Remediation of the Alluvial Aquifer of the Sardas Landfill (Sabiñánigo, Huesca) by Surfactant Application
Abstract
:1. Introduction
2. Materials & Methods
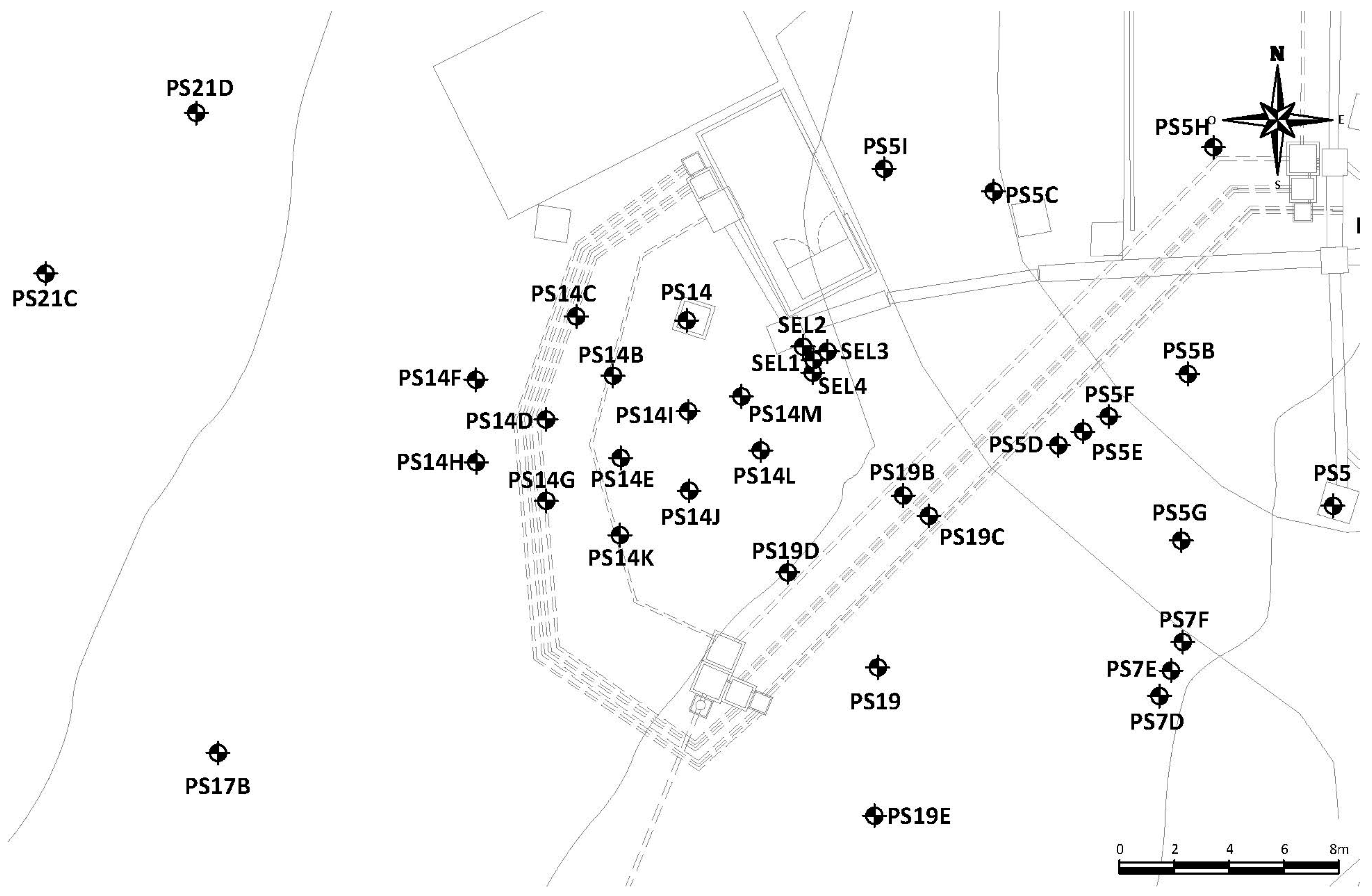
2.1. Site Description
2.2. Surfactant Injections in the Alluvial
2.3. Analytical Methods
2.3.1. COC Analysis
2.3.2. Bromide (Tracer) and Conductivity Analysis
2.3.3. Surfactant Concentration
3. Results and Discussion
3.1. DNAPL Composition
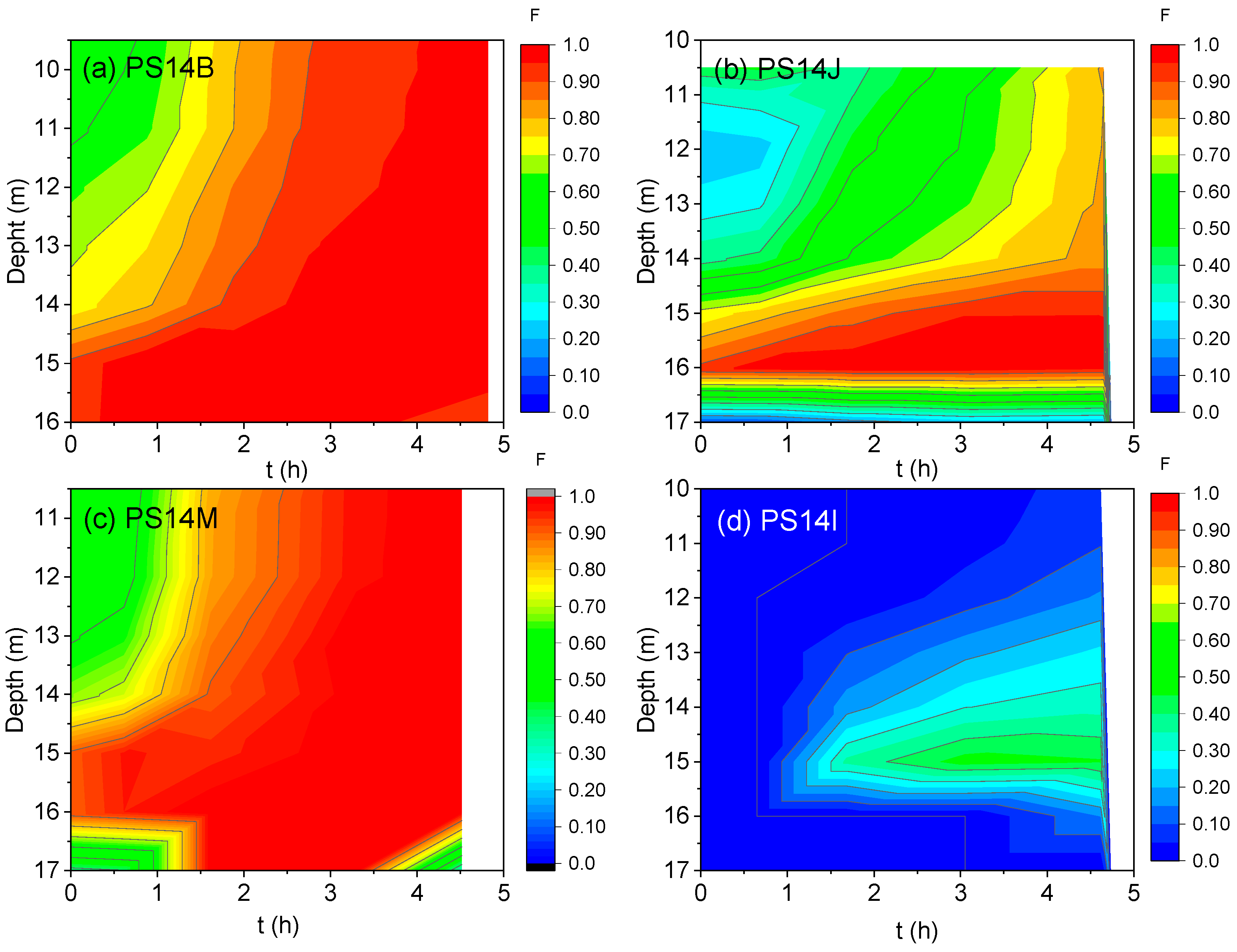
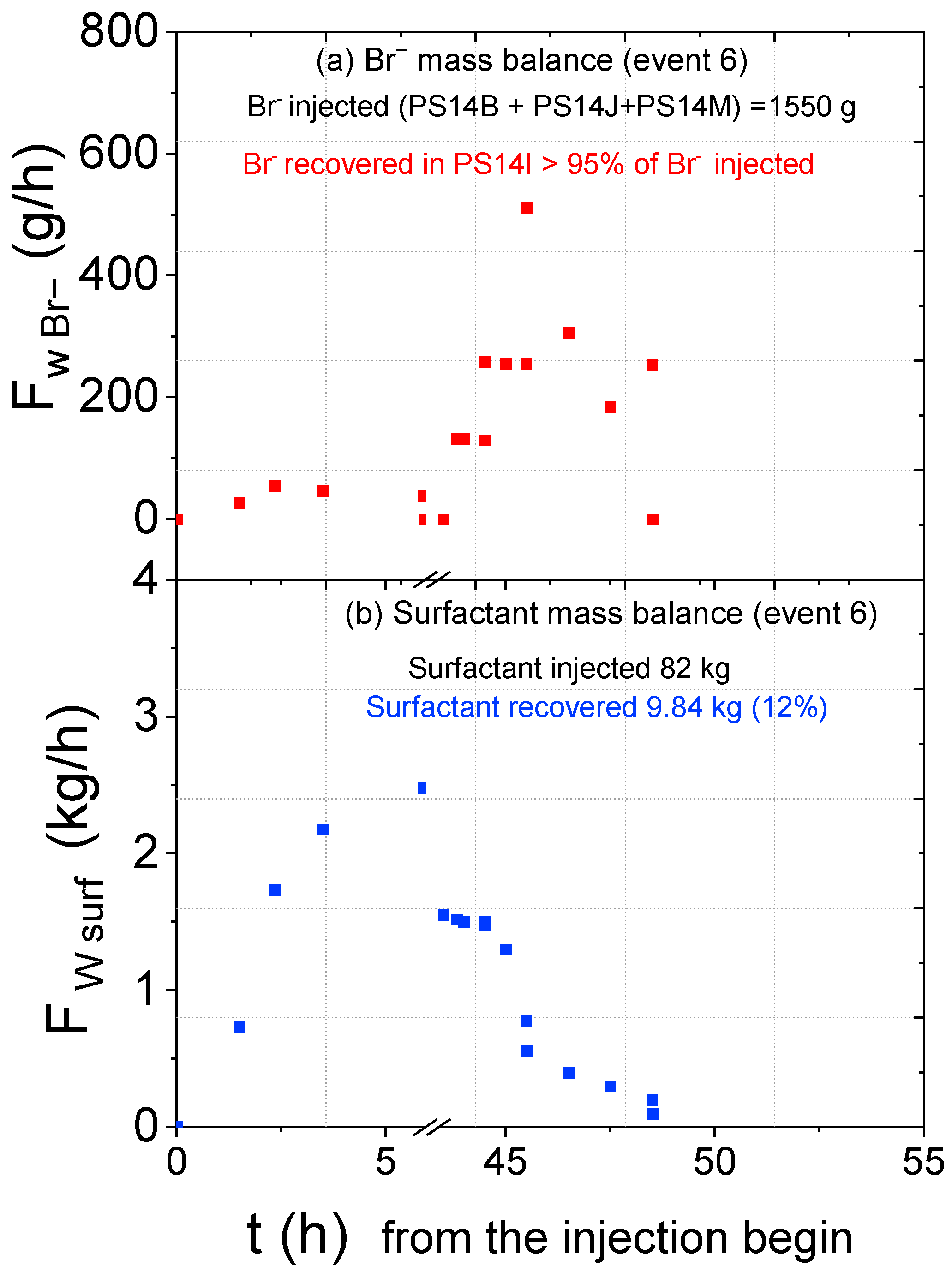
3.2. Results Obtained in the Injection Events
3.3. Groundwater Monitoring in 2021
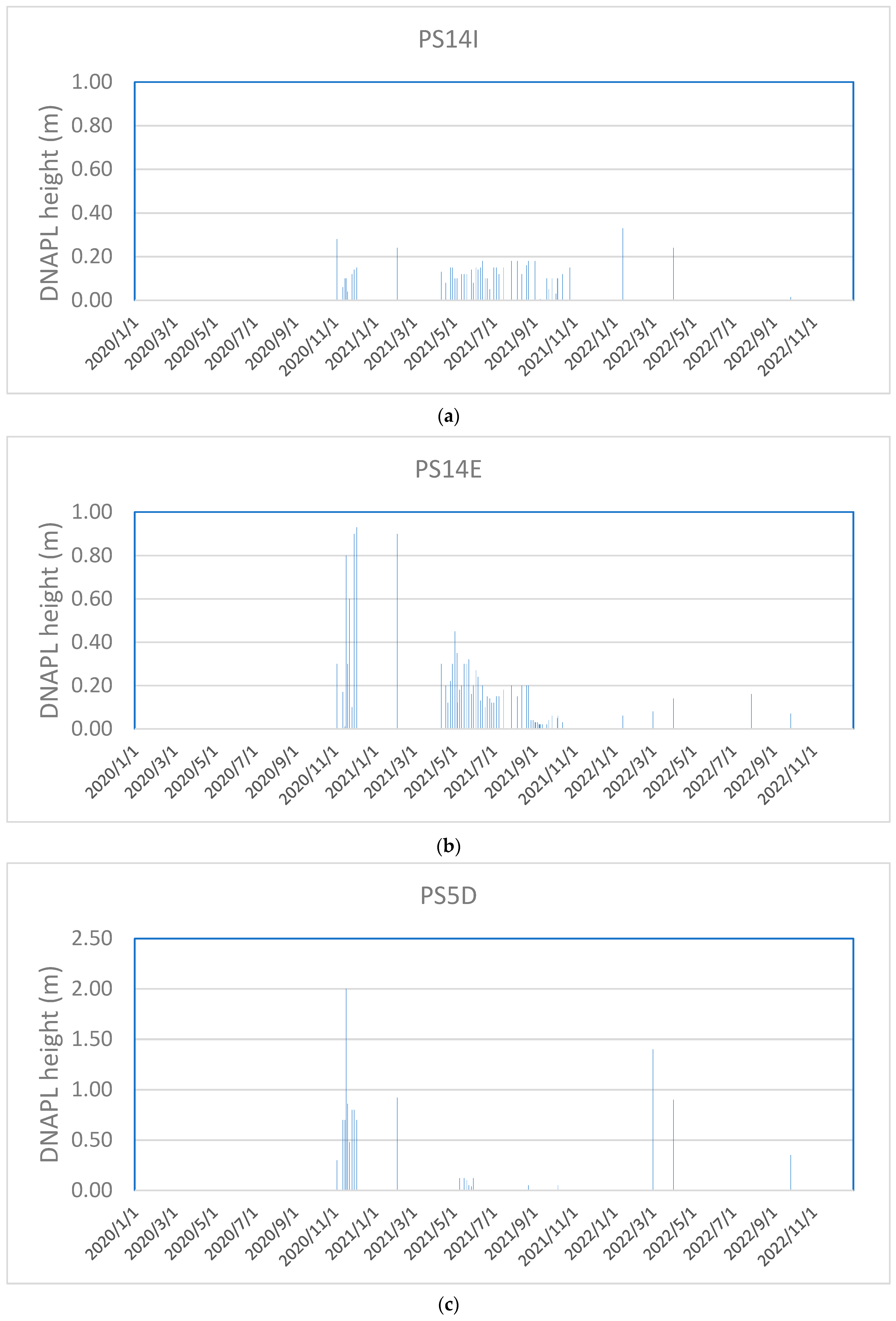
3.4. DNAPL Accumulation in PS14x Wells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, R.L.; Crimi, M.; Simpkin, T.J. In Situ Chemical Oxidation for Groundwater Remediation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 3. [Google Scholar]
- Stroo, H.F.; Leeson, A.; Marqusee, J.A.; Johnson, P.C.; Ward, C.H.; Kavanaugh, M.C.; Sale, T.C.; Newell, C.J.; Pennell, K.D.; Lebrón, C.A. Chlorinated ethene source remediation: Lessons learned. Environ. Sci. Technol. 2012, 46, 6438–6447. [Google Scholar] [CrossRef]
- Tressler, A.; Uchrin, C. Mathematical simulation of chlorinated ethene concentration rebound after in situ chemical oxidation. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2014, 49, 869–881. [Google Scholar] [CrossRef]
- Tomlinson, D.W.; Rivett, M.O.; Wealthall, G.P.; Sweeney, R.E.H. Understanding complex LNAPL sites: Illustrated handbook of LNAPL transport and fate in the subsurface. J. Environ. Manag. 2017, 204, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLU-IN. Dense Nonaqueous Phase Liquids (Dnapls). Available online: https://clu-in.org/contaminantfocus/default.focus/sec/dense_nonaqueous_phase_liquids_(dnapls)/cat/Treatment_Technologies/ (accessed on 7 March 2019).
