Establishment of a City-Based Index to Communicate Air Pollution-Related Health Risks to the Public in Bangkok, Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overall Process
2.2. Air Pollution and Meteorological Data
2.3. Mortality Data
2.4. Statistical Analysis
2.5. Constructing the AQHI Equations
2.6. Evaluation of the AQHI Validity
3. Results
3.1. Descriptive Analysis
3.2. Formation of the AQHI
3.3. Validity of the AQHI
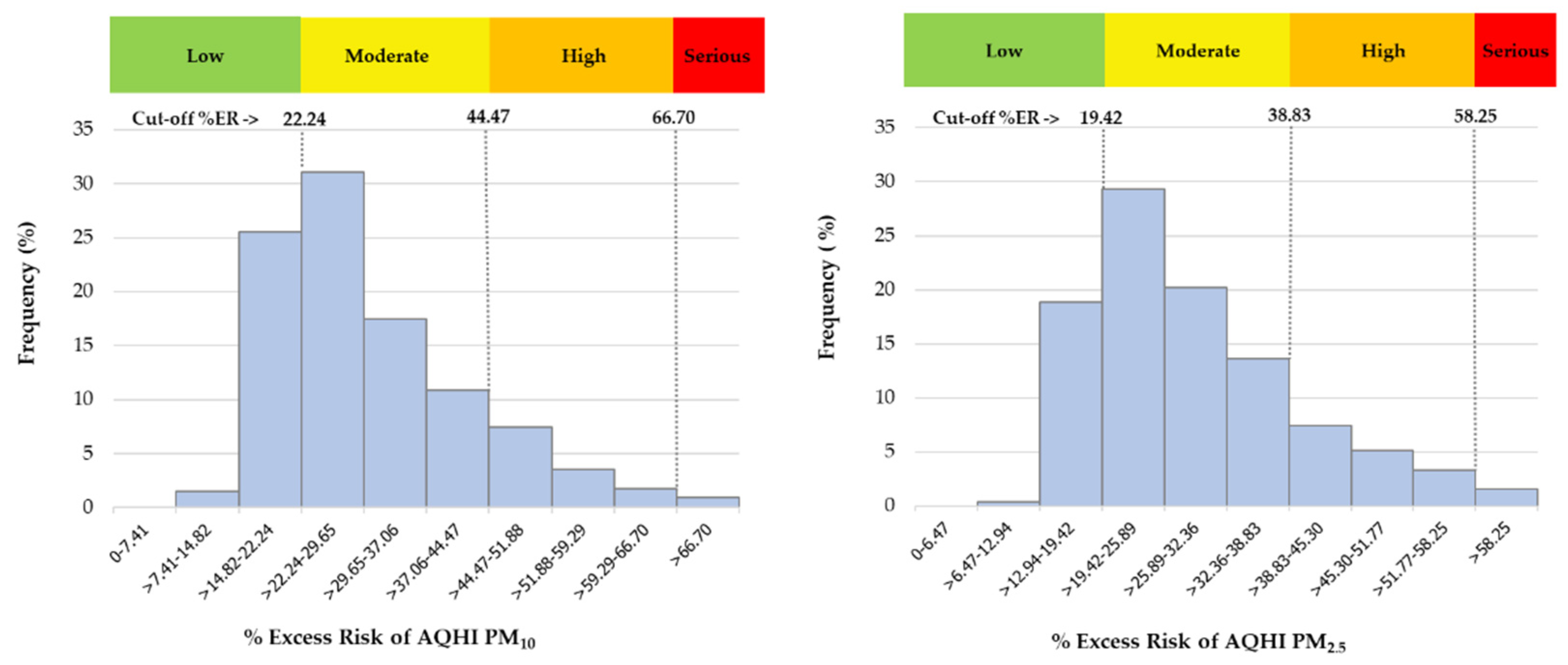
3.4. Banding the AQHI for Air Quality Reports Aimed at the Public
4. Discussion
4.1. Associations between Air Pollution and Respiratory Disease-Related Deaths
4.2. Excess Risk of Mortality
4.3. Establisment of the AQHI
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaniabadi, Y.O.; Sicard, P.; Takdastan, A.; Hopke, P.K.; Taiwo, A.M.; Khaniabadi, F.O.; De Marco, A.; Daryanoosh, M. Mortality and morbidity due to ambient air pollution in Iran. Clin. Epidemiol. Global Health 2019, 7, 222–227. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 25 December 2021).
