Abstract
Insectivorous bats may play a significant role in regulating populations of agricultural pests. Currently, few methods are available to enhance the activity of bats in agroecosystems. We asked whether synthetic sex pheromones, used in integrated pest management (IPM) to impede the mating success of major moth pests in vineyards and apple orchards, could also enhance the activity and richness of insectivorous bats, their natural enemies. We hypothesized that applying concentrated sex pheromones of pest moths will alter the movement patterns of male moths, indirectly affecting bat richness and activity. We compared the effect of sex pheromones on bats under two different agricultural management systems: conventional farming and IPM. We used synthetic sex pheromones of Lobesia botrana or Cydia pomonella; both are among the most destructive moth pests in vineyards and apple orchards, respectively. Using passive acoustic monitoring, we compared species richness and bat activity in plots without and with additional pheromones. In both IPM vineyards and IPM apple orchards, total bat activity and species richness significantly increased after applying the pheromone treatment, with a positive correlation between total bat activity and the numbers of moth pests in the vineyards. In conventional vineyards, bat species richness increased significantly, but not total bat activity. IPM vineyards had significantly higher species richness than conventional vineyards, both before and after the pheromone treatment. Our study shows that moth pheromone lures, commonly used as a pest control method, may also attract insectivorous bats, which in turn may further suppress the pests. These findings highlight the potential of insectivorous bats as pest control agents and call for further research directed at integrating them in IPM practices.
1. Introduction
Intensive agriculture has negative effects on biodiversity, soil health, water quality, and human health [1], which have led to the development of sustainable agricultural practices such as integrated pest management (IPM). IPM is recognized as a science-based management system to support pest control, implementing chemical and biological means, as well as agrotechnical approaches, to reduce the negative effects of pesticides on environmental health and economic costs [2]. A known pest control practice is the use of insect sex pheromones, which disrupt mating interactions and may contribute to a long-term reduction in insect populations [3]. Synthetic pheromones used in mating-disrupting pest control are typically species-specific, with no serious known adverse effects on non-target organisms [4,5]. Pheromones lures and traps are integrated into various crops and are principally based on the knowledge that many agricultural pests depend on sex pheromones for mate allocation and hence for their reproductive success [6]. Synthetic sex pheromones of female insects can generate aggregations of insect males near the pheromone source [7]. Insect aggregations, in general, attract different guilds of bats; for example, dense insect aggregations near artificial light sources attract various species of bats which feed on the aggregating insects [8]. Aggregations of a major pine defoliator, the pine processionary moth (Thaumetopoea pityocampa), generated by increasing concentrations of pheromone lures, were shown to attract forest-dwelling insectivorous bats, as well as to control the pine processionary moth through the association between emergent moths and foraging activity of the bats [9].
Insectivorous bats forage and feed in various types of agroecosystems [10] and can potentially provide pest suppression services in various agricultural crops, such as corn, cotton [11,12,13,14], rice [15], pecan, macadamia [16,17,18], grapes [19,20,21], and apples [22]. For example, Rodríguez-San Pedro et al. (2022) [20] showed that insectivorous bats could reduce the damage to organic grapes in Chile, saving approximately 600 kg/ha/year of berries from pest infections.
Currently, a major focus of research on bats in agroecosystems is to develop bat-friendly management schemes, which can enhance bat activity and species richness and promote the recovery of threatened bat species [23,24,25,26]. Several studies have strongly indicated that bat activity is negatively affected by insecticide use, therefore, a reduction in such practices would benefit bats [27,28,29,30]. Enhancement of landscape heterogeneity, by increasing patches of natural vegetation through the creation of hedgerows or distinct field edges, has also been found to enhance bat activity [31,32,33,34,35]. Other methods include the construction of water troughs for drinking and foraging sites [36,37], as well as placing bat houses as alternative roosts [24] in various crop habitats, have been shown as effective measures for increasing populations of bats and suppressing moth pest populations [24].
In the present study, we asked whether the use of pheromone lures, in both IPM and conventional vineyards and in IPM apple orchards will increase the activity of insectivorous bats. We hypothesized that synthetic female sex pheromones of the tortricid moth (Lobesia botrana) and the codling moth (Cydia pomonella) alter the movement patterns of the male moths and in turn affect the foraging activity of insectivorous bats. We predicted that concentrated pheromone odors will increase insectivorous bat species richness and their foraging activity in the treated plantations.
2. Materials and Methods
2.1. Study Areas and Bat Diversity
The experiments were carried out in vineyards and apple orchards at two locations in Northern Israel (Figure 1A), characterized by a mesic Mediterranean climate. IPM (N = 6) and conventional (N = 6) vineyard plots were located in Hanadiv Valley (32°52′ N, 34°975′ E, Figure 1B), a diversified ecosystem comprising natural, semi-natural, and agricultural patches in the southern part of Mount Carmel. IPM apple orchards (N = 6) were located in Kibbutz El-Rom in the Golan Heights (33°18′ N, 35°76′ E, Figure 1C), a basaltic grassland plateau on the eastern side of the northern Jordan Valley, dominated by open habitats, apple orchards, cherry, and vineyards. Plots of conventional apple orchards were not available in Kibbutz El-Rom. In Hanadiv Valley, conventional farming involves the usage of herbicides for weed control and pesticides for pest control, while IPM farming is based on reduced use of pesticides with the implementation of pheromone-mediated mating disruption. Ground vegetation is either left untouched or removed with machinery (Korine, personal communication). Approximately 23 species of insectivorous bats were recorded in the study areas of North Israel [38], dominated by the synanthropic Kuhl’s pipistrelle (Pipistrellus kuhlii). Most of the other bat species are endangered and occur at the southern edge of their range of distribution [38,39].
