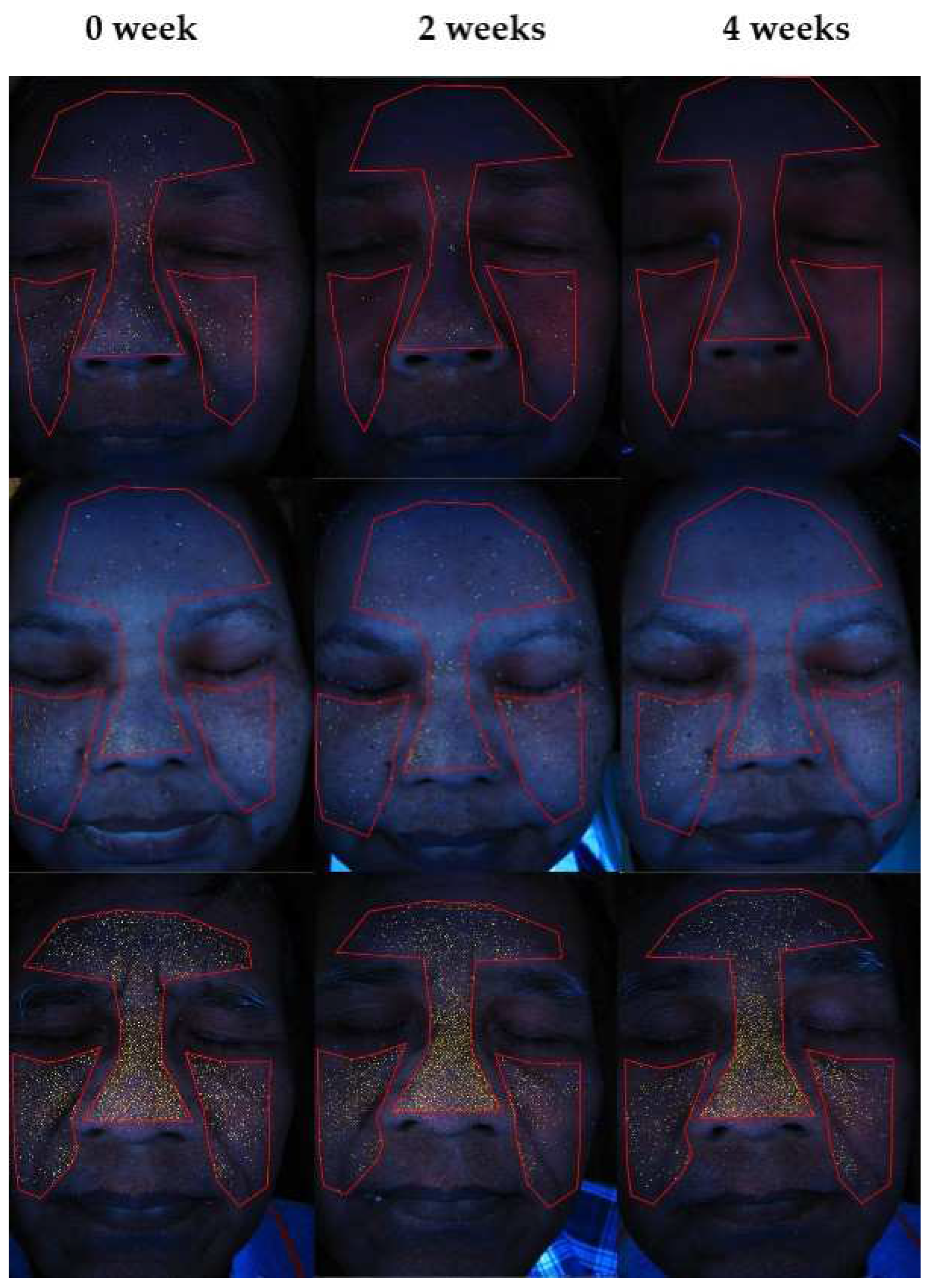
3.6. Facial Skin Evaluation
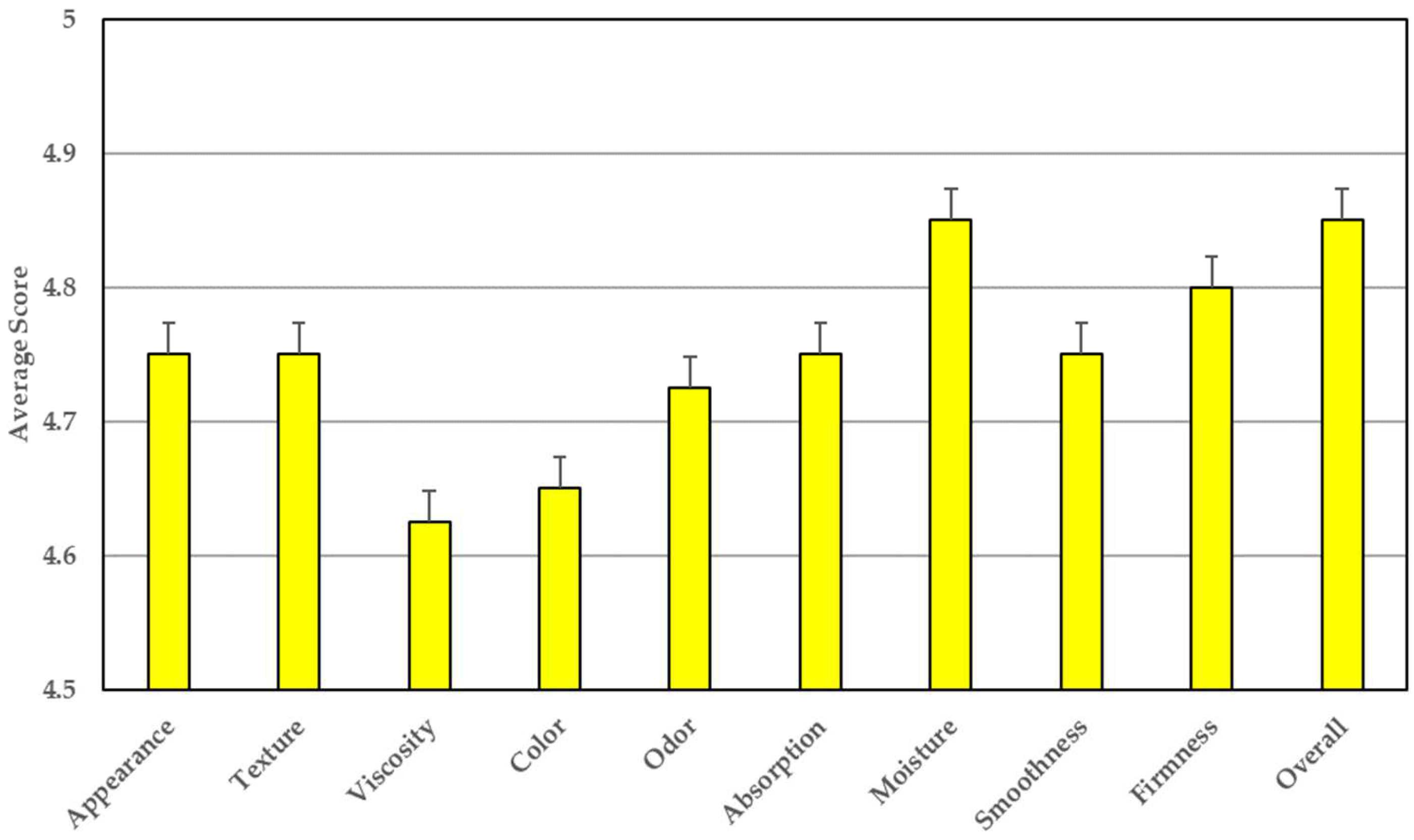
3.7. Satisfaction Questionnaire of HC Serum from Volunteers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Production and characterization of odorless antioxidative hydrolyzed collagen from seabass (Lates calcarifer) skin without descaling. Waste Biomass Valor. 2018, 9, 549–559. [Google Scholar]
- Sae-Leaw, T.; Benjakul, S.; O’Brien, N.M. Effect of pretreatments and drying methods on the properties and fishy odor/flavor of gelatin from seabass (Lates calcarifer) skin. Dry. Technol. 2016, 34, 53–65. [Google Scholar]
- Sae-leaw, T.; Karnjanapratum, S.; O’Callaghan, Y.C.; O’Keeffe, M.B.; FitzGerald, R.J.; O’Brien, N.M.; Benjakul, S. Purification and identification of antioxidant peptides from gelatin hydrolysate of seabass skin. J. Food Biochem. 2017, 41, 1–11. [Google Scholar]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from Lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int. J. Food Sci. Technol. 2018, 53, 1871–1879. [Google Scholar]
- Chotphruethipong, L.; Sukketsiri, W.; Aluko, R.E.; Sae-leaw, T.; Benjakul, S. Effect of hydrolyzed collagen from defatted Asian sea bass (Lates calcarifer) skin on fibroblast proliferation, migration and antioxidant activities. J. Food Sci. Technol. 2020, 58, 541–551. [Google Scholar]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of hydrolyzed collagen from defatted sea bass skin on proliferation and differentiation of preosteoblast MC3T3-E1 cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen based materials in cosmetic applications: A review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Aggnihotri, S. Formulation and development of botanicals-based herbal serum. Pharmaspire. 2021, 13, 211–217. [Google Scholar]
- Benjakul, S.; Morrissey, M.T. Protein hydrolysates from Pacific whiting solid wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar]
- Amnuaikit, T.; Khakhong, S.; Khongkow, P. Formulation development and facial skin evaluation of serum containing jellose from tamarind seeds. J. Int. Pharm. Res. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- The European Pharmacopoeia Commission. Microbiological examination of non-sterile products: Test for specified microorganisms. In European Pharmacopoeia, 9th ed.; EDQM & HealthCare Publications: Strasbourg, France, 2017; pp. 199–204. [Google Scholar]
- Thai Herbal Pharmacopoeia Appendix 10.2: Microbial Limit Tests in Thai Herbal Pharmacopoeia Volume I. Available online: https://www.bdn.go.th/tp/ebook/qQMcA3t0pR9gC3q0GT5gMJq0qT5co3uw (accessed on 1 October 2021).