- Fold, N.R.; Allison, M.R.; Wood, B.C.; Thao, P.T.B.; Bonnet, S.; Garivait, S.; Kamens, R.; Pengjan, S. An assessment of annual mortality attributable to ambient PM2.5 in Bangkok, Thailand. Int. J. Environ. Res. Public Health 2020, 17, 7298. [Google Scholar] [CrossRef] [PubMed]
- Kamma, J.; Manomaiphiboon, K.; Aman, N.; Thongkamdee, T.; Chuangchote, S.; Bonnet, S. Urban heat island analysis for Bangkok: Multi-scale temporal variation, associated factors, directional dependence, and cool island condition. ScienceAsia 2020, 46, 213–223. [Google Scholar] [CrossRef]
- Aman, N.; Manomaiphiboon, K.; Suwattiga, P.; Assareh, N.; Limpaseni, W.; Suwanathada, P.; Soonsin, V.; Wang, Y. Visibility, aerosol optical depth, and low-visibility events in Bangkok during the dry season and associated local weather and synoptic patterns. Environ. Monit. Assess. 2022, 194, 322. [Google Scholar] [CrossRef] [PubMed]
- Wannalai, S.; Nokaew, S.; Siriwong, W. Assessment of Knowledge and Perception of Adverse Health Effects Associated With Self-Prevention From Air Pollution in Traffic Policemen in Bangkok, Thailand. J. Health Res. 2016, 30, 147–152. [Google Scholar]
- Pollution Control Department (PCD). Air Quality Inndex. Available online: air4thai.pcd.go.th/webV3/#/AQIInfo (accessed on 25 December 2021). (In Thai).
- United States Environmental Protection Agency (U.S.EPA). Air Quality Index Report. Available online: https://www.airnow.gov/aqi/aqi-basics/ (accessed on 27 December 2021).
- Cromar, K.; Gladson, L.; Jaimes Palomera, M.; Perlmutt, L. Development of a health-based index to identify the association between air pollution and health effects in Mexico city. Atmosphere 2021, 12, 372. [Google Scholar] [CrossRef]
- Stieb, D.M.; Burrnett, R.T.; Smith-Doiron, M.; Brion, O.; Hwashin, H.S.; Economou, V. A new multipollutant, no-threshold air quality health index based on short-term associations observed in daily time-series analyses. J. Air Waste Manag. Assoc. 2008, 58, 435–450. [Google Scholar] [CrossRef] [Green Version]
- Government of Canada. Air Quality Health Index. Available online: https://weather.gc.ca/airquality/pages/index_e.html (accessed on 17 June 2022).
- Environmental Protection Department. Forecast of Health Risk Maximums. Available online: https://www.aqhi.gov.hk/en.html (accessed on 17 June 2022).
- Olstrup, H. An air quality health index (AQHI) with different health outcomes based on the air pollution concentrations in stockholm during the period of 2015–2017. Atmosphere 2020, 11, 192. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.W.; Tam, W.W.S.; Yu, I.T.S.; Lau, A.K.H.; Pang, S.W.; Wong, A.H.S. Developing a risk-based air quality health index. Atmos. Environ. 2013, 76, 52–58. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.; Meng, X.; Hua, J.; Zhou, Z.; Chen, B.; Kan, H. Communicating air pollution-related health risks to the public: An application of the Air Quality Health Index in Shanghai, China. Environ. Int. 2013, 51, 168–173. [Google Scholar] [CrossRef]
- Du, X.; Chen, R.; Meng, X.; Liu, C.; Niu, Y.; Wang, W.; Li, S.; Kan, H.; Zhou, M. The establishment of National Air Quality Health Index in China. Environ. Int. 2020, 138, 105–594. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; Lin, H.; Liu, T.; Qian, Z.; Zeng, W.; Guo, L.; Ma, W. The construction and validity analysis of AQHI based on mortality risk: A case study in Guangzhou, China. Environ. Pollut. 2017, 220, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Guaita, R.; Pichiule, M.; Maté, T.; Linares, C.; Díaz, J. Short-term impact of particulate matter (PM2.5) on respiratory mortality in Madrid. Int. J. Environ. Health Res. 2011, 21, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, 69–74. [Google Scholar]
- Grigorieva, E.; Lukyanets, A. Combined effect of hot weather and outdoor air pollution on respiratory health: Literature review. Atmosphere 2021, 12, 790. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; Limsukon, A.; Tajarernmuang, P.; Phetsuk, N. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. J. Thorac. Dis. 2019, 11, 3070–3083. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Air Quality and Health. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/policy-progress/sustainable-development-goals-air-pollution (accessed on 15 June 2022).