- Kavanaugh, M.C.; Suresh, P.; Rao, C. The DNAPL Remediation Challenge: Is there a Case for Source Depletion? Environmental Protection Agency: Washington, DC, USA, 2003. [Google Scholar]
- Maire, J.; Joubert, A.; Kaifas, D.; Invernizzi, T.; Marduel, J.; Colombano, S.; Cazaux, D.; Marion, C.; Klein, P.Y.; Dumestre, A.; et al. Assessment of flushing methods for the removal of heavy chlorinated compounds DNAPL in an alluvial aquifer. Sci. Total Environ. 2018, 612, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, L.; Liu, G.; Yang, X.; Ahmad, Z.; Zhong, H. Surfactant-enhanced aquifer remediation: Mechanisms, influences, limitations and the countermeasures. Chemosphere 2020, 252, 126620. [Google Scholar] [CrossRef] [PubMed]
- Abriola, L.M.; Christ, J.A.; Pennell, K.D.; Ramsburg, C.A. Source remediation challenges. In Delivery and Mixing in the Subsurface: Processes and Design Principles for In Situ Remediation; Kitanidis, P.K., McCarty, P.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 239–276. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- McCray, J.E.; Tick, G.R.; Jawitz, J.W.; Gierke, J.S.; Brusseau, M.L.; Falta, R.W.; Knox, R.C.; Sabatini, D.A.; Annable, M.D.; Harwell, J.H.; et al. Remediation of NAPL Source Zones: Lessons Learned from Field Studies at Hill and Dover AFB. Ground. Water 2011, 49, 727–744. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Lim, H.S.; Gao, Y.; Kang, J.; Jeong, H.Y. Evaluation of ethoxylated nonionic surfactants for solubilization of chlorinated organic phases: Effects of partitioning loss and macroemulsion formation. J. Contam. Hydrol. 2019, 223, 103475. [Google Scholar] [CrossRef]