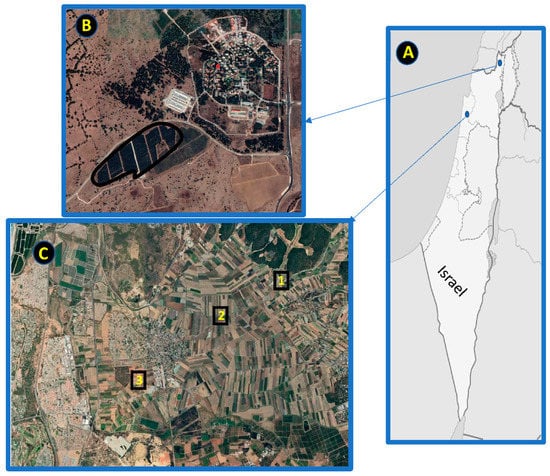
Figure 1.
A map of Israel (panel (A)) and the study areas in Kibbutz El-Rom indicated by red circle (panel (B)) and Hanadiv Valley (panel (B)). The location of the apple-growing area in (panel (B)) is indicated by a black border line. The locations of the vineyard production in (panel (C)) are indicated by three black squares. The plots of the apple orchards (n = 6) are managed by integrated pest management (IPM). The plots of the vineyards were dispersed in the black squares (1, 2, and 3); each contained 4 plots managed either by conventional (n = 6) or IPM (n = 6). For further details see methods.
2.2. Experimental Design
The experiment comprised two consecutive stages of five nights each: (1) control, without additional pheromones, and (2) treatment with a concentrated pheromone odor, 10 times the standard concentration used by farmers in both regions in the IPM fields. The length of application of the treatment was chosen based on the findings that male moths as well as bats respond within short time periods to pheromone lures or to a high density of insects, respectively [40,41]. It should be noted that in the control stage, the conventional plots were not treated with pheromones, while the control stage at the IPM plots included treatment with the standard concentration of pheromones used by the farmers as described below.
In Hanadiv Valley IPM vineyards pheromone ropes were placed by the farmers as a routine practice in March 2018, approximately three months before the beginning of our experiment. The farmers evenly distributed the pheromone dispensers (75 ropes ha−1) at a canopy height of ∼1.30 m above the ground and used polyethylene rope dispensers (Shin-Etsu Tokyo, Japan) containing 170 mg of -9,7-Z, E, dodecadienyl acetate, the major component of L. botrana female sex pheromone (Shin-Etsu Tokyo, Japan) [42]. Lobesia botrana is the most destructive pest in vineyards and causes damage directly by feeding on the grapes and indirectly by increasing the susceptibility of the berries to fungal disease [43]. In Kibbutz EL-Rom IPM apple orchards, pheromone ropes were placed by the farmers in April 2018 at a density of 70 ropes ha−1 at a canopy height of ∼3.5 m above the ground, approximately four months before the beginning of our experiment. They used polyethylene rope dispensers containing 120 mg of codlemone, E8, 10-12OH of the female C. pomonella sex pheromone (Shin-Etsu Tokyo, Japan), one of the most widespread and economically important pests in apple orchards [44].
In our experiment, we concentrated the odor of L. botrana or C. pomonella sex pheromones by braiding 10 of the same pheromone ropes used by the farmers in both study sites. We placed the pheromone braids at the edge of the plots since bats prefer to forage at the edge of agricultural fields [30,35]. For standardization, we placed the braids in the north eastern corner of all the plots.
Based on previous bat monitoring throughout 2017 in both plantations in the studied areas (Korine, personal observations), we found that bat activity and species richness were the highest during summer (June–August), which may have been related to high moth pest activity [41]. We performed the experiments in July and August in the vineyards and apple orchards in 2018, respectively. For each type of crop and management system (i.e., both IPM and conventional vineyards and IPM apple orchard), we randomly selected plots (a typical plot area is approximately 10 ha in the IPM and conventional vineyards, and in IPM apple orchard), with a mean inter-plot distance of >800 meters to avoid recording the same individual bats by our bat detectors. The vineyard plots were distributed across the Hanadiv Valley, while the plots of the apple orchards were located in a large apple-growing area in Kibbutz El-Rom (Figure 1). All of the plots in the study area were surrounded by plots of the same fruit crop (i.e., vineyards or apples) except for four vineyards plots that were located at the eastern part of Hanadiv Valley (square 1, Figure 1) near Mediterranean maquis.
We placed a pheromone trap near each pheromone braid both in IPM and conventional vineyards and counted the moths at the end of each of the experimental periods. Although pheromone-based mating disruption was applied in these plots, pheromone traps could be effectively used since our experiment started only 3 to 4 months after the treatment by the farmers, and the traps remained effective up to 3–4 months after they were placed [45].
