Effects of the Application of Nutrients on Soil Bacterial Community Composition and Diversity in a Larix olgensis Plantation, Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sample Collection
2.3. Soil Chemical Properties and Biological Analysis
2.3.1. Chemical Properties Analysis
2.3.2. DNA Extraction and PCR Amplification
2.4. Bioinformatics
2.5. Statistical Analyses
3. Results
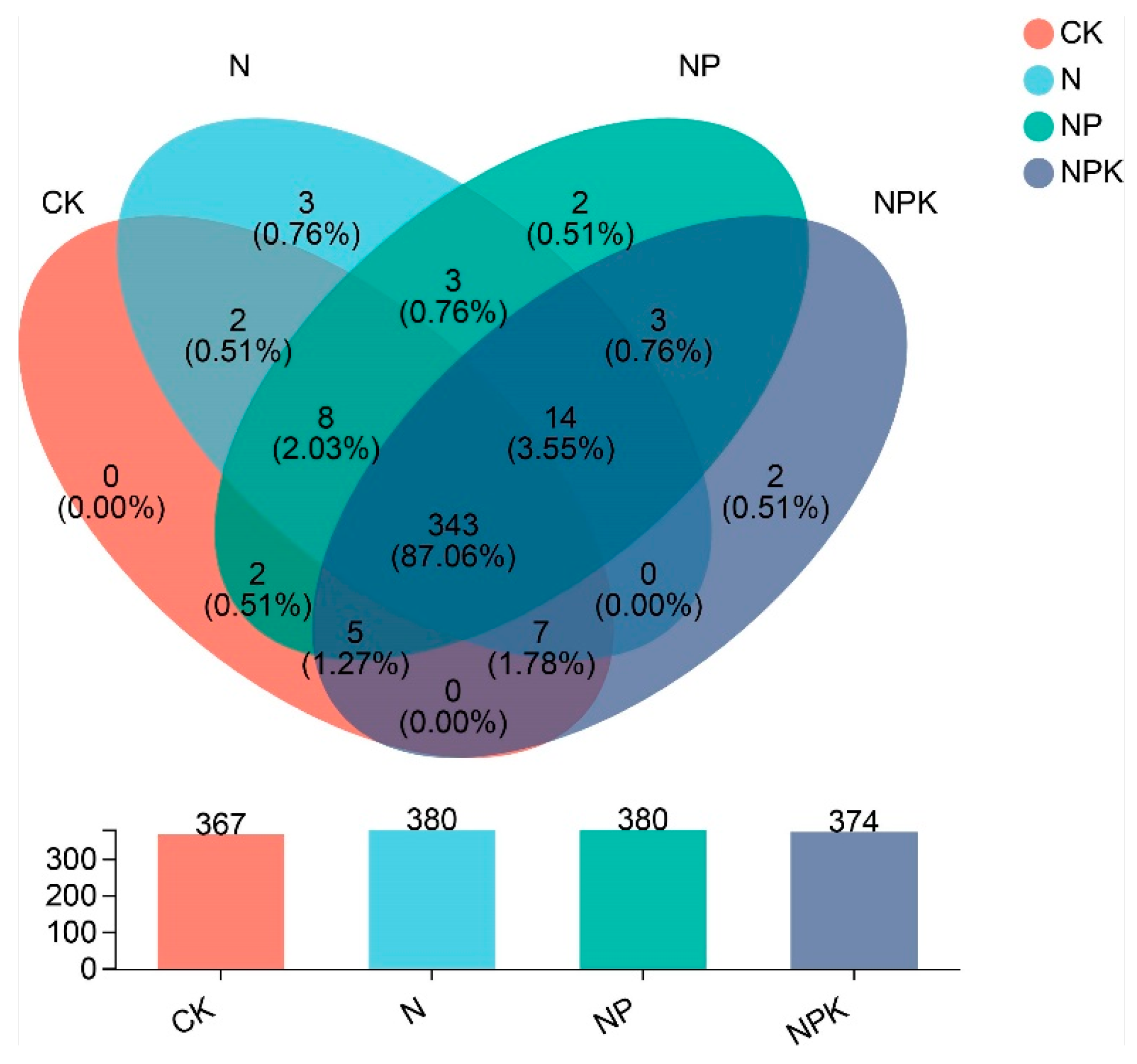
3.1. Characteristics of Soil Bacterial Diversity under Different Nutrient Application Measures
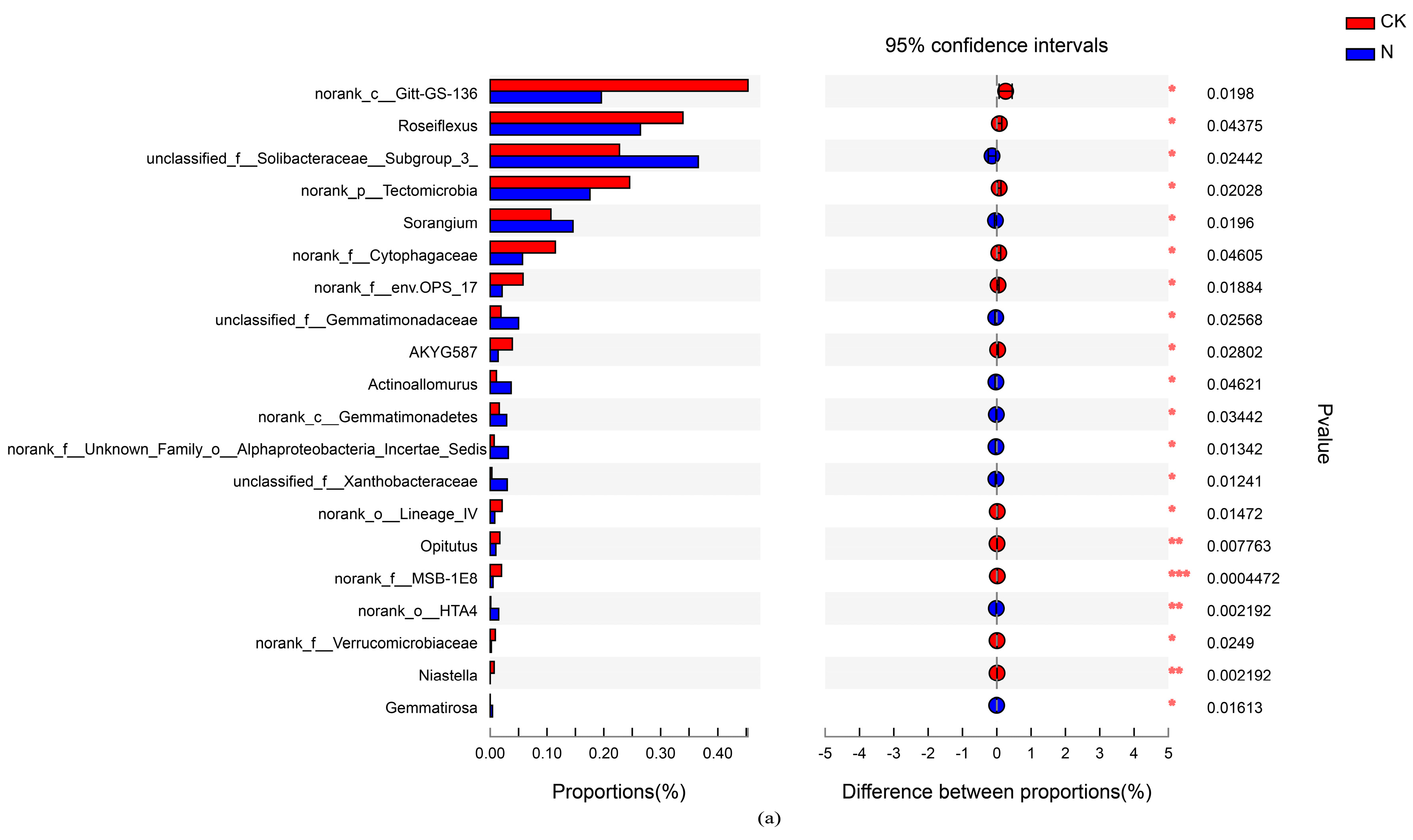
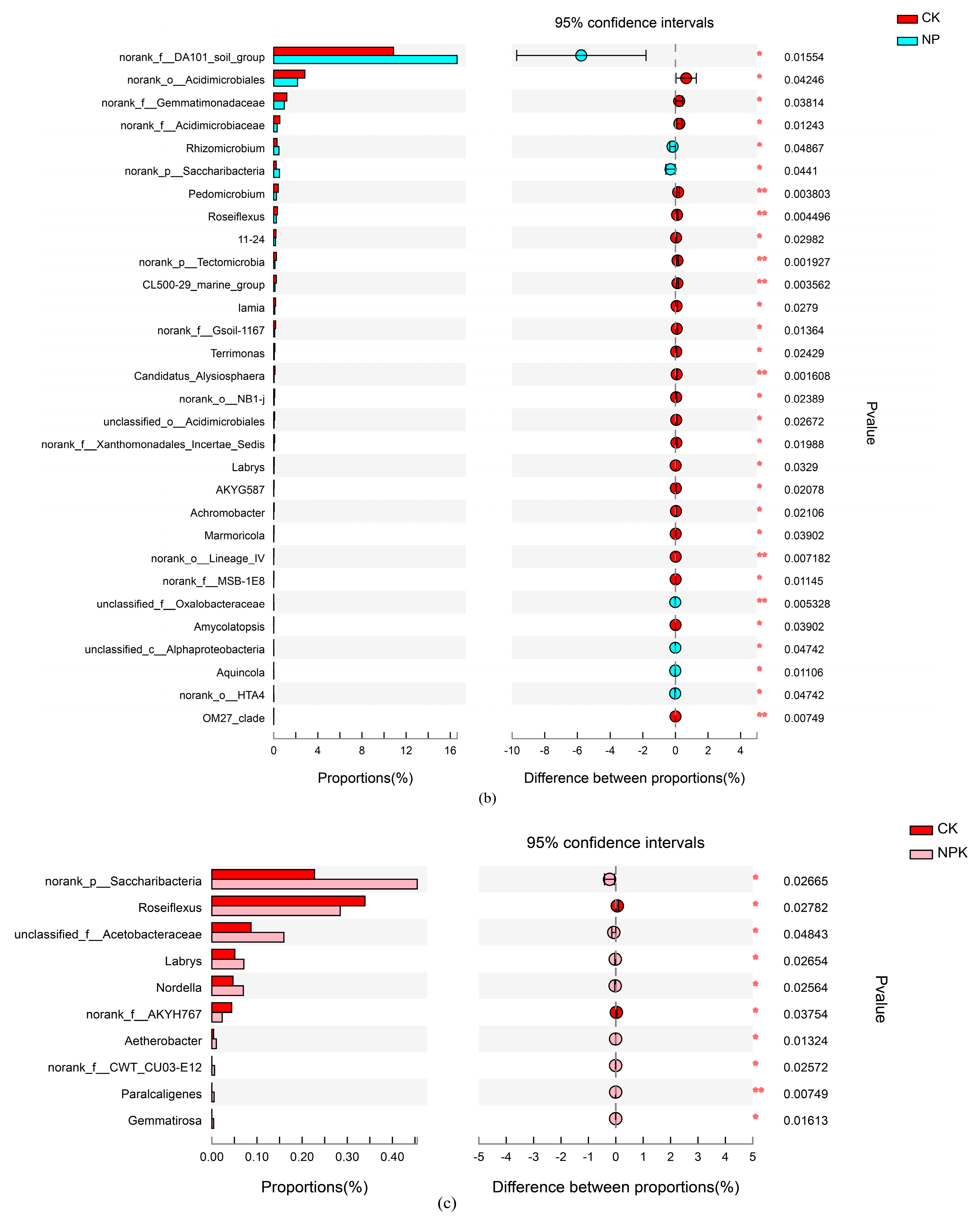
3.2. Effects of the Application of Nutrients on Soil Bacterial Community Composition
3.3. The Relationship between the Soil Bacterial Community Structure and Soil Chemical Properties
4. Discussion
4.1. Response of Soil Chemical Properties to the Different Nutrient Application Regimes
4.2. Soil Bacterial Diversity under Nutrient Application Regimes
4.3. Changes in the Main Soil Bacterial Community under Different Nutrient Application Measures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lui, Z.F.; Fu, B.; Liu, G.H. Key Laboratory of System Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences. Acta Ecol. Sin. 2006, 26, 901–913. [Google Scholar]
- Dilly, O.; Munch, J.C. Ratios between estimates of microbial biomass content and microbial activity in soils. Biol. Fertil. Soils 1998, 27, 374–379. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, P.; Xu, Z. Soil microbial functional diversity under intensively managed bamboo plantations in southern China. J. Soils Sediments 2008, 8, 177–183. [Google Scholar] [CrossRef]
- Ding, X.; Jing, R.; Huang, Y.; Chen, B.; Ma, F. Bacterial structure and diversity of rhizosphere and bulk soil of Robinia pseudoacacia forests in Yellow River Delta. Acta Pedol. Sin. 2017, 54, 1293–1302. [Google Scholar]
- Sheng, W.T.; Fan, S.H. Study on the Mechanism of Maintaining Long-term Productivity of Plantation: Background, Present Condition and Trends. For. Res. 2004, 17, 105–115. [Google Scholar]