- Pennell, K.D.; Capiro, N.L.; Walker, D.I. Surfactant and cosolvent flushing. In Chlorinated Solvent Source Zone Remediation; Kueper, B.H., Stroo, H.F., Vogel, C.M., Ward, C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 7, pp. 353–394. [Google Scholar]
- Yang, J.S.; Yang, J.W. Partitioning effects of nonionic surfactants on the solubilization of single or binary chlorinated solvents: Batch and column experiments. J. Ind. Eng. Chem. 2018, 58, 140–147. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Kibbey, T.C.G.; Cowell, M.A.; Hayes, K.F. Partitioning of ethoxylated nonionic surfactants into nonaqueous-phase organic liquids: Influence on solubilization behavior. Environ. Sci. Technol. 1999, 33, 169–176. [Google Scholar] [CrossRef]
- Paria, S. Surfactant-enhanced remediation of organic contaminated soil and water. Adv. Colloid Interface Sci. 2008, 138, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Londergan, J.; Yeh, L. Surfactant-Enhanced Aquifer Remediation (SEAR) Implementation Manual; Intera Inc.: Austin, TX, USA, 2003. [Google Scholar]
- Abriola, L.M.; Drummond, C.D.; Hahn, E.J.; Hayes, K.F.; Kibbey, T.C.; Lemke, L.D.; Pennell, K.D.; Petrovskis, E.A.; Ramsburg, C.A.; Rathfelder, K.M. Pilot-scale demonstration of surfactant-enhanced PCE solubilization at the Bachman road site. 1. Site characterization and test design. Environ. Sci. Technol. 2005, 39, 1778–1790. [Google Scholar] [CrossRef]
- Atteia, O.; Estrada, E.D.; Bertin, H. Soil flushing: A review of the origin of efficiency variability. Rev. Environ. Sci. Bio-Technol. 2013, 12, 379–389. [Google Scholar] [CrossRef]
