Abstract
The prevention and control of root-knot nematode disease is a worldwide challenge and there are not many varieties of pesticides for nematode control. To meet the huge market demand, the development of new nematicides is urgently needed. The lethal effects of soil fumigant dimethyl disulfide (DMDS) mixed with the chemical compounds copper sulfate (CuSO4) and ammonium bicarbonate (NH4HCO3) on Meloidogyne incognita were tested using the immersion method. The results showed that the LC50 of DMDS, CuSO4, and NH4HCO3 on the second stage juveniles (J2) of M. incognita were 19.28, 187.42, and 213.49 mg/L, respectively. The lethal effect on J2 were enhanced with the combination of DMDS and CuSO4 or NH4HCO3. The compound uses of DMDS (2.5 mg/L) and CuSO4 (46.58 mg/L) or NH4HCO3 (80.25 mg/L) have obvious synergistic effects on the control of M. incognita, with corrected mortalities of 97.09% and 94.00%, respectively. The synergistic effect of fumigant and chemical compounds on M. incognita was investigated to provide a new concept for the control of root-knot nematode disease.
1. Introduction
Plant-parasitic nematodes (PPN) are among the main invasive pathogens of plants. PPN are distributed worldwide and cause damage to important crops. More than 100 species of nematodes cause serious harm to agriculture, forestry, and economically important crops in China. The damage caused by PPN to some crops even exceeds that of other diseases, insects, and weeds [1], causing approximately USD 157 billion in losses to global agriculture every year [2], of which USD 10 billion is caused by Meloidogyne spp. [3]. Meloidogyne spp. (root-knot nematodes, RKN) are considered the most destructive species of PPN in the world and include four major species, namely, M. arenaria, M. hapla, M. incognita, and M. javanica [4]. Nematode control is subject to integrated pest management (IPM) [5,6].
Several management practices can be used to control nematode diseases. These include cultural, chemical, and biological controls and host resistance. The effective management of PPN has relied upon the application of chemical nematicides as a short-term control means by suppressing nematode population densities in soil to levels below known economic damage thresholds [7,8]. Chemical nematicides have been used singly or in combination with other nematode control practices since the late 19th century [9]. Nematicides can be classified into fumigants and non-fumigants, according to their action modes. Fumigant nematicides are applied as a liquid or gas and generate lethal volatiles that diffuse as gas into the soil and kill the nematodes [10]. Most fumigant nematicides are currently banned in many countries over concerns about their impacts on human health and the environment. For example, the fumigant methyl bromide damages the atmospheric ozone layer [11]. Others, such as dazomet, meta sodium, chloropicrin, 1,3-dichloropropene, sulfuryl fluoride, and dimethyl disulfide are still in use for controlling nematode diseases [12]. Other non-fumigant, but highly toxic, nematicides, such as aldicarb [13] and carbopol [14], are banned because of environmental problems. Only 10 nematicides are commonly used, although more than 30 active ingredients are known [1]. Therefore, the chemical control strategies of PPN face severe barriers in their application.
Focusing on the development of new environmentally friendly pesticides has become a research focus in the control of RKN disease in recent years. The scientific use of organic and inorganic compounds have a certain control effect on RKN [15]. Humic acid and trace elements such as Fe, Mn, and Cu have obvious inhibitory effects on the survival of M. incognita second-stage juveniles (J2) [16]. N, P, Cu, Mn, and K at very high concentrations have significant mortality rates on the Heterodera avenae J2 [17].
In a world where food safety and environmental concerns are increasing, assessing the compatibility of mixtures with other crop protection products and compounds to minimize the number of required applications is an important task. Therefore, the toxicity of the soil fumigant dimethyl disulfide (DMDS) combined with chemical compounds copper sulfate (CuSO4) and ammonium bicarbonate (NH4HCO3) to M. incognita was evaluated to provide a novel concept for the prevention and control of RKN.