- Sun, C.Z. Study on the problems of forest plantation productivity of China Ⅱ. —To probelems into the social and other non-nature factors debasing the plantation productivity. Sci. Silvae Sin. 2001, 37, 26–34. [Google Scholar]
- Heilongjiang Forestry Society Communication; Heilongjiang Forestry Society: Harbin, China, 2011; Volume 5.
- Bergh, J.; Nilsson, U.; Allen, H.; Johansson, U.; Fahlvik, N. Long-term responses of Scots pine and Norway spruce stands in Sweden to repeated fertilization and thinning. For. Ecol. Manag. 2014, 320, 118–128. [Google Scholar] [CrossRef]
- Fernandez, I.; Álvarez-González, J.G.; Carrasco, B.; Ruíz-González, A.D.; Cabaneiro, A. Post-thinning soil organic matter evolution and soil CO2 effluxes in temperate radiata pine plantations: Impacts of moderate thinning regimes on the forest C cycle. Can. J. For. Res. 2012, 42, 1953–1964. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Chen, B.D.; Fu, W. Research frontiers in soil ecology. Sci. Technol. Rev. 2022, 40, 25–31. [Google Scholar]
- Liu, Y.R.; Zhang, J.W.; Bao, Y.Y.; Lin, X.G.; Feng, Y.Z. Convergent Responses of Paddy Soil Microbial Community to Fertilization at Regional Scale. Soils 2022, 54, 968–977. [Google Scholar]
- Guan, Z.; Lin, D.; Dong, C.; Guo, Y. Soil microbial communities response to different fertilization regimes in young Catalpa bungei plantation. Front. Microbiol. 2022, 13, 948875. [Google Scholar] [CrossRef] [PubMed]
- LI, C.; Xu, Y.; Wu, Z. Short-term Effects of Different Eucalyptus Plantation Fertilization Treatments on Soil Bacterial Community Structure and Diversity. Eucalypt Sci. Technol. 2020, 37, 10–17. [Google Scholar]
- Guo, P.; Huang, X.; Wu, W.; Zheng, L.; Fang, X.; Yi, Z.G. Effects of different nitrogen application methods and levels on soil bacterial communities of Pinus massoniana and Schima superba seedling roots. Acta Ecol. Sin. 2021, 41, 149–161. [Google Scholar]