- Kostarelos, K.; Lenschow, S.R.; Stylianou, M.A.; de Blanc, P.C.; Mygind, M.M.; Christensen, A.G. Jet A fuel recovery using micellar flooding: Design and implementation. Sci. Total Environ. 2016, 563, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kang, H.; Do, W. Application of nonionic surfactant-enhanced in situ flushing to a diesel contaminated site. Water Res. 2005, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Domínguez, C.M.; Lorenzo, D.; García-Cervilla, R.; Lominchar, M.A.; Fernández, J.; Gómez, J.; Guadaño, J. Soil flushing pilot test in a landfill polluted with liquid organic wastes from lindane production. Heliyon 2019, 5, e02875. [Google Scholar] [CrossRef] [Green Version]
- Svab, M.; Kubala, M.; Muellerova, M.; Raschman, R. Soil flushing by surfactant solution: Pilot-scale demonstration of complete technology. J. Hazard. Mater. 2009, 163, 410–417. [Google Scholar] [CrossRef]
- Sharma, P.; Kostarelos, K.; Lenschow, S.; Christensen, A.; de Blanc, P.C. Surfactant flooding makes a comeback: Results of a full-scale, field implementation to recover mobilized NAPL. J. Contam. Hydrol. 2020, 230, 103602. [Google Scholar] [CrossRef]
- Liu, J.-W.; Wei, K.-H.; Xu, S.-W.; Cui, J.; Ma, J.; Xiao, X.-L.; Xi, B.-D.; He, X.-S. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef]
- Santos, A.; Fernandez, J.; Guadaño, J.; Lorenzo, D.; Romero, A. Chlorinated organic compounds in liquid wastes (DNAPL) from lindane production dumped in landfills in Sabiñanigo (Spain). Environ. Pollut. 2018, 242, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Arjol, M.; Cacho, C. POP-contaminated sites from HCH production in Sabiñánigo, Spain. Environ. Sci. Pollut. Res. 2013, 20, 1937–1950. