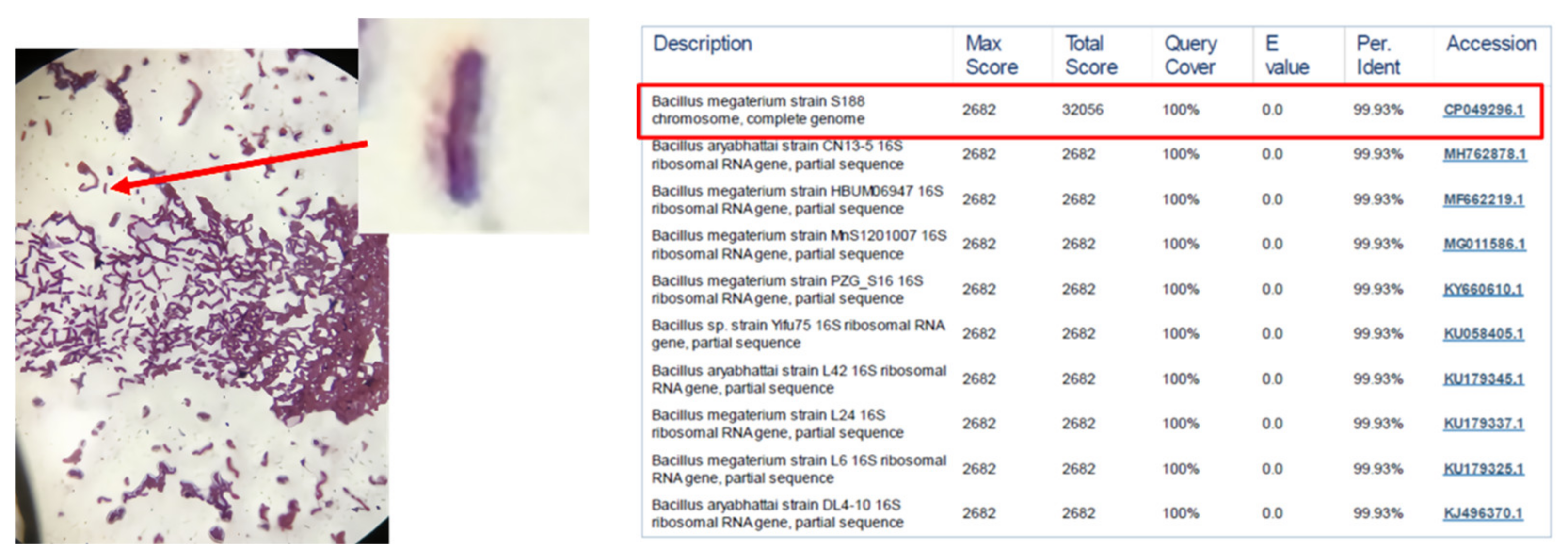
3.1. Beneficial Microorganisms
In order to verify that the salt-tolerant beneficial microorganisms isolated from Penghu have sufficient conditions for subsequent experiments, we conducted a series of physiological and biochemical assays on the beneficial microorganisms in the early stage of the experiment. The amylolytic activity, cellulolytic activity, proteolytic activity, lipolytic activity and phosphorus-dissolving activity of beneficial microorganisms were measured (
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8). After testing
Bacillus amyloliquefaciens,
Bacillus megaterium A and
Bacillus megaterium B the amylolytic activity, proteolytic activity, cellulolytic activity test, Lipolytic activity test and phosphorus-dissolving activity, the experimental results show that
Bacillus amyloliquefaciens has amylolytic activity, proteolytic activity, cellulolytic activity test and phosphorus-dissolving activity;
Bacillus megaterium A It has proteolytic activity, cellulolytic activity test and phosphorus-solubilizing activity;
Bacillus megaterium B has amylolytic activity, proteolytic activity, cellulolytic activity test and phosphorus-solubilizing activity (
Table 3). Based on the experimental results, the follow-up research will use two microorganisms,
Bacillus amyloliquefaciens and
Bacillus megaterium B, as the target for the development of beneficial microorganisms.