2.3. Acoustic Bat Monitoring
We recorded bat activity using AnaBat detectors (Titley Electronics, Ballina, Australia) placed on the ground at a 45° angle, 1 m from the pheromone braid, in each of the experimental plots. We measured foraging activity by counting bat passes and identifying the species according to their calls [46]. Prior to the recording sessions, all detectors were calibrated to a sensitivity level of 7 in a new SD2 AnaBat detector, using a constant and monotonic ultrasonic signal. Calls were analyzed manually using the software AnalookW version 3.8v (https://users.lmi.net/corben/ (accessed on 10 November 2022)). Identification of a bat call and species was carried out according to known species-specific acoustic characteristics [47,48,49]. Due to the high call overlap between the species of the genus Myotis, all Myotis calls were identified to the genus level. Recordings were made on each experimental night from sunset (19:30) to sunrise (05:30). Recordings were not made during the period of five days before and after the full moon, and we ensured that insecticides were not applied starting five nights before the beginning of the experiment until its end. To avoid bias due to the sampling date, recordings were made in the IPM and conventional vineyards in the same 10 experimental nights.
2.4. Data Analysis
The data were analyzed using generalized linear mixed models (GLMMs) with total bat activity (passes per night) and species richness (number of species per night) as the dependent variables. Species richness and bat activity were calculated as an average of five days before and after the addition of the pheromone braid. We assessed the effect of the treatment on total bat activity and species richness using the treatment (control or pheromone application) as a fixed factor and the plot as a random factor, distinguishing between the two farming methods (conventional or IPM). Using a t-test, we compared the number of pests in the pheromone traps, before and after placing the pheromone braid, and examined the relationships between bat activity and pest density by a regression analysis after checking for normality and constant variance. In the IPM plots, one of the pheromone traps collapsed during the treatment, and therefore our sample size was 11 instead of 12 traps. We examined changes in the community composition before and after the treatment using the ‘adonis’ function in the ‘Vegan’ package [50] to perform a permutational multivariate analysis of variance (PERMANOVA) based on Bray–Curtis dissimilarities [51] and analyzed the cumulative sum for each species with R program version 4.1.2 [52]. Since bat activity can potentially vary due to differences in ambient temperatures and wind speeds, we compared the average night ambient temperature, mean and maximum night ambient temperature, and wind speed for each experimental stage (before and after adding the pheromone braid). We found no significant differences between the various temperatures and wind speeds in each site (p = 0.32, p = 0.09, p = 0.27, and p = 0.11, respectively). We chose p < 0.05 as the minimum acceptable level of significance.
3. Results
3.1. Effect of Pheromone Treatment in IPM and Conventional Vineyards
In the IPM vineyards, both bat activity (F1,5 = 19.1, p = 0.007, Figure 2A) and species richness (F1,5 = 9.2, p = 0.03, Figure 2B) significantly increased after the treatment with the pheromone braids. In the conventional vineyards, bat activity did not change after the treatment (p = 0.34, Figure 2C), while species richness increased significantly (F1,5 = 5.3, p = 0.047, Figure 2D). Bat activity, but not species richness (p = 0.41), also varied significantly (F1,5 = 6.3, p = 0.029) between the IPM plots.
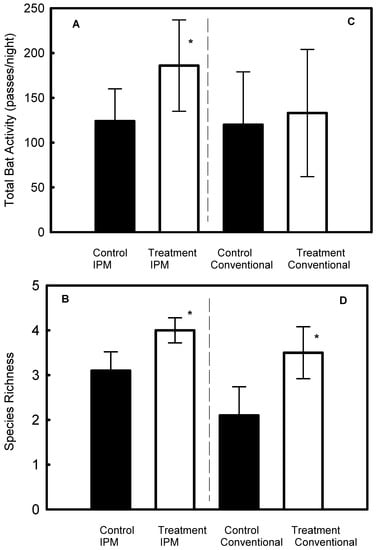
Figure 2.
Mean ± SD of bat activity and species richness before (control) and after the introduction of the pheromone odor (treatment) in integrated pest management (IPM) vineyards (A,B), and in conventional vineyards (C,D). Asterisks indicate significant differences. Experiments took place in Hanadiv Valley, Israel.
The number of moths in the pheromone traps increased significantly after the addition of the pheromone braid (1.1 ± 2.2, 5.7 ± 3.5, respectively, t = 2.9, df = 10, p = 0.016). In the IPM and conventional vineyards, the relationship between bat activity and moth numbers in the traps was significantly positive, but only after applying the pheromone braid (F11 = 5.73, p = 0.04, r2 = 0.4; Figure 3).
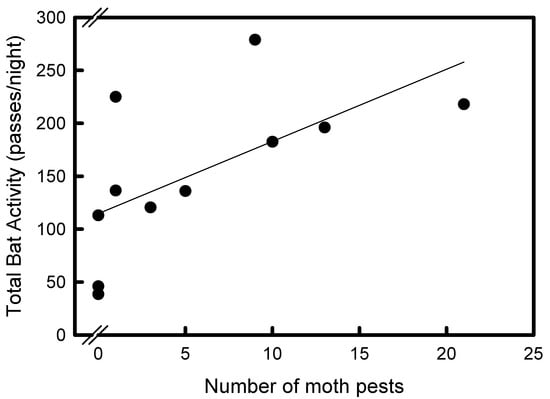
Figure 3.