- Wei, J.; Zhang, J.; Wang, W.; Wang, Z.; Gu, J. Effect of Nitrogen Fertilization on Soil Microbial Biomass and Community Structure of Picea koraiensis Plantation in Maoershan Mountains of Northeastern China; Northeast Forestry University: Harbin, China, 2016; Volume 44, pp. 52–56. [Google Scholar]
- Serita, D.; Melissa, K.; Jeri, L.P.; Rodney, T.S. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar]
- Sun, C.; Shen, G.; Li, J.; Jia, L. Study on the Present Condition and the Potentialities of the Productivity of Main Tree Species Plantation of China. For. Res. 2001, 6, 657–667. [Google Scholar]
- Smolander, A.; Kitunen, V.; Tamminen, P.; Kukkola, M. Removal of logging residue in Norway spruce thinning stands: Long-term changes in organic layer properties. Soil Biol. Biochem. 2010, 42, 1222–1228. [Google Scholar] [CrossRef]
- Eilers, K.; Debenport, S.; Anderson, S. Fierer, N. Digging deeper to find unique microbial communities: The strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol. Biochem. 2012, 50, 58–65. [Google Scholar] [CrossRef]
- Jia, S.; Zhao, Y.; Li, C.; Wang, Z. Effects of nitrogen fertilization on seasonal dynamics of soil microbial biomasscar bonandni-trogenin Larixgme liniiand Fraxinusm and shurica plantations. Chin. J. Appl. Ecol. 2009, 20, 2063–2071. [Google Scholar]
- Nelson, D.; Sommers, L. Carbon and organic matter. In Methods of Soil Analysis-Part 2: Chemical and Microbiological Properties; Page, A.L., Mille, R.H., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 561–579. [Google Scholar]
- Ravel, J.; Gajer, P.; Abdo, Z. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.; Tiedje, J.; Cole, J. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J. Vegan: An Introduction to Ordination; The Natural Resources Institute Finland, Itäinen Pitkäkatu: Turku, Finland, 2015. [Google Scholar]