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [Green Version]
- Vajanapoom, N.; Shy, C.M.; Neas, L.M.; Loomis, D. Associations of particulate matter and daily mortality in Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 2002, 33, 389–399. [Google Scholar] [PubMed]
- Vichit-Vadakan, N.; Vajanapoom, N.; Ostro, B. The Public Health and Air Pollution in Asia (PAPA) Project: Estimating the mortality effects of particulate matter in Bangkok, Thailand. Environ. Health Perspect. 2008, 116, 1179–1182. [Google Scholar] [CrossRef]
- Ravindra, K.; Rattan, P.; Mor, S.; Aggarwal, A.N. Generalized additive models: Building evidence of air pollution, climate change and human health. Environ. Int. 2019, 132, 104–987. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, S.; Tawatsupa, B.; Punnasiri, K.; Jaakkola, J.J.K.; Williams, G. The association between air pollution and mortality in Thailand. Sci. Rep. 2014, 4, 5509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.N. Generalized Linear Models An Introduction with R. In Statistical Models in S, 2nd ed.; Blitzstein, J.K., Faraway, J.J., Tanner, M., Zidek, J., Eds.; Chapman and Hall/CRC: New York, NY, USA, 2017; pp. 1–492. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning with Applications in R. In Springer Texts in Statistics, 1st ed.; Casella, G., Fienberg, S., Olkin, I., Eds.; Springer: New York, NY, USA, 2013; pp. 1–426. [Google Scholar]
- Chen, R.; Yin, P.; Meng, X.; Liu, C.; Wang, L.; Xu, X.; Ross, J.A.; Tse, L.A.; Zhao, Z.; Kan, H.; et al. Fine particulate air pollution and daily mortality: A nationwide analysis in 272 Chinese cities. Am. J. Respir. Crit. Care Med. 2017, 196, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, H.; Zhao, Z.; Xiang, X.; Li, M.; Juan, J.; Song, J.; Cao, Y.; Wang, X.; Chen, L.; et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: A nationwide time-series analysis. PLoS Med. 2018, 15, e1002668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskaran, K.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Global Air Quality Guidelines. Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 15 December 2021).
- Meteorological. Climate Statistics for the Period of 30 Years (1981–2010). Available online: http://climate.tmd.go.th/statistic/stat30y (accessed on 15 March 2021).
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Liu, B.; Sun, X.; Zhang, J.; Bi, X.; Li, Y.; Li, L.; Dong, H.; Xiao, Z.; Zhang, Y.; Feng, Y. Characterization and spatial source apportionments of ambient PM10 and PM2.5 during the heating period in tian’jin, China. Aerosol Air Qual. Res. 2020, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Sharma, S.K.; Vijayan, N.; Mandal, T.K. Seasonal characteristics of aerosols (PM2.5 and PM10) and their source apportionment using PMF: A four year study over Delhi, India. Environ. Pollut. 2020, 262, 114–337. [Google Scholar] [CrossRef]
- Jiang, N.; Yin, S.; Guo, Y.; Li, J.; Kang, P.; Zhang, R.; Tang, X. Characteristics of mass concentration, chemical composition, source apportionment of PM2.5 and PM10 and health risk assessment in the emerging megacity in China. Atmos. Pollut. Res. 2018, 9, 309–321. [Google Scholar] [CrossRef]
- World Health Organization. Health Effects of Particulate Matter. Available online: https://www.euro.who.int/__data/assets/pdf_file/0006/189051/Health-effects-of-particulate-matter-final-Eng.pdf (accessed on 18 March 2021).