The relationship between bat activity and pest numbers of Lobesia botrana in integrated pest management and conventional vineyards, Hanadiv Valley, Israel. Bat activity was positively correlated with the number of pests captured in the pheromone traps.
3.2. Comparison between IPM Vineyards and Conventional Vineyards
Prior to the treatment, bat activity did not differ between the IPM and conventional vineyards (p = 0.34, Figure 4A), while species richness was significantly higher in the IPM vineyards compared to the conventional vineyards (F1,5 = 7.9, p = 0.019, Figure 4C). After applying the pheromone braid, bat activity still did not differ between the IPM vineyards and the conventional vineyards (p = 0.25, Figure 4B), and species richness was significantly higher in the IPM vineyards (F1,5 = 7.5, p = 0.021, Figure 4D).
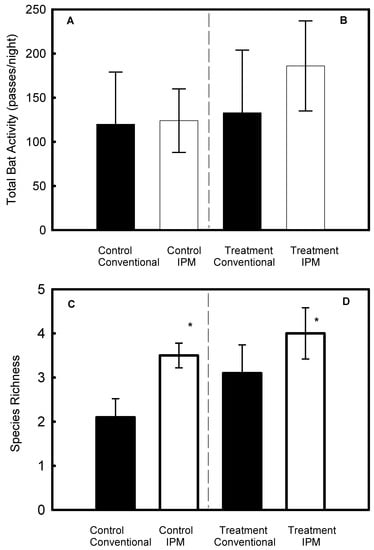
Figure 4.
Comparison of bat activity between the controls (A) and treatments (B) of conventional and integrated pest management (IPM) vineyards, and comparison of species richness between the controls (C) and treatments (D) of conventional and integrated pest management (IPM) vineyards. The treatment was based on an introduction of the pheromone odor. Results are presented as mean ± SD. Asterisks indicate significant differences. Experiments took place in Hanadiv Valley, Israel.
3.3. Community Structure
For the changes in the community structure, we pooled the results of specific species of bat activity from the IPM and conventional vineyards since bat activity did not differ between the two vineyard types. Following the treatment, changes in community structure were significant (Permanova, p = 0.01). In total, seven bat species were detected before adding the pheromone braid, while nine species were detected after the treatment. The main cumulative sum effect was attributed to the increase in activity of Pipistrellus kuhlii (average of 323 to 423 calls per night, Figure 5). The common bent-wing bat (Miniopterus schreibersii) and the genus Myotis were only detected after the treatment. The activity of the serotine bat (Eptesicus serotinous) was reduced after the treatment (average of 3 to 4 calls per night, while the activity of the four other species increased. The activity of both the greater horseshoe bat (Rhinolophus ferrumequinum) and the lesser horseshoe bat (R. hipposideros) was the same before and after the treatment, but both species were hardly detected (Figure 5).
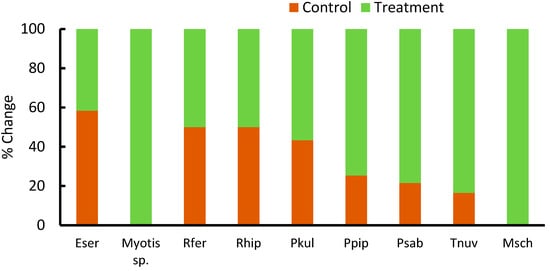
Figure 5.
Percent changes in species-specific bat activity in both integrated pest management and conventional vineyards before (control) and after the introduction of the pheromone odor (treatment). Experiments took place in Hanadiv Valley, Israel. Eser: Eptesicius serotinus, Rfer: Rhinolophus ferrumequinum, Rhip: Rhinolophus hipposideros, Pkul: Pipistrellus kulii, Ppip: Pipistrellus pipistrellus Psab: Pipistrellus savii, Tnuv: Taphozous nudiventris, and Msch: Miniodterus schreibersi.
3.4. Apple Orchards
Both species richness (F1,5 = 8.9, p = 0.031) and bat activity (F1,5 = 9.9, p = 0.026) increased significantly after the pheromone braid was applied (Figure 6). Bat activity (p = 0.3) and species richness (p = 0.5) did not vary between the plots. Seven and eight species of bats were recorded before and after the treatment, respectively. Species that were recorded before and after the manipulation included E. serotinus, Kuhl’s pipistrelle (Pipistrellus kuhlii), common pipistrelle (P. pipistrellus), Savi’s pipistrelle (P. savii), European free-tailed bat (Tadarida teniotis), T. nudiventris, and the genus, Myotis, while R. ferrumequinum was only recorded after the pheromone braid treatment.