3.2. Biochar Preparation
To produce biochar suitable for this research purpose, we used agricultural residues such as
Syzygium samarangense,
Leucaena leucocephala, and
Ziziphus jujuba as crude materials. After initial crushing and carbonization at 500 °C, biochar and crushed oyster shells are mixed 1:1, and then re-modified and activated at 900 °C. After crushing and screening, 1–2 mm biochar was screened for subsequent follow-up. It can be known from the fertility analysis that the biochar fired in the experiment basically has basal fertilizer parts (
Table 4 and
Table 5), but the contained amount still needs to be fertilized as an aid if the effect of increasing crop yield is to be achieved [
28].
In addition, the suitable conductivity for the growth of general crops is about 0.7~2.0 mS/cm. The conductivity of the biochar fired in the experiment is high, and the pH value of the biochar is highly alkaline due to the high temperature firing. Therefore, the biochar must be upgraded before application and bacteria loading (
Table 5). Cation exchange capacity (CEC) refers to the cations adsorbed by soil particles and the internal and external surfaces of organic matter, so the exchangeable cations in the soil (especially in the soil solution) can be freely exchanged. The soil exchangeable one-hundredth mole of cations is expressed in units (cmol (+)/kg soil). Because of the large surface area of clay and humus in the soil, they carry more charges, which dominate the cation exchange capacity of the soil. Different organic matter and clay minerals have different CEC values. The larger the value, the stronger the ability of soil to adsorb cations.
The factors that generally affect the soil CEC value are: (1) the content of organic matter, (2) the type of clay minerals, (3) the content of clay particles, and (4) the degree of weathering of soil minerals. Taking kaolinite, illite (fine mica), montanite (or bentonite), vermiculite and organic matter as examples, the CECs are 3–30, 10–40, 80–120, 100–150 and >500~1000 cmol (+)/kg soil; therefore, the cation exchange capacity of the biochar produced in this experiment is weak (10.4, 6.8, 13.3 cmol (+)/kg soil), and may not be effective as a soil conditioner. However, biochar produced from agricultural wastes focuses on whether the pore size is suitable for the ingrowth of beneficial microorganisms and serves as a breeding base for beneficial microorganisms in the soil. Therefore, the “heterogeneous material binding effect” between beneficial microorganisms and biochar is the focus of this experiment. At the same time, when helping crops to survive the stress of salinity, the biochar produced in this experiment is not primarily to improve the soil effect, so it can be indirectly confirmed that the main reason for improving the growth of crops is from beneficial microorganisms.
3.3. Composite Materials of Biochar Micro-Coated Soil Beneficial Microorganisms
The pH value of the three biochars was adjusted to 7.3 ± 0.2, and the specific surface areas (BET) were 315.09, 213.53 and 318.53 m
2/g, respectively. The specific surface area of biochar produced in general is about 20–50 m
2/g, and the specific surface area of the biochar fired in this experiment is all greater than 100 m
2/g. The possible reason is that the firing temperature is high (900 °C), and during the firing process, oyster shells (calcium carbonate mixture) are mixed, and carbon dioxide is released through high temperature sintering, which collides with the surface of biochar in a microscopic environment, forming numerous small pores (<2 nm) and increasing the specific surface area. In addition, the fixed carbon contents of the three types of biochars were 92.16, 93.69 and 87.13 Wt%, respectively, with a higher proportion of carbon, which was presumably caused by the volatilization of most of the other elements during the carbonization process. The pore size of biochar was analyzed by Mercury intrusion porosimetry (MIP). The ratios were 2.58% and 97.42%, respectively, and the ratios of mesopore and macro-pore in
Ziziphus jujuba were 2.19% and 97.81%, respectively. The three biochars have high pore size ratios, which are very suitable as a habitat for the growth of beneficial microorganisms and as a carrier for micro-coated beneficial microorganisms (
Table 6).
Observed by Scanning Electron Microscope (SEM), the three biochars are all porous structures, and their pore sizes are about 13.31–23.44 × 16.88–23.06 μm, 15.94–25.31 × 20.91–26.63 μm, 8.53–16.68 × 11.44–17.16 μm, respectively. Taking
Bacillus amyloliquefaciens as an example, the cell length is about 0.7–0.9 × 1.8–3.0 μm, and the cells are often linear. The size of other microorganisms of the genus
Bacillus is similar. The pore size of biochar is suitable for the growth of beneficial microorganisms (
Figure 9,
Figure 10 and
Figure 11).