2. Materials and Methods
2.1. Nematode Source
The nematodes were collected from infected tomato roots (Solanum lycopersicum L., Variety Provence) in Nanhe village, Dashiwo Town, Fangshan District, Beijing (115°48′2″ E, 39°31′33″) and identified as M. incognita via morphological and molecular identification. The root system was cleaned, single females were isolated and perineal patterns were prepared and observed, according to the methods of Zhang et al. [18] and Feng [19]. The DNA of J2 was extracted according to the method of Feng et al. [20]. A specific primer (MI-F: 5′-GTGAGGATTCAGCTCCCCAG-3′, MI-R: 5′-ACGAGGAACATACTTCTCCC-3′) [21] was used for PCR amplification. This primer was designed based on the specific RAPD fragments OP26-011200, which amplified a fragment of 995 bp from M. incognita [22]. Following the procedure of Hussey and Barker [23], fresh egg masses were picked from tomato roots. The eggs were extracted by applying 0.5% NaClO for 3 min, washed thrice with sterile water, and then placed into 24-well culture plates. The nematodes were incubated at room temperature (25 °C) to promote the hatching of J2. The freshly hatched J2 were collected every day and stored at 4 °C until further use.
2.2. Test Compounds
Dimethyl disulfide (98%, Shanghai Maclean Biochemical Technology Co., Ltd., Shanghai, China), copper sulfate (CuSO4·5H2O, Analytical Purity, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and ammonium bicarbonate (NH4HCO3, 99%, Beijing Coolman Technology Co., Ltd., Beijing, China) were used as test compounds.
2.3. Determination of the Toxicity of DMDS, CuSO4, and NH4HCO3 on M. incognita J2
In the immersion method, 0.5 mL of each test compound was added to 0.5 mL of nematode suspension (approximately 50 J2), resulting in final DMDS concentrations of 2.5, 5, 10, 20, 40, and 80 mg/L; final CuSO4 concentrations of 11.65, 23.29, 46.58, 93.16, 186.33, and 372.66 mg/L; and final NH4HCO3 concentrations of 80.25, 160.5, 321, 642, 1284, and 2568 mg/L. The control treatment consisted of the 50 J2s maintained in 1 mL distilled water alone. Each treatment was replicated 6 times. The samples were maintained at 25 °C for 24 h. Approximately 0.5 mL of the mixture from each replicate was observed under a microscope and the number of total and dead nematodes was recorded. The nematodes were classified as dead when they were straight and did not move when touched with a hairpin.
2.4. Determination of Toxicity of DMDS with CuSO4 or NH4HCO3 on M. incognita J2
The concentration gradients of DMDS, CuSO4, and NH4HCO3 were diluted as follows. Approximately 0.5 mL of nematode suspension with 100 J2/mL was added to a 2 mL centrifuge tube, followed by 0.5 mL of a mixture of DMDS with CuSO4 or DMDS with NH4HCO3. The final concentrations of DMDS were 2.5, 10, and 20 mg/L. The final concentrations of CuSO4 were 46.58 and 186.33 mg/L and those of NH4HCO3 were 80.25 and 241 mg/L. The samples were cultured at 25 °C for 24 h. Approximately 0.5 mL of the mixture from each replicate was observed under an anatomical microscope and the number of total and dead nematodes was recorded. The nematodes were classified as dead when they were straight and did not move when touched with a hairpin.
2.5. Statistical Analysis of Data
The data were used to calculate the corrected mortality using Formulas (1) and (2) [24]:
where P is the mortality (%), K is the number of dead nematodes, and N is the number of total nematodes;
where E is the corrected mortality (%), Pt is the mortality of treatments (%), and P0 is the mortality of the controls (%).
The dose–response probabilistic model (PROBIT) was used to calculate the half-lethal median concentration LC50 compound and its 95% confidence limit using the IBM SPSS Statistic V22.0.0 Software.
According to the Colby [25] method, the corresponding theoretical effect was calculated and compared with the real effect of the mixture. Formula (3) was used:
where E is the real measured efficacy of the combination, E0 is the expected efficacy of the combination, X1 is the real measured efficacy of the first compound, and X2 is the real measured efficacy of the second compound. If E > E0, the combinations were synergistic; if E < E0, the combinations were antagonistic.
The experimental data on E − E0 were subjected to one-way analysis of variance (ANOVA) and the means were compared using Duncan’s multiple range test. The data in percentages were normalized with an arcsine square-root transformation.
3. Results and Discussion
3.1. Lethal Effect of DMDS, CuSO4, and NH4HCO3 on M. incognita J2
The DMDS, CuSO4, and NH4HCO3 had lethal effects on the M. incognita J2 and these effects increased with the concentration. The LC50 on the J2 of M. incognita differed from each compound. After 24 h, the LC50 values of DMDS, CuSO4, and NH4HCO3 were 19.28, 187.42, and 213.49 mg/L, respectively (Table 1).