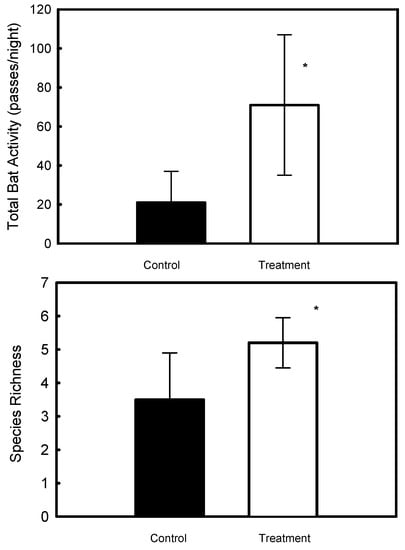
Figure 6.
Mean ± SD of bat activity and species richness before (control) and after the introduction of the pheromone odor (treatment) in integrated pest management apple orchards, Kibbutz El-Rom, Israel. Asterisks indicate significant differences.
4. Discussion
In this study and in accord with our prediction, we show that bat activity increased after the pheromone treatment, in both IPM vineyards and IPM apple orchards, which differ from each other in many respects but are both used by bats as foraging habitats. In addition, we show that bat activity was positively related to pest moth density after the treatment with the pheromone both in the IPM and conventional vineyard, indicating that bats may have responded to the increase in pest numbers. The principal ecological mechanism behind the use of pheromone lures in the present study is that bats are attracted to insect pest aggregations, as was shown in several studies [13,21,35,39,53,54]. Pheromone lures were successfully used to enhance the activity of forest-dwelling bats, as reported by Charbonnier et al. [9], who showed that the increased foraging activity of bats near the pheromone lures contributed to a significant decrease in reproductive success of the moths. Although their results and the results of our study need further long-term evaluation, our results indicate the potential of applying this method in agroecosystems or in forestry systems [9].
The direct role of pheromones in mating disruption and their potential indirect effect on the enhancement of bat activity as found in our study may have a synergistic effect in reducing pest numbers and crop damage. However, a detailed study on the diet of the bat species involved in these kinds of experimental manipulations is needed to quantify the potential pest control services of the bats. Since the effectiveness of sex pheromones on mating disruption diminishes as the pest’s population density increases [55], enhancement of insectivorous bat activity may also be useful in keeping the pest population density below the level at which pheromone-mediated mating disruption becomes ineffective. Furthermore, this method is easy to apply since the preparation and placement of the pheromone braid on a fruiting tree is a quick and simple procedure that has limited, if any, impacts on other management practices. However, applying pheromones is generally more expensive than pesticides, which depend on the number of spraying events and the type of pesticide in use. Since mating disruption is generally less effective when pest densities are high [55], further studies are needed to reveal whether bat activity will increase when pheromones of other moth pest species are used, and whether a combination of pheromones manipulations for various pests would be more effective for the indirect attraction of the bats. Our study took place in plantations that are relatively small, and it would be interesting to determine whether this method can achieve similar results in large-sized fields, such as corn or cotton fields. In addition, in the present study, we did not have replications over time, and the study was only conducted during summer, when late generations of the moth occur. Thus, more experiments that test the response of the bats over time and consider changes in the diet of the bats (i.e., occurrence of the pests) are needed to support the results of the present study.
The increase in bat activity, under the pheromone treatment, may be related to changes in moth density. However, we cannot rule out the possibility that the bats were directly attracted to the smell of the synthetic pheromone. The chemical signals of the pheromones, which the natural enemies exploit, are referred to as kairomones [56,57]. However, it is unlikely that insectivorous bats use olfaction cues to locate the prey [58,59].
Our results show that in both vineyards and apple orchards, species richness changed following the application of concentrated pheromone lures, with the appearance of the genus Myotis and M. schreibersii in the vineyards and R. ferrumequinum in the apple orchards. These species, as well as all the other species that were recorded in the study sites, except for P. kuhlii and T. teniotis, are defined as nearly threatened or vulnerable according to the IUCN (International Union for the Conservation of Nature and Nature Resources) Red List. These results, in addition to our main findings, may indicate that a long-term IPM approach better supports populations of insectivorous bats in Mediterranean habitats, as was demonstrated with organic farming [19−20,23]. Methods for enhancing bat activity may cause changes in the composition of bat communities due to increased interspecific competition [60], but our results did not indicate changes in species-specific activity due to the pheromone treatment.
Applying enhancement methods to increase the activity of natural enemies must be combined with a friendly pesticide spraying regime. Pesticides are widely used in both conventional and IPM agricultural systems and are known to have adverse effects on natural enemies [61]. Enhancement methods aiming to draw natural enemies from the surrounding area might create an ecological trap, negatively affecting populations of natural enemies in the short and long term. Thus, combining natural enemies as pest control agents requires adaptations in the spraying regime, including the selection of compatible pesticides and the timing of pesticide application [62].
In conclusion, we show that pheromone lures used by farmers to control pests may also indirectly attract their natural enemies, insectivorous bats. In many IPM systems, where pheromones are already used, our proposed method to use pheromones in a concentrated manner can increase bat activity and may contribute to pest control. Nevertheless, using this method for the purpose of pest control by bats requires additional large-scale studies to establish our understanding of the mechanisms underlining the effect of moth pheromones on bat activity, and how these interactions can be used for pest suppression. In this respect, our findings highlight the potential of integrating bat-friendly agricultural management schemes into IPM to benefit both pest suppression and bat conservation.