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cervilla, R.; Santos, A.; Romero, A.; Lorenzo, D. Simultaneous addition of surfactant and oxidant to remediate a polluted soil with chlorinated organic compounds: Slurry and column experiments. J. Environ. Chem. Eng. 2022, 10, 107625. [Google Scholar] [CrossRef]
- Garcia-Cervilla, R.; Santos, A.; Romero, A.; Lorenzo, D. Partition of a mixture of chlorinated organic compounds in real contaminated soils between soil and aqueous phase using surfactants: Influence of pH and surfactant type. J. Environ. Chem. Eng. 2021, 9, 105908. [Google Scholar] [CrossRef]
- Garcia-Cervilla, R.; Santos, A.; Romero, A.; Lorenzo, D. Compatibility of nonionic and anionic surfactants with persulfate activated by alkali in the abatement of chlorinated organic compounds in aqueous phase. Sci. Total Environ. 2021, 751, 141782. [Google Scholar] [CrossRef]
- Garcia-Cervilla, R.; Romero, A.; Santos, A.; Lorenzo, D. Surfactant-Enhanced Solubilization of Chlorinated Organic Compounds Contained in DNAPL from Lindane Waste: Effect of Surfactant Type and pH. Int. J. Environ. Res. Public Health 2020, 17, 4494. [Google Scholar] [CrossRef]
- Lorenzo, D.; Santos, A.; Domínguez, C.M.; Guadaño, J.; Gómez, J.; Fernández, J. Transport Model of Fluids Injected in a Landfill Polluted with Lindane Wastes. Comput. Aided Chem. Eng. 2020, 48, 613–618. [Google Scholar]
- StopLindano. Memoria Anual 2020. Servicio de Seguimiento Hidrogeológico de Sardas, 2020–2022. Available online: http://www.stoplindano.es/app/uploads/2021/07/Memoria%20anual%20Sardas%202020%20completo%20red.pdf (accessed on 1 November 2022).