Table 1.
Effects of DMDS, CuSO4, and NH4HCO3 on M. incognita J2.
The nematode population densities were reduced after DMDS treatment in both indoor and field experiments. The LC50 values reported for DMDS against M. incognita were 29.865, 0.086, and 6.348 mg/L depending on the application method, namely, the small tube method, desiccator fumigation, and soil fumigation, respectively [26]. In addition, DMDS has a good control effect against RKN on various crops. For example, DMDS was used at 300 a.i. kg/ha in greenhouse experiments and obtained a control efficiency of 63–97% against the M. incognita, M. javanica, and Heterodera schachtii that occurred on tomatoes and sugar beets [27]. Low doses (112 kg/ha) of DMDS showed good effects on RKN during the grape growth period [28]. The DMDS acts on nematodes by affecting cytochrome oxidase in mitochondria [29]. Similarly, Dugravot et al. [30] found that DMDS reduces intracellular ATP concentration by inhibiting the mitochondrial respiratory chain complex IV (cytochrome oxidase) of Periplaneta americana. Subsequently, the activated neuronal KATP channels mediate membrane hyperpolarization and decrease neuronal activity to control soil pests. Therefore, DMDS can show good activity against nematodes.
CuSO4 is often used in agricultural production as a fungicide and micronutrient and, when used in moderate concentrations, it can replenish copper in the soil [31]. Moreover, trace elements such as Fe, Mn, and Cu significantly inhibit the survival of M. incognita J2 [16]. Similarly, CuSO4 has a strong inhibitory effect on the survival of M. incognita J2 under in vitro conditions, thus significantly reducing their movement behavior, shortening their body length, and lengthening their transparent tails [32]. CuSO4 has a strong toxic effect on Bursaphelenchus xylophilus and it is speculated that the killing mechanism may be through the combination of copper ions and proteins in the nematodes to form copper complexes. This process denatures and precipitates the proteins, causing enzyme inactivation, thereby hindering and inhibiting the metabolism and finally killing the nematode [33].
Ammonium nitrogen is a chemical compound that is often used in agricultural production and it is expected to become a nematicide [34,35]. The pot experiments of Oka and Pivonia [36] showed that among the 10 ammonium compounds tested, NH4OH, NH4H2PO4, and NH4HCO3 had significant nematicidal activity against M. javanica. The field experiments of Su et al. [37] showed that the total number of nematodes after treatment with NH4HCO3 combined with lime decreased by nearly 50% compared with NH4HCO3 alone and 66.2% compared with the control. The combination of lime bicarbonate and NH4HCO3 can exert a significant killing effect when the soil water content is low and in a wide temperature range. After application, the soil pH and the ammonium nitrogen, nitrate nitrogen, and total nitrogen contents can be significantly increased. The combined treatment of ammonium sulfate and alkaline-stable biosolids had a more significant effect than the single product. Although ammonium salts do not directly kill nematodes, they can form ammonia that are highly toxic to nematodes in alkaline soils [38]. Ammonia can be produced by organic matter with high nitrogen content and also by marine organisms with high chitin content, which can promote the dissolution of chitin on the surface of RKN and lead to the death of nematodes [39].
3.2. Lethal Effect of DMDS with CuSO4 or NH4HCO3 on M. incognita J2
Based on the concentration of the single product, CuSO4 and NH4HCO3 were mixed with DMDS (Table 2, Figure 1). The combinations of DMDS with CuSO4 and NH4HCO3 enhanced the lethal effect on M. incognita J2. The expected efficacy of nematodes was 0.58–17.60% when DMDS was combined with CuSO4. Actually, the corrected mortality of nematodes was more than 97% when combined with CuSO4. The expected efficacy of nematodes was 1.15–18.32% when combined with NH4HCO3. However, the corrected mortality of nematodes was more than 95% when combined with NH4HCO3. When E > E0, the combinations were all synergistic. In the combination of DMDS with CuSO4, the effects of DMDS (2.5,10 mg/L) + CuSO4 (46.58 mg/L) and DMDS (2.5 mg/L) + CuSO4 (186.33 mg/L) were significantly different from the other concentrations. In the combination of DMDS with NH4HCO3, the effects of DMDS (2.5,10 mg/L) + NH4HCO3 (80.25, 241 mg/L) were significantly different from the other concentrations.

Table 2.