Author Contributions
Methodology, C.K., Y.C. and I.K.; Investigation, C.K., Y.C. and I.K.; Data curation, C.K.; Writing—original draft, C.K.; Writing—review & editing, Y.C. and I.K.; Funding acquisition, C.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Israel Ministry of Agriculture and Rural Development, # 857071314 and by Yad Hanadiv, # 2018.
Institutional Review Board Statement
Our study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are part of a larger unpublished data set which will be available once published.
Acknowledgments
We thank Avi Goldstein and Ronen Refaely for their incredible assistance in the field research by providing access to the vineyards and the apple orchards and on the information about pesticide applications. We also thank Yuval Arazi for the discussion. This study was supported by Yad Hanadiv (CK) and by the Israel Ministry of Agriculture and Rural Development, # 857071314 (CK). This is publication 1119 of the Mitrani Department of Desert Ecology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M.; Bajwa, W.I. Integrated pest management: A global reality? Ann. Soc. Entomol. Bras. 1999, 28, 01–25. [Google Scholar] [CrossRef]
- Burkholder, W.E.; Ma, M. Pheromones for monitoring and control of stored-product insects. Ann. Rev. Entomol. 1985, 30, 257–272. [Google Scholar] [CrossRef]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Stenberg, J.A. A conceptual framework for integrated pest management. Trends. Plant Sci. 2017, 22, 759–769. [Google Scholar] [CrossRef]
- Cardé, R.T.; Minks, A.K. Insect Pheromone Research: New Directions; Chapman and Hall: New York, NY, USA, 1977. [Google Scholar]
- Landolt, P.J. Sex attractant and aggregation pheromones of male phytophagous insects. Am. Entomol. 1997, 43, 12–22. [Google Scholar] [CrossRef]
- Rydell, J. Exploitation of insects around streetlamps by bats in Sweden. Func. Ecol. 1992, 6, 744–750. [Google Scholar] [CrossRef]
- Charbonnier, Y.; Barbaro, L.; Theillout, A.; Jactel, H. Numerical and functional responses of forest bats to a major insect pest in pine plantations. PLoS ONE 2014, 9, e109488. [Google Scholar] [CrossRef]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef]
- Cleveland, C.J.; Betke, M.; Federico, P.; Frank, J.D.; Hallam, T.G.; Horn, J.; Lopez, J.D., Jr; McCracken, G.F.; Medellín, R.A.; Moreno-Valdez, A.; et al. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front Ecol. Environ. 2006, 4, 238–243. [Google Scholar] [CrossRef]
- Maine, J.J.; Boyles, J.G. Bats initiate vital agroecological interactions in corn. Proc. Nat. Acad. Sci. USA 2015, 112, 12438–12443. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Bar-David, S.; Nielsen, M.; Bohmann, K.; Korine, C. An appetite for pests: Synanthropic insectivorous bats exploit cotton pest irruptions and consume various deleterious arthropods. Mol. Ecol. 2020, 29, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Kolkert, H.; Smith, R.; Rader, R.; Reid, N. Insectivorous bats provide significant economic value to the Australian cotton industry. Ecosyst. Serv. 2021, 49, 101280. [Google Scholar] [CrossRef]
- Puig-Montserrat, X.; Flaquer, C.; Gómez-Aguilera, N.; Burgas, A.; Mas, M.; Tuneu, C.; López-Baucells, A. Bats actively prey on mosquitoes and other deleterious insects in rice paddies: Potential impact on human health and agriculture. Pest Manag. Sci. 2020, 76, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; de Torrez, E.B.; McCracken, G.F. Crop pests eaten by bats in organic pecan orchards. Crop Prot. 2015, 67, 66–71. [Google Scholar] [CrossRef]
- Taylor, P.J.; Grass, I.; Alberts, A.J.; Joubert, E.; Tscharntke, T. Economic value of bat predation services—A review and new estimates from macadamia orchards. Ecosyst. Serv. 2018, 30, 372–381. [Google Scholar] [CrossRef]
- Williams-Guillén, K.; Perfecto, I.; Vandermeer, J. Bats limit insects in a neotropical agroforestry system. Science 2008, 320, 70. [Google Scholar] [CrossRef]
- Froidevaux, J.S.; Louboutin, B.; Jones, G. Does organic farming enhance biodiversity in Mediterranean vineyards? A case study with bats and arachnids. Agric. Ecosyst. Environ. 2017, 249, 112–122. [Google Scholar] [CrossRef]
- Rodríguez-San Pedro, A.; Allendes, J.L.; Beltrán, C.A.; Chaperon, P.N.; Saldarriaga-Córdoba, M.M.; Silva, A.X.; Grez, A.A. Quantifying ecological and economic value of pest control services provided by bats in a vineyard landscape of central Chile. Agric. Ecosyst. Environ. 2020, 302, 107063. [Google Scholar] [CrossRef]