- Hartmann, M.; Brunner, I.; Hagedorn, F.; Bardgett, R.D.; Stierli, B.; Herzog, C. A decade of irrigation transforms the soil microbiome of semi-arid pine forest. Mol. Ecol. 2017, 26, 1190–1206. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Walthert, L.; Perez, M.C.; Stierli, B.; Koechli, R.; Dharmarajah, A.; Brunner, I. Deep soil layers of drought-exposed forests harbor poorly known bacterial and fungal communities. Front. Microbiol. 2021, 12, 674160. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Brunner, I.; Widmer, F.; Zeyer, J.; Frey, B. Vertical distribution of the soil microbiota along a successional gradient in a glacier forefield. Mol. Ecol. 2015, 24, 1091–1108. [Google Scholar] [CrossRef]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. Fems Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ma, M.; Jiang, X.; Yang, X.; Li, J. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Sanon, A.; Andrianjaka, Z.N.; Prin, Y. Rhizosphere microbiota interfers with plant-plant interactions. Plant Soil 2009, 321, 259–278. [Google Scholar] [CrossRef]
- Cusack, D.F.; Karpman, J.; Ashdown, D. A Global change effects on humid tropical forests: Evidence for biogeochemical and biodiversity shifts at an ecosystem scale. Rev. Geophys. 2016, 54, 523–610. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.C.; Yan, S.; Li, C. Effect of different potassium fertilizer application mass on olive root development and rhizospheric soil micro environment. Non-Wood For. Res. 2020, 38, 153–163. [Google Scholar]
- Krasilnikov, N. On the role of soil bacteria in plant nutrition. J. Gen. Appl. Microbiol. 2006, 7, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Sibyl, M.; Hilszczańska, D. Biodiversity and the role of soil bacteria in a forest environment. Sylvan 2017, 161, 155–162. [Google Scholar]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar]
- Jiang, X.; Hou, X.; Zhou, X.; Xin, X.; Wright, A.; Jia, Z. pH regulates key players of nitrification in paddy soils. Soil Biol. Biochem. 2015, 81, 9–16. [Google Scholar] [CrossRef]