Effects of DMDS combination with CuSO4 or NH4HCO3 on M. incognita J2 mortality.
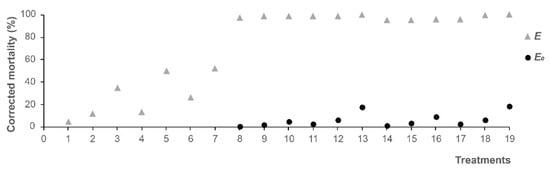
Figure 1.
Effects of DMDS combination with CuSO4 or NH4HCO3 on M. incognita J2 mortality.
Currently, most RKN are controlled by chemical agents, resulting in most nematicides facing problems such as reduced control effectiveness and pest resistance. In order to achieve the efficient and safe management of RKN, this test used copper sulfate and DMDS mixtures with CuSO4 or NH4HCO3 to determine their toxicity against M. incognita. The combination of DMDS (2.5 mg/L) and CuSO4 (46.58 mg/L) not only showed the best results with an E − E0 value of 97.09% and a corrected mortality of 97.67%, but also has the lowest dosage. CuSO4 mixed with other chemical nematicides also showed synergistic effects. CuSO4 combined with deltamethrin and abamectin on B. xylophilus showed additive or synergistic effects [33]. Copper is an indispensable trace element for plant growth and plays a pivotal role, although the demand is small. In addition to mixing CuSO4 as a trace fertilizer with pesticides, copper can also be used as an additive or nanomaterial and mixed into pesticides. Copper oxide nanopowders were mixed into cypermethrin to determine the toxic and synergistic effect on Spodoptera litura Fabricius and the results showed that the nanomaterials mixed with cypermethrin insecticide have synergistic effects. Nanotechnology held great promise for mitigating the harmful effects of pesticides on the environment and human health, since it can provide systems enabling the controlled release of active compounds, thus increasing the efficiency and safety of products, while reducing the quantities required in field applications [40]. Therefore, further experiments are needed regarding the form of CuSO4 mixed with DMDS for use in the field, alongside its significant synergistic effect indoors.
In the present study, the combination of DMDS (2.5 mg/L) and NH4HCO3 (80.25 mg/L) showed a synergistic effect on the control of M. incognita, with an E − E0 value of 94.00% and a corrected mortality of 95.15%, and the usage is minimal. Similarly, the application of the ammonium sulfate and chitin in combination with the neem extracts reduced the root galling of M. javanica significantly [41]. A nanopesticide was formed by emamectin benzoate and glycine methyl ester was used as an organic nitrogen source. The biological experiments also showed that nanopesticides can maintain high insecticidal activity [42]. Furthermore, the data combined from 2019 and 2020 suggested that fluopyram seed treatment + (NH4)2 SO4 + Vydate + Max-In (R) Sulfur was effective at increasing seed cotton yields in the Rotylenchulus reniformis microplot trials. In M. incognita field trials, imidacloprid and thiodicarb + 28-0-0-5 + Vydate + Max-In (R) Sulfur supported the largest lint yields [43]. The integrated application of soil fumigation combined with fertigation and biocontrol agents could improve the RKN disease control efficacy further, demonstrated by reductions in diseases of 82.7–85.1% [44]. Therefore, the compound use of fumigant DMDS and nitrogen compounds has certain reference significance for practical production. DMDS, CuSO4, and NH4HCO3 have nematocidal activities by themselves.
4. Conclusions
As chemical compounds, CuSO4 and NH4HCO3 are absorbed by crops and they can control RKN. In this experiment, the results showed that CuSO4 and NH4HCO3 had strong lethal activity on M. incognita J2 and the combination of DMDS and the two compounds had a synergistic effect. Although this experiment showed a good effect under indoor conditions, its practical application is limited by various factors such as different environments and soils. Therefore, further research is needed on the field control effect of these mixtures.