- Baroja, U.; Garin, I.; Vallejo, N.; Aihartza, J.; Rebelo, H.; Goiti, U. Bats actively track and prey on grape pest populations. Ecol. Indic. 2021, 126, 107718. [Google Scholar] [CrossRef]
- Jay, M.; de Roincé, C.B.; Ricard, J.M.; Garcin, A.; Mandrin, J.F.; Lavigne, C.; Bouvier, J.C.; Tupinier, Y.; Puechmaille, S. Functional biodiversity in apple orchards: Are the bats eating the pests? Infos-Ctifl 2012, 86, 28–34. [Google Scholar]
- Wickramasinghe, L.P.; Harris, S.; Jones, G.; Vaughan, N. Bat activity and species richness on organic and conventional farms: Impact of agricultural intensification. J. App. Ecol. 2003, 40, 984–993. [Google Scholar] [CrossRef]
- Puig-Montserrat, X.; Torre, I.; López-Baucells, A.; Guerrieri, E.; Monti, M.M.; Ràfols-García, R.; Ferrer, X.; Gisbert, D.; Flaquer, C. Pest control service provided by bats in Mediterranean rice paddies: Linking agroecosystems structure to ecological functions. Mamm. Biol. 2015, 80, 237–245. [Google Scholar] [CrossRef]
- Krings, C.H.; Darras, K.; Hass, A.; Batáry, P.; Fabian, Y. Not only hedgerows, but also flower fields can enhance bat activity in intensively used agricultural landscapes. Basic Appl. Ecol. 2022, 63, 23–35. [Google Scholar] [CrossRef]
- Russo, D.; Bosso, L.; Ancillotto, L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: Research frontiers and management implications. Agric. Ecosyst. Environ. 2018, 266, 31–38. [Google Scholar] [CrossRef]
- Bayat, S.; Geiser, F.; Kristiansen, P.; Wilson, S.C. Organic contaminants in bats: Trends and new issues. Environ. Int. 2014, 63, 40–52. [Google Scholar] [CrossRef]
- Park, K.J. Mitigating the impacts of agriculture on biodiversity: Bats and their potential role as bioindicators. Mamm. Biol. 2015, 80, 191–204. [Google Scholar] [CrossRef]
- Williams-Guillén, K.; Olimpi, E.; Maas, B.; Taylor, P.J.; Arlettaz, R. Bats in the anthropogenic matrix: Challenges and opportunities for the conservation of Chiroptera and their ecosystem services in agricultural landscapes. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 151–186. [Google Scholar]
- Kahnonitch, I.; Lubin, Y.; Korine, C. Insectivorous bats in semi-arid agroecosystems− effects on foraging activity and implications for insect pest control. Agric. Ecosyst. Environ. 2018, 261, 80–92. [Google Scholar] [CrossRef]
- Pasek, J.E. Influence of wind and windbreaks on local dispersal of insects. Agric. Ecosyst. Environ. 1988, 2, 539–554. [Google Scholar] [CrossRef]
- Boughey, K.L.; Lake, I.R.; Haysom, K.A.; Dolman, P.M. Improving the biodiversity benefits of hedgerows: How physical characteristics and the proximity of foraging habitat affect the use of linear features by bats. Biol. Cons. 2011, 144, 1790–1798. [Google Scholar] [CrossRef]
- Angell, R.L.; Langton, S.D.; MacDonald, M.A.; Skates, J.; Haysom, K.A. The effect of a Welsh agri-environment scheme on bat activity: A large-scale study. Agric. Ecosyst. Environ. 2019, 275, 32–41. [Google Scholar] [CrossRef]
- Froidevaux, J.S.; Boughey, K.L.; Hawkins, C.L.; Broyles, M.; Jones, G. Managing hedgerows for nocturnal wildlife: Do bats and their insect prey benefit from targeted agri-environment schemes? J. Appl. Ecol. 2019, 56, 1610–1623. [Google Scholar] [CrossRef]
- Korine, C.; Niv, A.; Axelrod, M.; Dahan, T. Species richness and activity of insectivorous bats in cotton fields in semi-arid and mesic Mediterranean agroecosystems. Mamm. Biol. 2020, 100, 3–80. [Google Scholar] [CrossRef]
- Tuttle, S.R.; Chambers, C.L.; Theimer, T.C. Potential effects of livestock water-troughs modifications on bats in Northern Arizona. Wildl. Soc. Bull. 2006, 34, 602–608. [Google Scholar] [CrossRef]
- Korine, C.; Adams, R.; Russo, D.; Fisher-Phelps, M.; Jacobs, D. Bats and water: Anthropogenic alterations threaten global bat populations. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 215–241. [Google Scholar]
- Yom-Tov, Y.; Kadmon, R. Analysis of the Distribution of Insectivorous Bats in Israel. Divers. Distrib. 1998, 4, 63–70. [Google Scholar] [CrossRef]
- Dolev, A.; Perevolotsky, A. Vertebrates in Israel: The Red Book; Israel Nature and Parks Authority: Jerusalem, Israel, 2004. [Google Scholar]
- Jacobson, M. Insect Sex Pheromones; Elsevier: Cambridge, UK, 2012. [Google Scholar]
- Cvikel, N.; Berg, K.E.; Levin, E.; Hurme, E.; Borissov, I.; Boonman, A.; Amichai, E.; Yovel, Y. Bats aggregate to improve prey search but might be impaired when their density becomes too high. Curr. Biol. 2015, 25, 206–211. [Google Scholar] [CrossRef]