According to the polycyclic aromatic hydrocarbons (PAHs) report, most of the polycyclic aromatic hydrocarbons in
Syzygium samarangense biochar were not detected, except naphthalene (CAS No.: 91-20-3), which was detected at 0.289 ppm (
Figure 7); the allowable amount was determined by comparing the two major international biochars—European Biochar Certificate (EBC) [
29,
30] and International Biochar Initiative (IBI) [
31] Basic: <12 mg/kg, Premium grade: <4 mg/kg and 6–300 mg/kg; 3 mg/kg B(a)p-TEQ, respectively. In addition, the dioxin test result (PCDD/Fs) of
Syzygium samarangense biochar was 0.208 pg/kg WHO2005-TEQ (Annex 2), while EBC and IBI set the tolerable levels to be <20 ng/kg I-TEQ and <17 ng/kg WHO-TEQ, shows that the dioxin content of this
Syzygium samarangense biochar is far lower than the allowable amount set by the international mass organization. Therefore, based on the above biochar test results, the use of agricultural waste the biochar produced is very safe.
In previous studies, a multifunctional cyclic embedded technology was developed through the carbonization technology of recycled biomass materials and the microbial culture and fermentation technology, and a composite material of biochar micro-coated soil beneficial microorganisms was successfully prepared.
Figure 12 shows the micro-coating results of
Syzygium samarangense embedded with
Bacillus amyloliquefaciens; it can be clearly seen that there are short rod-shaped
Bacillus amyloliquefaciens in the pores. The presence of
Bacillus amyloliquefaciens was also clearly observed in the SEM images of the
Leucaena leucocephala and the
Ziziphus jujuba biochar (
Figure 13 and
Figure 14). These results successfully confirmed that
Bacillus amyloliquefaciens can indeed enter the pores of biochar for growth and reproduction. The micro-coated beneficial microorganisms with biochar using the multi-functional cycle embedded technology can have a bacterial count of more than 10
8 CFU/g, and some of the culture medium up to 10
10 CFU/mL.
To test whether beneficial microorganisms can inhibit pathogenic microorganisms and prevent crop diseases, the fermented beneficial microorganisms and tomato wilt fungus (
Fusarium oxysporum f. sp. Lycopersici) were placed in the pre-cultured beneficial microorganism medium for confrontation in the experiment. The results showed that the results of the five-day confrontation culture were obtained (
Figure 15).
Bacillus amyloliquefaciens has inhibitory effect on tomato wilt pathogen. It can be seen from the figure that the inhibition circle is complete, and it should be used as a biological agent for future development. While
Bacillus megaterium A and
Bacillus megaterium B can make the edge of tomato wilt mycelium sparse, the inhibitory effect is not obvious, and other functions should be tested.
3.4. Pot Experiment of Tomato Wilt Fungus
With the demand for the reduction of fertilizer and pesticides, there is an urgent need to reduce the use of environmentally unfriendly pesticides and agrochemicals (Wei et al., 2019). Pathogen invasion can drive community-wide dynamics in tomato endophytic bacterial composition.
Bacillus sp., which is enriched in the seedling phase, may play a key role for the suppression of
F. oxysporum based on Lefse analysis [
32,
33]. We tested biochar blended with
Bacillus amyloliquefaciens to see if the beneficial microbe-embedded biochar had a slowing effect on tomato wilt. The test seedlings were tomato seeds (
Lycopersicon esculentum Mill.). The habit of this variety is a one or two-year-old herb. The optimum temperature for germination is 20~30 °C, and the optimum temperature for growth is 15~30 °C. It is a cow tomato with full red and large fruits, light green fruit shoulder and a high yield. The fruit weighs about 200 g, and the fruit is hard and not easy to crack. In the experiment, the disease severity of tomato was recorded on days 0, 7, 14, 21 and 28, and the wilting disease incidence index was divided into five grades [
26]: grade 0 was healthy plants with no symptoms (0%); grade 1 is plant dwarfing and yellowing of cotyledons (less than 25% of leaves have symptoms); grade 2 is yellowing of lower leaves (26–50% of leaves have symptoms); grade 3 is death of lower leaves and some upper leaves are yellow with degradation or wilting (51–75% of leaves show signs of disease); grade 4 is the death of lower leaves and withering of upper leaves (76–100% of leaves show signs of disease); grade 5 is the death of the whole plant. The calculation formula is Disease severity (%) = (∑ni × i)/5N × 100. i: incidence grade; ni: the number of plants with disease i level; N: the total number of plants under investigation.