- Laha, S.; Tansel, B.; Ussawarujikulchai, A. Surfactant-soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: A review. J. Environ. Manag. 2009, 90, 95–100. [Google Scholar] [CrossRef]
- Ussawarujikulchai, A.; Laha, S.; Tansel, B. Synergistic Effects of Organic Contaminants and Soil Organic Matter on the Soil-Water Partitioning and Effectiveness of a Nonionic Surfactant (Triton X-100). Bioremediat. J. 2008, 12, 88–97. [Google Scholar] [CrossRef]
- Fytianos, K.; Voudrias, E.; Papamichali, A. Behavior and fate of linear alkylbenzene sulfonate in different soils. Chemosphere 1998, 36, 2741–2746. [Google Scholar] [CrossRef]
- Kang, S.; Jeong, H.Y. Sorption of a nonionic surfactant Tween 80 by minerals and soils. J. Hazard. Mater. 2015, 284, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cruz, M.S.; Sanchez-Martin, M.J.; Sanchez-Camazano, M. A comparative study of adsorption of an anionic and a non-ionic surfactant by soils based on physicochemical and mineralogical properties of soils. Chemosphere 2005, 61, 56–64. [Google Scholar] [CrossRef]
- Ramsburg, C.A.; Pennell, K.D.; Abriola, L.M.; Daniels, G.; Drummond, C.D.; Gamache, M.; Hsu, H.-l.; Petrovskis, E.A.; Rathfelder, K.M.; Ryder, J.L.; et al. Pilot-Scale Demonstration of Surfactant-Enhanced PCE Solubilization at the Bachman Road Site. 2. System Operation and Evaluation. Environ. Sci. Technol. 2005, 39, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Robert, T.; Martel, R.; Conrad, S.H.; Lefebvre, R.; Gabriel, U. Visualization of TCE recovery mechanisms using surfactant–polymer solutions in a two-dimensional heterogeneous sand model. J. Contam. Hydrol. 2006, 86, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.; Acosta, E.; Annable, M.D.; Brooks, M.C.; Enfield, C.G.; Harwell, J.H.; Hasegawa, M.; Knox, R.C.; Rao, P.S.C.; Sabatini, D.A.; et al. Field demonstration of surfactant-enhanced solubilization of DNAPL at Dover Air Force Base, Delaware. J. Contam. Hydrol. 2006, 82, 1–22. [Google Scholar] [CrossRef]
- Bettahar, M.; Ducreux, J.; Schäfer, G.; Van Dorpe, F. Surfactant enhanced in situ remediation of LNAPL contaminated aquifers: Large scale studies on a controlled experimental site. Transp. Porous Media 1999, 37, 255–276. [Google Scholar] [CrossRef]
- Besha, A.T.; Bekele, D.N.; Naidu, R.; Chadalavada, S. Recent advances in surfactant-enhanced In-Situ Chemical Oxidation for the remediation of non-aqueous phase liquid contaminated soils and aquifers. Environ. Technol. Innov. 2018, 9, 303–322. [Google Scholar] [CrossRef]

| Injections | |||||||
|---|---|---|---|---|---|---|---|
| Event | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Injection wells | PS14B, PS14E, PS14I | PS5D | PS14E | PS14E | PS14I | PS14B, PS14J, PS14M | PS14D |
| Date/time injection | 20 April 2021 8:00 | 11 May 2021 10:30 | 24 May 2021 13:30 | 7 June 2021 10:35 | 14 June 2021 11:50 | 20 September 2021 12:07 | 5 October 2021 13:30 |
| Surfactant (g/L) | 20 | 25 | 5.4 | 25 | 25 | 30 | 53 |
| Bromide (Y/N) | Yes (260 mg/L) | No | No | No | No | Yes (538 mg/L) | N |