Author Contributions
Conceptualization, Q.W. (Qing Wang) and D.Y.; methodology, Z.S.; literature search, L.R.; software, W.F.; validation, Q.W. (Qiuxia Wang); formal analysis, Y.L.; investigation, Q.W. (Qing Wang); data curation, D.Y.; data interpretation, D.Z.; writing—original draft preparation, Q.W. (Qing Wang); writing—review and editing, D.Y.; visualization, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation project of China (32172462), Beijing Talents Foundation (2018000021223ZK47).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qinge, L.I.U. Research Progress in the Control of Plant Nematode. J. Anhui Agric. Sci. 2006, 34, 4644–4645, 4664. [Google Scholar]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Sasser, J.N. Plant-Parasitic Nematodes: The Farmer’s Hidden Enemy; Excel India Publishers: New Delhi, India, 1989. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Peshin, R.; Dhawan, A.K. Integrated Pest Management: Innovation-Development Process; Springer: Dordrecht, The Netherlands, 2009; p. 688. [Google Scholar]
- Ntalli, N.G.; Caboni, P. Botanical Nematicides: A Review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, Q.X.; Song, B.A. Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef]
- Hajihassani, A. Chemical Nematicides for Control of Plant-Parasitic Nematodes in Georgia Vegetable Crops; University of Georgia: Athens, GA, USA, 2018. [Google Scholar]
- Desaeger, J.; Wram, C.; Zasada, I. New reduced-risk agricultural nematicides—Rationale and review. J. Nematol. 2020, 52, e2020-91. [Google Scholar] [CrossRef]
- MBTOC. Methyl Bromide Technical Options Committee 2018 Assessment Report; United Nations Environment Programme: Nairobi, Kenya, 2019. [Google Scholar]
- Wang, Q.; Yan, D.; Wang, X.; Xiang, L.P.; Li, X.; Cao, A. Research advances in soil fumigants. J. Plant Prot. 2017, 44, 529–543. [Google Scholar]
- EPA. Aldicarb; Cancellation Order for Amendments to Terminate Usesfed. Regist; EPA: Washington, DC, USA, 2012. [Google Scholar]
- EPA. Carbofuran; Product Cancellation Orderfed. Regist; EPA: Washington, DC, USA, 2009. [Google Scholar]
- Hemmati, S.; Saeedizadeh, A. Root-knot nematode, Meloidogyne javanica, in response to soil fertilization. Braz. J. Biol. 2020, 80, 621–630. [Google Scholar] [CrossRef]
- Kesba, H.H.; Al-Shalaby, M.E.M. Survival and reproduction of Meloidogyne incognita on tomato as affected by humic acid. Nematology 2008, 10, 243–249. [Google Scholar]
- Wang, Z.; Shi, Y.; Yuan, H.; Yang, W.; Li, H. The effects of nutrient elements on the hatching of cyst and vitality of secondary stage juvenile of cereal cyst nematode. Acta Phytopathol. Sin. 2013, 43, 333–336. [Google Scholar]
- Zhang, K.; Jia, Z. Method of Dyeing Perineal-striae of Root-knot Nematiodes. North. Hortic. 2008, 3, 207–208. [Google Scholar]
- Feng, Z. Plant Nematology; China Agriculture Press: Beijing, China, 2001; pp. 135–154. [Google Scholar]
- Feng, G.; Dong, L.; Chen, Y.; Shang, H.; Liu, Y.; Li, J.; Yang, J.; Cui, X.; Yang, P. PCR detection of nematode isolated from Panax notoginseng. Southwest China J. Agric. Sci. 2008, 21, 100–102. [Google Scholar]
- Zhang, F.; Yang, M.; Sun, J.; Hong, B.; Zhang, S. Specific Molecular Assay for the Detection of Greenhouse Vegetables Meloidogyne spp. in Shaanxi. Chin. Agric. Sci. Bull. 2014, 30, 136–140. [Google Scholar]
- Meng, Q.; Long, H.; Xu, J. PCR assays for rapid and sensitive identification of three major root-knot nematodes, Meloidogyne incognita, M. javanica and M. arenaria. Acta Phytopathol. Sin. 2004, 34, 204–210. [Google Scholar]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Mao, L.G.; Wang, Q.X.; Yan, D.D.; Xie, H.W.; Li, Y.; Guo, M.X.; Cao, A.C. Evaluation of the combination of 1,3-dichloropropene and dazomet as an efficient alternative to methyl bromide for cucumber production in China. Pest Manag. Sci. 2012, 68, 602–609. [Google Scholar] [CrossRef]