- Asean Guidelines on Limits of Contaminants for Cosmetics Version 3. Available online: https://www.hsa.gov.sg/docs/default-source/hprg-cosmetics/asean-guidelines-on-limits-of-contaminants-for-cosmetics-ver-3.pdf (accessed on 1 October 2021).
- Nilsuwan, K.; Chantakun, K.; Chotphruethipong, L.; Benjakul, S. Development of hydrolysis and defatting processes for production of lowered fishy odor hydrolyzed collagen from fatty skin of sockeye salmon (Oncorhynchus nerka). Foods. 2021, 10, 2257. [Google Scholar] [CrossRef]
- Leclercq, E.; Dick, J.R.; Taylor, J.F.; Bell, J.G.; Hunter, D.; Migaud, H. Seasonal variations in skin pigmentation and flesh quality of Atlantic salmon (Salmo salar L.): Implications for quality management. J. Agric. Food Chem. 2010, 58, 7036–7045. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Boil. Macromol. 2015, 77, 369–374. [Google Scholar]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Hillebrand, G.G.; Raleigh, P.; Li, J.; Marmor, M.J.; Bertucci, V.; Grimes, P.E.; Mandy, S.H.; Perez, M.I.; Weinkle, S.H.; et al. A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br. J. Dermatol. 2010, 162, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Amount (% w/w) |
|---|---|
| HC | 2.5 |
| Vitamin C | 0.3 |
| Vitamin E | 0.5 |
| Vitamin B3 | 1 |
| Sodium carboxymethyl cellulose | 0.5 |
| Disodium EDTA | 0.1 |
| Polysorbate 20 | 7 |
| Propylene glycol | 20 |
| Phenoxyethanol | 0.5 |
| 10% w/v Sodium hydroxide | 0.5 |
| Perfume | 0.8 |
| Water | 66.3 |
| pH | Viscosity (cps) * | DPPH (%) |
|---|---|---|
| 7.77 ± 0.02 | 1333 ± 144 | HC serum (100 µg/mL) 90.04 ± 1.00 |
| Ascorbic acid (10 µg/mL) 98.13 ± 0.74 |
| Physicochemical Properties | Freeze–Thaw Cycle | Room Temperature |
|---|---|---|
| Color | Clear pale yellow | Clear pale yellow |
| pH | 7.68 ± 0.03 * | 7.74 ± 0.04 |
| Viscosity (cps) | 1250 ± 72 | 1291 ± 0 |
| % DPPH (HC serum 100 µg/mL) | 86.51 ± 5.64 | 88.78 ± 0.95 |
| Heavy Metals | MDL/LOQ (mg/kg) * | Amount (ppm) | Criteria (ppm) |
|---|---|---|---|
| Mercury (Hg) | 0.001 | Not found | ≤ 1 |
| Arsenic (As) | 0.125 | < 0.125 | ≤ 5 |
| Cadmium (Cd) | 0.01 | Not found | ≤ 3 |
| Lead (Pb) | 0.0425 | Not found | ≤ 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amnuaikit, T.; Shankar, R.; Benjakul, S. Hydrolyzed Fish Collagen Serum from By-Product of Food Industry: Cosmetic Product Formulation and Facial Skin Evaluation. Sustainability 2022, 14, 16553. https://doi.org/10.3390/su142416553
Amnuaikit T, Shankar R, Benjakul S. Hydrolyzed Fish Collagen Serum from By-Product of Food Industry: Cosmetic Product Formulation and Facial Skin Evaluation. Sustainability. 2022; 14(24):16553. https://doi.org/10.3390/su142416553
Chicago/Turabian StyleAmnuaikit, Thanaporn, Rajeev Shankar, and Soottawat Benjakul. 2022. "Hydrolyzed Fish Collagen Serum from By-Product of Food Industry: Cosmetic Product Formulation and Facial Skin Evaluation" Sustainability 14, no. 24: 16553. https://doi.org/10.3390/su142416553
APA StyleAmnuaikit, T., Shankar, R., & Benjakul, S. (2022). Hydrolyzed Fish Collagen Serum from By-Product of Food Industry: Cosmetic Product Formulation and Facial Skin Evaluation. Sustainability, 14(24), 16553. https://doi.org/10.3390/su142416553