- Harari, A.R.; Zahavi, T.; Gordon, D.; Anshelevich, L.; Harel, M.; Ovadia, S.; Dunkelblum, E. Pest management programmes in vineyards using male mating disruption. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 769–775. [Google Scholar] [CrossRef]
- Gordon, D.; Zahavi, T.; Anshelevich, L.; Harel, M.; Ovadia, S.; Dunkelblum, E.; Harari, A.R. Mating disruption of Lobesia botrana (Lepidoptera: Tortricidae): Effect of pheromone formulations and concentrations. J. Econ. Entomol. 2005, 98, 135–142. [Google Scholar] [CrossRef]
- Ju, D.; Mota-Sanchez, D.; Fuentes-Contreras, E.; Zhang, Y.L.; Wang, X.Q.; Yang, X.Q. Insecticide resistance in the Cydia pomonella (L.): Global status, mechanisms, and research directions. Pestic. Biochem. Physiol. 2021, 178, 104925. [Google Scholar] [CrossRef]
- Gavara, A.; Navarro-Llopis, V.; Primo, J.; Vacas, S. Influence of weather conditions on Lobesia botrana (Lepidoptera: Tortricidae) mating disruption dispensers’ emission rates and efficacy. Crop Protec. 2022, 155, 105926. [Google Scholar] [CrossRef]
- Fenton, M.B. A technique for monitoring bat activity with results obtained from different environments in southern Ontario. Can. J. Zool. 1970, 48, 847–851. [Google Scholar] [CrossRef]
- Benda, P.; Dietz, C.; Andreas, M.; Hotovy, J.; Lucan, R.K.; Maltby, A.; Meakin, K.; Truscott, J.; Vallo, P. Bats (Mammalia Chiroptera) of the Eastern Mediterranean and Middle East. Part 6. Bats of Sinai (Egypt) with some taxonomic, ecologic and echolocation data on this fauna. Acta Soc. Zool. Bohem. 2008, 72, 1–103. [Google Scholar]
- Russo, D.; Jones, G. Identification of twenty-two bat species (Mammalia: Chiroptera) from Italy by analysis of time-expanded recordings of echolocation calls. J. Zool. 2002, 258, 91–103. [Google Scholar] [CrossRef]
- Hackett, T.D.; Holderied, M.W.; Korine, C. Echolocation call description of 15 species of Middle-Eastern desert dwelling insectivorous bats. Bioacoustics 2017, 26, 217–235. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.4-1; 2016. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 December 2021).
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). Wiley Statsref: Statistics Reference Online. 2015. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/9781118445112.stat07841 (accessed on 1 December 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 December 2021).
- McCracken, G.F.; Westbrook, J.K.; Brown, V.A.; Eldridge, M.; Federico, P.; Kunz, T.H. Bats track and exploit changes in insect pest populations. PLoS ONE 2012, 7, e43839. [Google Scholar] [CrossRef]
- Blažek, J.; Konečný, A.; Bartonička, T. Bat aggregational response to pest caterpillar emergence. Sci. Rep. 2021, 11, 13634. [Google Scholar] [CrossRef]
- Welter, S.; Pickel, C.; Millar, J.; Cave, F.; Van Steenwyk, R.; Dunley, J. Pheromone mating disruption offers selective management options for key pests. Calif. Agric. 2005, 59, 16–22. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Pirk, C.W.; Yusuf, A.A.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Exploring the kairomone-based foraging behaviour of natural enemies to enhance biological control: A review. Front. Ecol. Evol. 2021, 9, 143. [Google Scholar]
- Dicke, M.; Sabelis, M.W. Infochemical terminology: Based on cost-benefit analysis rather than origin of compounds? Func. Ecol. 1988, 2, 131–139. [Google Scholar] [CrossRef]
- Teeling, E.C.; Jones, G.; Rossiter, S.J. Phylogeny, genes, and hearing: Implications for the evolution of echolocation in bats. In Bat Bioacoustics; Fenton, M.B., Grinnell, A.D., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2016; pp. 25–54. [Google Scholar]
- Brokaw, A.F.; Smotherman, M. Role of ecology in shaping external nasal morphology in bats and implications for olfactory tracking. PLoS ONE 2020, 15, e0226689. [Google Scholar] [CrossRef]
- Razgour, O.; Korine, C.; Saltz, D. Pond characteristics as determinants of species diversity and community composition in desert bats. Anim. Conserv. 2010, 13, 505–513. [Google Scholar] [CrossRef]
- Bommarco, R.; Miranda, F.; Bylund, H.; Björkman, C. Insecticides suppress natural enemies and increase pest damage in cabbage. J. Econ. Entomol. 2011, 104, 782–791. [Google Scholar] [CrossRef] [PubMed]
- El-Wakeil, N.; Gaafar, N.; Sallam, A.; Volkmar, C. Side effects of insecticides on natural enemies and possibility of their integration in plant protection strategies. In Insecticides: Development of Safer and More Effective Technologies Agricultural and Biological Sciences; Trdan, S., Ed.; Tech Open Access Publisher: Rijeka, Croatia, 2013; pp. 1–56. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).