- Colby, S.R. Calculating synergistic and antagonistic responses of herbicide combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Yan, D.; Cao, A.; Wang, Q.; Li, Y.; Canbin, O.; Guo, M.; Guo, X. Dimethyl disulfide (DMDS) as an effective soil fumigant against nematodes in China. PLoS ONE 2019, 14, e0224456. [Google Scholar] [CrossRef]
- Charles, P. DMDS: A New Alternative for Soil Disinfestation; MBAO: San Diego, CA, USA, 2003; pp. 1–4. [Google Scholar]
- Cabrera, J.A.; Wang, D.; Gerik, J.S.; Gan, J. Spot drip application of dimethyl disulfide as a post-plant treatment for the control of plant parasitic nematodes and soilborne pathogens in grape production. Pest Manag. Sci. 2014, 70, 1151–1157. [Google Scholar] [CrossRef]
- Charles, P. Biogenic Emission, Biological Origin, and Mode of Action of DMDS, a Natural Ubiquitous Fumigant; MBAO: San Diego, CA, USA, 2003; p. 138/1. [Google Scholar]
- Dugravot, E.; Grolleau, F.; Macherel, D.; Rochetaing, A.; Hue, B.; Stankiewicz, M.; Huignard, J.; Lapied, B. Dimethyl disulfide exerts insecticidal neurotoxicity through mitochondrial dysfunction and activation of insect K-ATP channels. J. Neurophysiol. 2003, 90, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ouzounidou, G. Copper-induced changes in growth, metal content and photosynthetic function of Alyssum montanum L. plants. Environ. Exp. Bot. 1994, 34, 165–172. [Google Scholar] [CrossRef]
- Bai, C.; Duan, Y.; Chen, L.; Liu, Y.; Yu, X. The modes of action of inorganic compounds to Meloidogyne incognita. Plant Prot. 2011, 37, 74–78. [Google Scholar]
- Long, D.; Wu, Y.; Li, W.; Wang, Q.; Qin, Q. Toxicities of eight chemicals on Bursaphelenchus xylophilus. J. Zhejiang For. Sci. Technol. 2006, 26, 39–42. [Google Scholar]
- Zuo, Q.; Wu, F.; Zhang, S.; Xing, L.; Li, J.; Xiao, Q.; Liu, J. Effect of different nitrogen on root-knot nematodes and its soil microorganisms. Plant Prot. 2022, 48, 329–336 + 376. [Google Scholar]
- Treskova, V.S. Microelements and nematicides in the control of root-knot nematodes. Zashchita Rastenii Vred. Bolezn. 1959, 4, 26–27. [Google Scholar]
- Oka, Y.; Pivonia, S. Use of ammonia-releasing compounds for control of the root-knot nematode Meloidogyne javanica. Nematology 2002, 4, 65–71. [Google Scholar] [CrossRef]
- Su, L.; Ruan, Y.; Yang, X.; Wang, K.; Li, R.; Shen, Q. Suppression on plant-parasitic nematodes using a soil fumigation strategy based on ammonium bicarbonate and its effects on the nematode community. Sci. Rep. 2015, 5, 17597. [Google Scholar] [CrossRef]
- Oka, Y.; Tkachi, N.; Shuker, S.; Rosenberg, R.; Suriano, S.; Roded, L.; Fine, P. Field studies on the enhancement of nematicidal activity of ammonia-releasing fertilisers by alkaline amendments. Nematology 2006, 8, 881–893. [Google Scholar] [CrossRef]
- Cao, A.; Guo, M.; Wang, Q.; Li, Y.; Yan, D. Progress of Soil Disinfection Technology in the World. China Veg. 2010, 21, 17–22. [Google Scholar]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; do Espirito Santo Pereira, A.; de Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Tkachi, N.; Shuker, S.; Yerumiyahu, U. Enhanced Nematicidal Activity of Organic and Inorganic Ammonia-Releasing Amendments by Azadirachta indica Extracts. J. Nematol. 2007, 39, 9–16. [Google Scholar] [PubMed]
- Zhao, M.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. A high-efficient nano pesticide-fertilizer combination fabricated by amino acid-modified cellulose based carriers. Pest Manag. Sci. 2022, 78, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.L.; Schrimsher, D.W.; Lawrence, K.S. Additional fertilizer and nematicide combinations on upland cotton to manage Rotylenchulus reniformis and Meloidogyne incognita in Alabama. J. Nematol. 2022, 54, 20220003. [Google Scholar]
- Yang, L.; Zhou, Q.; Wang, D.; Guo, R.; Fu, H.; Zhang, W.; Ge, B.; Li, S. Management of tomato diseases by a combined technique of soil fumigation with fertigation and biological agents in greenhouse. Chin. J. Biol. Control. 2022, 38, 890–895. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).