- Lin, H.; Tao, J.; Du, Y.; Liu, T.; Qian, Z.; Tian, L.; Di, Q.; Rutherford, S.; Guo, L.; Zeng, W.; et al. Particle size and chemical constituents of ambient particulate pollution associated with cardiovascular mortality in Guangzhou, China. Environ. Pollut. 2016, 208, 758–766. [Google Scholar] [CrossRef]
- Laumbach, R.J.; Cromar, K.R.; Adamkiewicz, G.; Carlsten, C.; Charpin, D.; Chan, W.R.; De Nazelle, A.; Forastiere, F.; Goldstein, J.; Gumy, S.; et al. Personal Interventions for Reducing Exposure and Risk for Outdoor Air Pollution: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Narita, D.; Oanh, N.T.K.; Sato, K.; Huo, M.; Permadi, D.A.; Chi, N.N.H.; Ratanajaratroj, T.; Pawarmart, I. Pollution Characteristics and Policy Actions on Fine Particulate Matter in a Growing Asian Economy: The Case of Bangkok Metropolitan Region. Atmosphere 2019, 10, 227. [Google Scholar] [CrossRef]
- Yitshak-Sade, M.; Bobb, J.F.; Schwartz, J.D.; Kloog, I.; Zanobetti, A. The association between short and long-term exposure to PM2.5 and temperature and hospital admissions in New England and the synergistic effect of the short-term exposures. Sci. Total Environ. 2018, 639, 868–875. [Google Scholar] [CrossRef]
- Ye, T.; Guo, Y.; Chen, G.; Yue, X.; Xu, R.; Coêlho, M.D.S.Z.S.; Saldiva, P.H.N.; Zhao, Q.; Li, S. Risk and burden of hospital admissions associated with wildfire-related PM2·5 in Brazil, 2000–2015: A nationwide time-series study. Lancet Planet. Health 2021, 5, 599–607. [Google Scholar] [CrossRef]
- Dastoorpoor, M.; Khanjani, N.; Bahrampour, A.; Goudarzi, G.; Aghababaeian, H.; Idani, E. Short-term effects of air pollution on respiratory mortality in Ahvaz, Iran. Med. J. Islam. Repub. Iran 2018, 32, 30. [Google Scholar] [CrossRef]
- Pinichka, C.; Makka, N.; Sukkumnoed, D.; Chariyalertsak, S.; Inchai, P.; Bundhamcharoen, K. Burden of disease attributed to ambient air pollution in Thailand: A GIS-based approach. PLoS ONE 2017, 12, e0189909. [Google Scholar] [CrossRef]
- Wu, H.; Lu, K.; Fu, J. A Time-Series Study for Effects of Ozone on Respiratory Mortality and Cardiovascular Mortality in Nanchang, Jiangxi Province, China. Front. Public Health 2022, 10, 864537. [Google Scholar] [CrossRef]
- Almeida, S.P.; Casimiro, E.; Calheiros, J. Short-term association between exposure to ozone and mortality in Oporto, Portugal. Environ. Res. 2011, 111, 406–410. [Google Scholar] [CrossRef]