| Q injection (m3/h) | 0.30 | 0.65 | 0.68 | 0.85 | 0.71 | 0.20 | Approx. 0.08 |
| Injection Depth (m) | 0.5 above contact with marls | 0.5 above contact with marls | 0.5 above contact with marls | 0.5 above contact with marls | 0.5 above contact with marls | Alluvial-marls contact | Alluvial-marls contact |
| Injection duration (h) | 8.0 | 4.0 | 3.5 | 3.0 | 3.5 | 5.0 | 2.5 |
| Total volume injected (m3) | 7.20 | 3.00 | 2.80 | 2.60 | 3.00 | 2.80 | 0.20 |
| Monitoring wells during injection | PS14, PS14B, PS14C, PS14D, PS14E, PS14F, PS14G, PS14H, PS14I, PS14J, PS14K PS14L, PS14M and PS21C | PS5D | PS14E | PS14, PS14B, PS14C, PS14D, PS14E, PS14I, PS14J, PS14K, PS14L, PS14M | |||
| Monitoring time (GW samples) (h) | 0, 8 and 24 | 0, 8, 24 | 0, 5.8, 24 | ||||
| Remarks | Simultaneous extraction in PS14I at approx. 0.18 m3/h. (total volume extracted 1.3 m3) | ||||||
| Extractions | |||||||
|---|---|---|---|---|---|---|---|
| Event | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Date/time extraction 1 | 21 April 2021 15:00 | 13 May 2021 9:00 | 27 May 2021 7:00 | 10 June 2021 11:30 | 17 June 2021 6:40 | 22 September 2021 7:20 | 6 October 2021 7:00 |
| Extraction wells | PS14, PS14B, PS14C, PS14D, PS14E, PS14G, PS14H, PS14I, PS14K, PS14M | PS5D | PS14E | PS14E | PS14I | PS14I | PS14D |
| Pumping depth | Bottom of piezometer | 0.5 m above alluvial-marl contact | Bottom of piezometer | Bottom of piezometer | Bottom of piezometer | Bottom of piezometer | Bottom of piezometer |
| Q extraction (m3/h) | 0.30 | 2 m3 | 3.00 | 3.00 | 3.00–4.50 | 1 m3/h (0–1 h) | 4.50 |
| 2 m3/h (1–2 h) | |||||||
| 3 m3/h (2–3 h) | |||||||
| 4 m3/h (3–4 h) | |||||||
| 5 m3/h (4–5 h) | |||||||
| Total volume extracted (m3) | 6.52 | 6.00 | 9.00 | 24.00 | 37.00 | 15.26 | 38.38 |
| Date/time extraction 2 | 6 May 2021 10:45 | 14 May 2021 7:00 | 22 September 2021 12:30 | ||||
| Extraction point(s) | PS14B, PS14E, PS14I | PS5D | PS14B, PS14M, PS14J | ||||
| Suction depth | Bottom of piezometer | 0.5 m above alluvial-marls contact | Bottom of piezometer | ||||
| Q extraction (m3/h) | 0.30 | Staggered 2 m3 | 4.50 | ||||
| Total volume extracted (m3) | 2.21 | 6.00 | 12.88 | ||||
| Time elapsed between injection and extraction (h) | 1st: 31; 2nd 391 | 1st: 47; 2nd 71 | 65.5 | 70 | 67 | 1st 48; 2nd 53 | 18 |
| Event | Surfactant Injected (kg) | V Injected (m3) | V Extracted (m3) | kg of DNAPL Recovered as Organic Phase | Mass of Surfactant in Extracted Fluids (kg) | kg COCs Solubilized in Extracted Fluids | Average Surfactant Concentration in Extracted Fluid (g/L) | Average COCs Concentration Solubilized in Extracted Fluid (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 144 | 7.2 | 6.52 | 53 | 2 | 0.52 | 0.31 | 79.75 |
| 2 | 75 | 3 | 6 | 10 | 9 | 0.75 | 1.50 | 125.00 |
| 3 | 18 | 2.8 | 9 | 4.5 | 0 | 0.81 | 0.00 | 90.00 |
| 4 | 60 | 2.6 | 24 | 15 | 0.06 | 1 | 0.00 | 41.67 |
| 5 | 60 | 3 | 37 | 12 | 1 | 0.19 | 0.03 | 5.14 |
| 6 | 84 | 2.8 | 16.06 | 280 | 10 | 0.54 | 0.62 | 33.62 |
| 7 | 10 | 0.2 | 38.4 | 0 | 6 | 1.8 | 0.16 | 46.88 |