Observed for 28 days after inoculation and counting the morbidity of tomato wilt disease (
Figure 16), it was found that the morbidity of the samples treated with the composite material (
Bacillus amyloliquefaciens + biochar) was the lowest, and only lower leaf yellowing occurred. In the treatments of adding water and biochar, the lower leaves died, the upper leaves withered, and the plants were lodging, but no complete plant death was found after 28 days. In addition, the disease degree of the biochar group was slightly higher than that of the water-added control group in the early stage of disease. It is speculated that because the biochar is not coated with microorganisms, the nutrients absorbed by itself can be used and grown by pathogenic bacteria, resulting in a high disease degree in the initial stage of the biochar treatment. The verification test results and analysis of the crop control effect of biochar-based biological fertilizers or preparations showed that the composite material treatment can effectively reduce the disease degree of tomato by 69.43%, compared with water treatment (
Figure 17).
In addition, the experiment also used the composite material made of
Syzygium samarangense biochar and
Bacillus amyloliquefaciens to test the anti-salt damage effect. In the experiment, CK (water) was used as the control group for the overall experiment, 100 mM NaCl was used as the source of salt damage, and leaf lettuce was used for comparison with CK,
Syzygium samarangense biochar, beneficial microorganisms and composite materials (
Figure 18) (
Table 7). Among them, 85 mM sodium chloride was added as the medium base when
Bacillus amyloliquefaciens were cultured in NA (Nutrient Agar) medium. It can be seen that
Bacillus amyloliquefaciens in this experiment can indeed grow under the stress of salt damage. The SPAD value (Soil and Plant Analyzer Development) is used to measure the relative greenness content or greenness degree of leaves at night. It shows that beneficial microorganisms can make the chlorophyll of plants relatively unaffected. The greenness of the plants under the untreated salt damage stress decreased by 65%, and the greenness of the plants with single addition of
Syzygium samarangense biochar decreased by 84%. It is speculated that the reason for this is that the biochar has not been modified, and it is a strong alkali and high EC. The
Syzygium samarangense biochar of value is caused by the direct growth of crops in multiple harsh environments during the seedling stage.
The fresh weight of the crops was weighed after about a month of growth. The fresh weights of CK (water), CK under salt damage,
Syzygium samarangense biochar, beneficial microorganisms and composite materials were 7.86, 0.64, 0.12, 2.02 and 0.77 g, respectively. It can be seen that under the condition of salt damage, the plants shrank; the dry weights were 0.83, 0.05, 0.01, 0.2 and 0.1 g, and the conversion ratios were 10.56, 7.81, 8.33, 9.90 and 12.99%, respectively. Although the plants shrank under the stress of salt damage, the dry/fresh weight ratio of the composite material was found to be higher than that of the control group with only water added through the test results. It can be seen that the composite material is indeed effective for crops to survive the stress of salt damage. There is a preliminary effect, followed by beneficial microorganism treatment, and the performance is excellent in all values. In terms of soil, the EC values of CK (water), CK under salt stress,
Syzygium samarangense biochar, beneficial microorganisms and composites were 1.5, 9.5, 9.6, 9.9 and 9.8 dS/m, respectively, and 100 mM NaCl was 9.4 dS/m, the soil pH value was roughly neutral, and this result showed that a large amount of salt accumulation was indeed the main cause of crop atrophy (
Table 7).
The beneficial microorganisms isolated in this experiment performed well in various values. In the experiments of salt damage and tomato wilt disease, it was shown that the beneficial microorganisms and composite materials can effectively improve the degree of tomato wilt disease and the rate of leaf atrophy. The situation may be used as a good material for the future development of agricultural and industrial projects.