- Chen, R.; Yin, P.; Meng, X.; Wang, L.; Liu, C.; Niu, Y.; Lin, Z.; Liu, Y.; Liu, J.; Qi, J.; et al. Associations Between Ambient Nitrogen Dioxide and Daily Cause-specific Mortality: Evidence from 272 Chinese Cities. Epidemiology 2018, 29, 482–489. [Google Scholar] [CrossRef]
- Chiusolo, M.; Cadum, E.; Stafoggia, M.; Galassi, C.; Berti, G.; Faustini, A.; Bisanti, L.; Vigotti, M.A.; Dessì, M.P.; Cernigliaro, A.; et al. Short-term effects of nitrogen dioxide on mortality and susceptibility factors in 10 Italian cities: The EpiAir study. Environ. Health Perspect. 2011, 119, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; She, L.; Guo, Y.; Zhao, Y.; Zhang, P.; Xiang, B.; Zeng, J.; Yang, M.; Wang, L. Association between ambient air pollution and mortality from chronic obstructive pulmonary disease in Wuhan, China: A population-based time-series study. Environ. Sci. Pollut. Res. 2021, 28, 33698–33706. [Google Scholar] [CrossRef]
- Hooper, L.G.; Kaufman, J.D. Ambient air pollution and clinical implications for susceptible populations. Ann. Am. Thorac. Soc. 2018, 15, 64–68. [Google Scholar]
- Ferguson, L.; Taylor, J.; Davies, M.; Shrubsole, C.; Symonds, P.; Dimitroulopoulou, S. Exposure to indoor air pollution across socio-economic groups in high-income countries: A scoping review of the literature and a modelling methodology. Environ. Int. 2020, 143, 105–748. [Google Scholar] [CrossRef]
- Fakkaew, N.; Bualert, S.; Thongyen, T.; Rungratanaubon, T. Ozone formation potential of ambient volatile organic compounds at roadside in Bangkok, Thailand. App. Envi. Res. 2021, 43, 14–28. [Google Scholar] [CrossRef]
- Choomanee, P.; Bualert, S.; Thongyen, T.; Duangmal, K.; Intaraksa, A.; Rungratanaubon, T.; Szymanski, W.W. Experimental assessment of tropical surface ozone related to land utilization in Central Thailand. Atmos. Environ. 2021, 11, 100–129. [Google Scholar] [CrossRef]
- Pollution Control Department (PCD). Situation and Management Air and Noise Pollution of Thailand in 2020. Available online: http://air4thai.pcd.go.th/webV2/download.php (accessed on 25 December 2021).
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Stieb, D.M.; Taylor, E.; Henderson, S.B. Assessment of the air quality health index (AQHI) and four alternate AQHIplus amendments for wildfire seasons in British columbia. Can. J. Public Health 2020, 111, 96–106. [Google Scholar] [CrossRef]