| SUM | 451.00 | 21.60 | 136.98 | 374.5 | 28.06 | 5.61 |
| Event | Well | C Surf (g/L) | COCs Tot (mg/L) | CBs + DCBS (mg/L) | TCBs + TetraCBS (mg/L) | NACs (mg/L) |
|---|---|---|---|---|---|---|
| April 2018 | PS14 | 0 | 71.17 | 50.96 | 5.65 | 14.57 |
| November 2020 | PS14 | 0 | 51.0 | 31.0 | 4.0 | 16.0 |
| 1 (April 2021) | PS14 | 0 | 26.50 | 16.45 | 5.48 | 4.57 |
| 6 (September 2021) | PS14 | 0 | 26.68 | 16.61 | 5.58 | 4.49 |
| April 2018 | PS14B | 0 | 89.21 | 67.68 | 6.34 | 15.19 |
| November 2020 | PS14B | 0 | 50.0 | 32.0 | 2.0 | 16.0 |
| 1 (April 2021) | PS14B | 0 | 17.32 | 11.1 | 3.2 | 3.02 |
| 6 (September 2021) | PS14B | 0 | 23.41 | 16.66 | 3.31 | 3.44 |
| April 2018 | PS14C | 0 | 77.92 | 57.25 | 5.08 | 15.60 |
| November 2020 | PS14C | 0 | 63.0 | 37.0 | 3.0 | 23.0 |
| 1 (Apr 2021) | PS14C | 0 | 21.26 | 12 | 5.1 | 4.16 |
| 6 (Sept 2021) | PS14C | 0 | 29.25 | 20.72 | 4.08 | 4.45 |
| Apr 2018 | PS14D | 0 | 108.47 | 83.73 | 6.58 | 18.16 |
| Nov 2020 | PS14D | 0 | 52.0 | 32.0 | 3.0 | 18.0 |
| 1 (April 2021) | PS14D | 0 | 19.5 | 13 | 3.4 | 3.1 |
| 6 (September 2021) | PS14D | 0 | 37.21 | 27.01 | 4.81 | 5.39 |
| November 2020 | PS14E | 0 | 59.0 | 43.0 | 3.0 | 13.0 |
| 1 (April 2021) | PS14E | 0 | 21 | 12 | 5 | 4 |
| 6 (September 2021) | PS14E | 0 | 23.78 | 18.41 | 3.09 | 2.28 |
| November 2020 | PS14I | 0 | 41.26 | 32.0 | 2.0 | 16.0 |
| 1 (April 2021) | PS14I | 0 | 20.2 | 12.9 | 3.8 | 3.5 |
| 6 (September 2021) | PS14I | 0 | 25.1 | 16.58 | 3.81 | 4.71 |
| 1 (April 2021) | PS14J | 0 | 21.2 | 14 | 3.8 | 3.4 |
| 6 (September 2021) | PS14J | 0 | 26.08 | 15.5 | 4.4 | 6.18 |
| November 2020 | PS14K | 0 | 20.3 | 15.5 | 0.8 | 4.0 |
| 1 (April 2021) | PS14K | 0 | 13.7 | 9 | 2.4 | 2.3 |
| 6 (September 2021) | PS14K | 0 | 7.64 | 6.77 | 0.57 | 0.3 |
| November 2020 | PS14L | 0 | 3.54 | 2.83 | 0.15 | 0.56 |
| 1 (April 2021) | PS14L | 0 | 9.8 | 6 | 2.1 | 1.7 |
| 6 (September 2021) | PS14L | 0 | 17.98 | 12.7 | 2.3 | 2.98 |
| November 2020 | PS14M | 0 | 32.1 | 23.8 | 1.4 | 6.9 |
| 1 (April 2021) | PS14M | 0 | 17.5 | 10.2 | 3.9 | 3.4 |
| 6 (September 2021) | PS14M | 0 | 18.83 | 14.85 | 2.37 | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadaño, J.; Gómez, J.; Fernández, J.; Lorenzo, D.; Domínguez, C.M.; Cotillas, S.; García-Cervilla, R.; Santos, A. Remediation of the Alluvial Aquifer of the Sardas Landfill (Sabiñánigo, Huesca) by Surfactant Application. Sustainability 2022, 14, 16576. https://doi.org/10.3390/su142416576
Guadaño J, Gómez J, Fernández J, Lorenzo D, Domínguez CM, Cotillas S, García-Cervilla R, Santos A. Remediation of the Alluvial Aquifer of the Sardas Landfill (Sabiñánigo, Huesca) by Surfactant Application. Sustainability. 2022; 14(24):16576. https://doi.org/10.3390/su142416576
Chicago/Turabian StyleGuadaño, Joaquín, Jorge Gómez, Jesús Fernández, David Lorenzo, Carmen M. Domínguez, Salvador Cotillas, Raúl García-Cervilla, and Aurora Santos. 2022. "Remediation of the Alluvial Aquifer of the Sardas Landfill (Sabiñánigo, Huesca) by Surfactant Application" Sustainability 14, no. 24: 16576. https://doi.org/10.3390/su142416576