- Hoorman, J.J. The Role of Soil Bacteria; The Ohio State University: Columbus, OH, USA, 2012; Volume 614, pp. 292–1868. [Google Scholar]
- Liu, C.; Xue, S. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 90, 40–49. [Google Scholar]
- Zeng, J.; Liu, X.; Song, L. Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Sun, S.; Xing, F.; Zhao, H.; Dong, Y. Response of bacterial community to simulated nitrogen deposition in soils and a unique relationship between plant species and soil bacteria in the songnen grassland in northeastern china. J. Soil Sci. Plant Nutr. 2014, 14, 565–580. [Google Scholar]
- Ling, N.; Chen, D.; Guo, H.; Wei, J.; Bai, Y.; Shen, Q. Differential responses of soil bacterial communities to long-term N and P inputs in a semi−arid steppe. Geoderma 2017, 292, 25–33. [Google Scholar]
- Liu, J.; Dang, P.; Gao, Y.; Zhu, H.; Zhu, H.; Zhao, F.; Zhao, Z. Effects of tree species and soil properties on the composition and diversity of the soil bacterial community following afforestation. For. Ecol. Manag. 2018, 427, 342–349. [Google Scholar] [CrossRef]
- Chodak, M.; Klimek, B.; Azarbad, H.; Ja´zwa, M. Functional diversity of soil microbial communities under Scots pine, Norway spruce, silver birch and mixed boreal forests. Pedobiologia 2015, 58, 81–88. [Google Scholar] [CrossRef]
- Peng, T.H.; Wen, S.Z. Effects of Phosphorus Assition on Soil Nutrient Elements and Microorganisms in Young Phoebe bournei Plantations by Peng Tieshuang; Central South University of Forestry and Technology: Changshai, China, 2022; p. 000410. [Google Scholar]
- Wang, N.; Zhang, T.; Zhang, F.; Wang, E.; He, J.; Ding, H.; Zhang, B.; Liu, J.; Ran, X.; Zhang, J. Diversity and structure of soil bacterial communities in the Fildes Region (maritime Antarctica) as revealed by 454 pyrosequencing. Front. Microbiol. 2015, 6, 11–88. [Google Scholar] [CrossRef] [Green Version]
- Insam, H.; Parkinson, D.; Domsch, K.H. Influence of macroclimate on soil microbial biomass. Soil Biol. Biochem. 1989, 21, 211–221. [Google Scholar] [CrossRef]