- Cao, R.; Wang, Y.; Huang, J.; Zeng, Q.; Pan, X.; Li, G.; He, T. The construction of the air quality health index (AQHI) and a validity comparison based on three different methods. Environ. Res. 2021, 197, 110–987. [Google Scholar] [CrossRef]
- Jarauta-Bragulat, E.; Hervada-Sala, C.; Egozcue, J.J. Air Quality Index Revisited from a Compositional Point of View. Math. Geosci. 2016, 48, 581–593. [Google Scholar]
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef]
- Jiang, X.Q.; Mei, X.D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, 31–40. [Google Scholar]
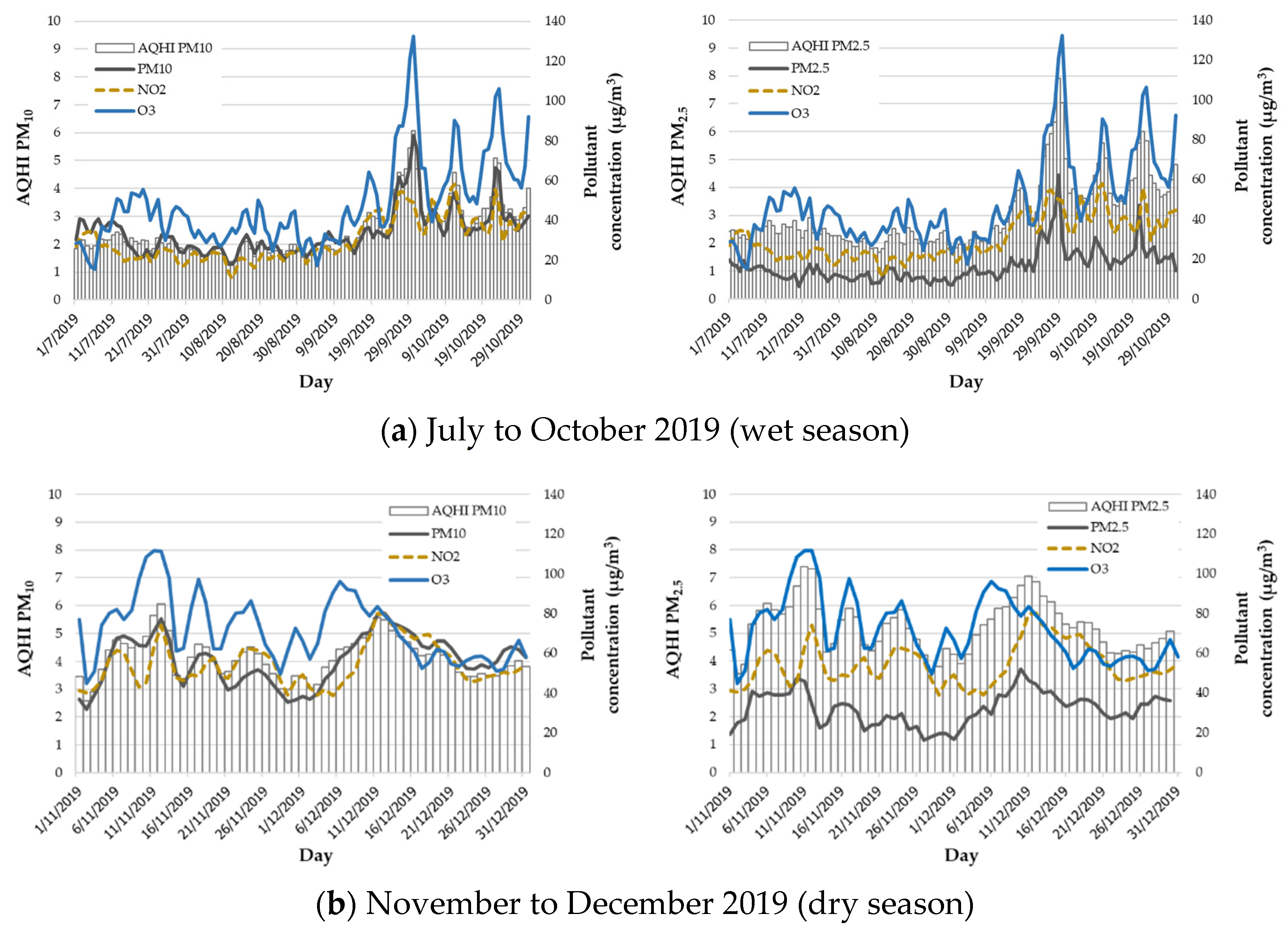
| Variable | Mean | SD | Min | Max | P25 | P50 | P75 | WHO Guideline |
|---|---|---|---|---|---|---|---|---|
| Number of deaths based on ICD-10 (J00–J99) b | 10.4 | 3.5 | 0.0 | 27.0 | 8.0 | 10.0 | 13.0 | |
| Air pollution | ||||||||
| PM2.5 (μg/m3) a | 24.8 | 13.6 | 5.4 | 90.0 | 14.9 | 20.5 | 30.9 | 15 |
| PM10 (μg/m3) b | 39.7 | 18.7 | 12.1 | 156.1 | 26.5 | 34.7 | 47.5 | 45 |
| SO2 (μg/m3) b | 6.2 | 3.1 | 0.4 | 30.7 | 4.0 | 5.5 | 7.8 | 40 |
| NO2 (μg/m3) b | 38.6 | 15.9 | 7.4 | 118.6 | 27.0 | 34.3 | 47.7 | 25 |
| O3 (μg/m3) b | 59.5 | 25.0 | 5.8 | 170.6 | 41.0 | 56.2 | 75.3 | NA |
| CO (ppm) b | 1.1 | 1.7 | 0.2 | 23.2 | 0.5 | 0.7 | 0.9 | 3.5 |
| Meteorology | ||||||||
| Temperature (°C) b | 29.1 | 1.8 | 18.2 | 34.3 | 28.1 | 29.3 | 30.3 | |
| Relative humidity (%) b | 70.2 | 8.8 | 37.8 | 96.6 | 64.5 | 69.9 | 75.9 |
| Spearman Correlation | PM2.5 (μg/m3) | PM10 (μg/m3) | SO2 (μg/m3) | NO2 (μg/m3) | O3 (μg/m3) | CO (ppm) |
|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) a | 1.000 | 0.913 ** | 0.469 ** | 0.649 ** | 0.644 ** | 0.478 ** |
| PM10 (μg/m3) b | 1.000 | 0.332 ** | 0.650 ** | 0.649 ** | 0.467 ** | |
| SO2 (μg/m3) b | 1.000 | 0.475 ** | 0.139 ** | 0.236 ** | ||
| NO2 (μg/m3) b | 1.000 | 0.407 ** | 0.631 ** | |||
| O3 (μg/m3) b | 1.000 | 0.240 ** | ||||
| CO (ppm) b | 1.000 |
| Lag Days | Poisson Regression Coefficient (95% CI) | |||||
|---|---|---|---|---|---|---|
| PM2.5 a | PM10 b | O3 b | SO2 b | NO2 b | CO b | |
| Lag0 | 0.0187 ** (0.0047, 0.0327) | 0.0188 *** (0.0118, 0.0258) | 0.0169 *** (0.0116, 0.0223) | 0.0298 (−0.0137, 0.0732) | 0.0193 *** (0.0109, 0.0276) | −0.0192 (−0.0970, 0.0586) |
| Lag1 | 0.0261 *** (0.0113, 0.0409) | 0.0177 *** (0.0104, 0.0252) | 0.0110 *** (0.0058, 0.0162) | 0.0271 (−0.0171, 0.0713) | 0.0209 *** (0.0122, 0.0297) | −0.0362 (−0.1144, 0.0420) |
| Lag2 | 0.0165 * (0.0016, 0.0314) | 0.0163 *** (0.0088, 0.0237) | 0.0114 *** (0.0064, 0.0165) | 0.0243 (−0.0195, 0.0681) | 0.0189 *** (0.0105, 0.0273) | −0.0294 (−0.1074, 0.0487) |
| Lag3 | 0.0213 ** (0.0070,0.0357) | 0.0129 *** (0.0056, 0.0201) | 0.0088 *** (0.0039, 0.0137) | 0.0422 (−0.0012, 0.0857) | 0.0161 *** (0.0079, 0.0243) | −0.0597 (−0.1384, 0.0189) |
| Lag01 | 0.0185 * (0.0041, 0.0329) | 0.0197 *** (0.0125, 0.0269) | 0.0204 *** (0.0147, 0.0261) | 0.0397 (−0.0071, 0.0864) | 0.0230 *** (0.0144, 0.0316) | −0.0320 (−0.1134, 0.0494) |
| Respiratory Disease-Related Mortality | AQHI PM10 | AQHI PM2.5 | AQI |
|---|---|---|---|
| Lag0 | 0.2603 (0.1814, 0.3393) | 0.2922 (0.1951, 0.3893) | 0.0086 (0.0059, 0.0113) |
| Lag1 | 0.3260 (0.2126, 0.4393) | 0.2425 (0.1472, 0.3378) | 0.0058 (0.0032, 0.0085) |
| Lag2 | 0.3056 (0.1939, 0.4174) | 0.2162 (0.1223, 0.3101) | 0.0062 (0.0036, 0.0088) |
| Lag3 | 0.2401 (0.1325, 0.3476) | 0.1776 (0.0873, 0.2680) | 0.0047 (0.0022, 0.0072) |
| Lag01 | 0.3926 (0.2802, 0.5050) | 0.4427 (0.3066, 0.5791) | 0.0091 (0.0063, 0.0119) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onchang, R.; Hirunkasi, K.; Janchay, S. Establishment of a City-Based Index to Communicate Air Pollution-Related Health Risks to the Public in Bangkok, Thailand. Sustainability 2022, 14, 16702. https://doi.org/10.3390/su142416702
Onchang R, Hirunkasi K, Janchay S. Establishment of a City-Based Index to Communicate Air Pollution-Related Health Risks to the Public in Bangkok, Thailand. Sustainability. 2022; 14(24):16702. https://doi.org/10.3390/su142416702
Chicago/Turabian StyleOnchang, Rattapon, Kannigar Hirunkasi, and Siriwan Janchay. 2022. "Establishment of a City-Based Index to Communicate Air Pollution-Related Health Risks to the Public in Bangkok, Thailand" Sustainability 14, no. 24: 16702. https://doi.org/10.3390/su142416702
APA StyleOnchang, R., Hirunkasi, K., & Janchay, S. (2022). Establishment of a City-Based Index to Communicate Air Pollution-Related Health Risks to the Public in Bangkok, Thailand. Sustainability, 14(24), 16702. https://doi.org/10.3390/su142416702