| Treat. | (g/kg) Total N | (g/kg) Total P | (g/kg) Total K | (g/kg) Organic C | (mg/kg) Available P | (mg/kg) Available K | pH |
|---|---|---|---|---|---|---|---|
| CK | 2.48 ± 0.28 a | 0.95 ± 0.21 a | 5.95 ± 1.85 a | 60.63 ± 22.53 a | 14.93 ± 6.72 a | 109.77 ± 33.09 b | 4.81 ± 0.25 a |
| N | 2.98 ± 1.01 a | 0.93 ± 0.44 a | 3.04 ± 1.48 b | 58.27 ± 8.90 a | 12.47 ± 4.55 a | 149.94 ± 29.04 b | 4.54 ± 0.30 ab |
| NP | 2.33 ± 0.76 a | 0.97 ± 0.23 a | 3.65 ± 1.00 ab | 62.44 ± 15.66 a | 18.74 ± 8.82 a | 199.15 ± 27.26 ab | 4.43 ± 0.04 b |
| NPK | 3.62 ± 0.89 a | 1.09 ± 0.37 a | 4.61 ± 1.13 ab | 59.64 ± 23.58 a | 15.49 ± 4.12 a | 286.15 ± 68.08 a | 4.55 ± 0.02 ab |
| Treat. | Chao1 Index | Shannon Index | Richness Index | Coverage |
|---|---|---|---|---|
| CK | 1717.01 ± 35.851 b | 5.83 ± 0.090 a | 1519.00 ± 42.46 a | 0.99 ± 0.00 a |
| N | 1790.50 ± 32.010 a | 5.80 ± 0.107 a | 1549.33 ± 36.23 a | 0.99 ± 0.00 a |
| NP | 1781.14 ± 23.490 a | 5.75 ± 0.108 a | 1544.67 ± 41.04 a | 0.99 ± 0.00 a |
| NPK | 1772.19 ± 39.659 ab | 5.81 ± 0.107 a | 1549.67 ± 13.58 a | 0.99 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Sun, Z.; Wang, Z.; Gong, L. Effects of the Application of Nutrients on Soil Bacterial Community Composition and Diversity in a Larix olgensis Plantation, Northeast China. Sustainability 2022, 14, 16759. https://doi.org/10.3390/su142416759
Cui J, Sun Z, Wang Z, Gong L. Effects of the Application of Nutrients on Soil Bacterial Community Composition and Diversity in a Larix olgensis Plantation, Northeast China. Sustainability. 2022; 14(24):16759. https://doi.org/10.3390/su142416759
Chicago/Turabian StyleCui, Jinyao, Zhihu Sun, Zixuan Wang, and Lifang Gong. 2022. "Effects of the Application of Nutrients on Soil Bacterial Community Composition and Diversity in a Larix olgensis Plantation, Northeast China" Sustainability 14, no. 24: 16759. https://doi.org/10.3390/su142416759





