Functional Properties of Egg White Protein and Whey Protein in the Presence of Bioactive Chicken Trachea Hydrolysate and Sodium Chloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Mixture Preparation
2.3. Foaming Ability of the Protein Mixtures in the System with and without Sodium Chloride
2.4. Emulsifying Capacity of the Protein Mixtures in the System with and without Sodium Chloride
2.5. Rheological Properties of the Protein Mixtures in the System with and without Sodium Chloride
2.6. Antioxidant Ability of the Protein Mixtures in the System with and without Sodium Chloride
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.6.3. FRAP Assay
2.7. ACE Inhibitor Ability of the Protein Mixtures in the System with and without Sodium Chloride
2.8. Statistical Analysis
3. Results and Discussion
3.1. CTH Properties
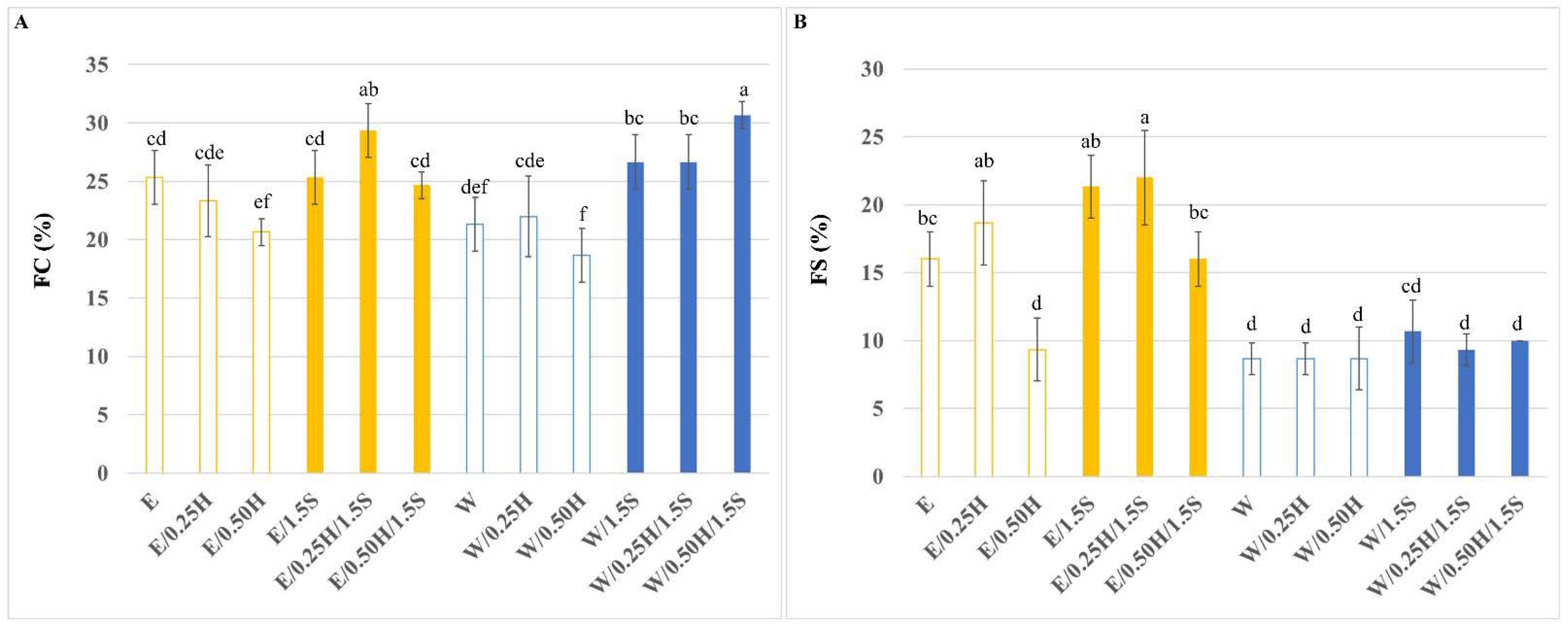
3.2. Foaming Ability of the Protein Mixtures in the System with and without Sodium Chloride
3.3. Emulsifying Capacity of the Protein Mixtures in the System with and without Sodium Chloride
3.4. Rheological Properties of the Protein Mixtures in the System with and without Sodium Chloride
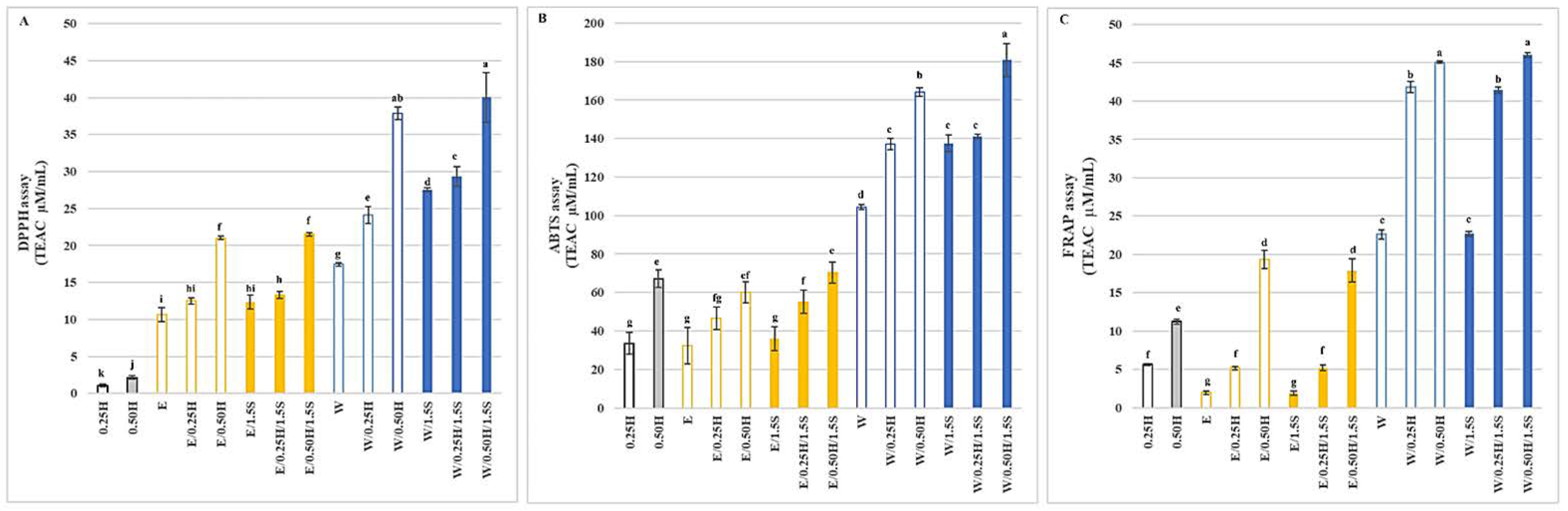
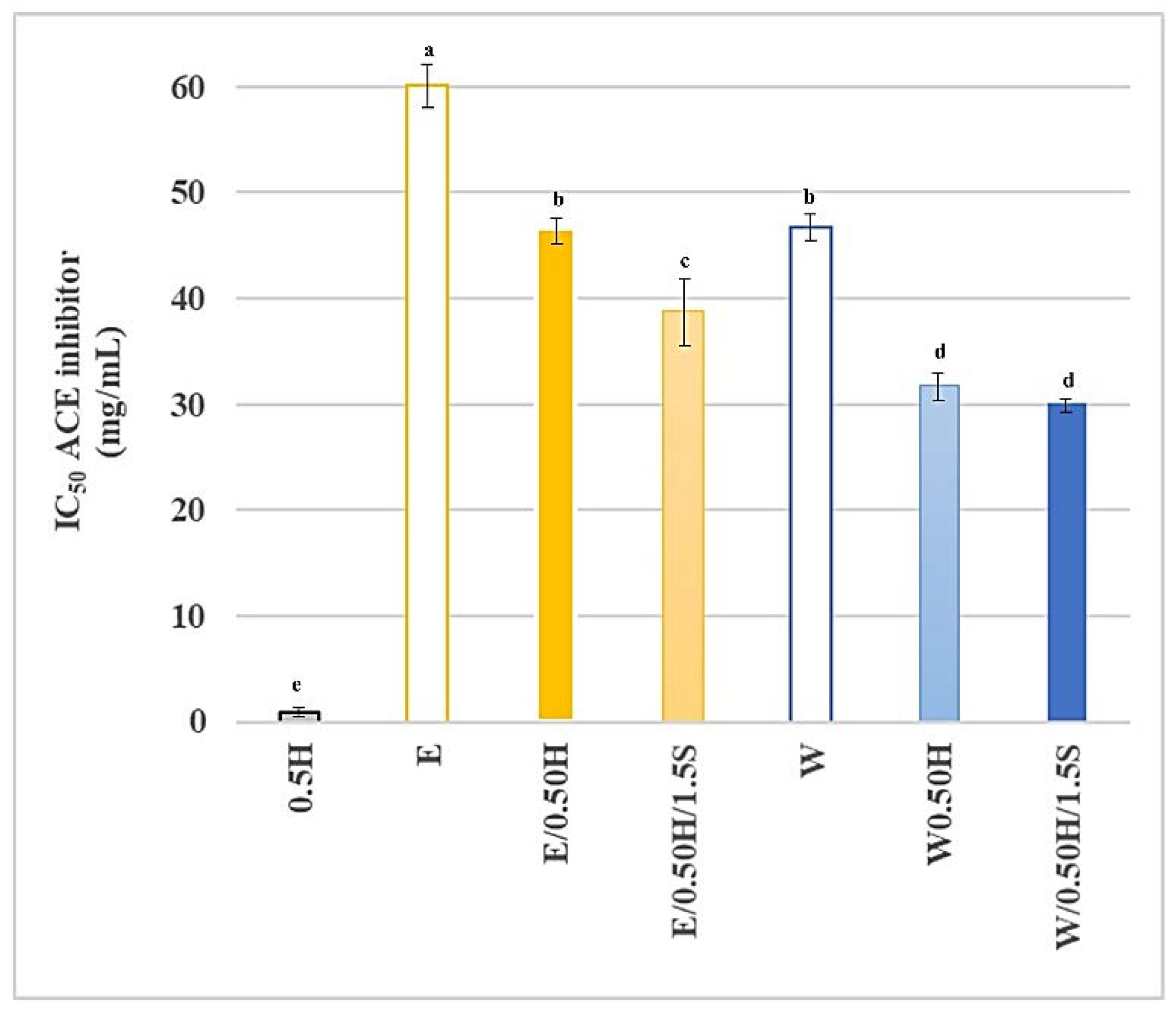
3.5. Antioxidant Ability and ACE Inhibitory Ability of the Protein Mixtures in the System with and without Sodium Chloride
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zegler, J.; Li, D.; Zanaza Bartalme, M.; Faulkner, D.; Koyenikan, A.; Mogelonky, M. 2022 Global Food and Drink Trends 2022; Mintel: London, UK, 2022; p. 28. [Google Scholar]
- Davis, J.P.; Foegeding, E.A. Comparisons of the foaming and interfacial properties of whey protein isolate and egg white proteins. Coll. Surf. B Biointerfaces 2007, 54, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Huang, X.; Ahn, D.U. Antioxidant, angiotensin-converting enzyme inhibitory activity and other functional properties of egg white proteins and their derived peptides—A review. Poult. Sci. 2018, 97, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.Y.; Jo, K.; Cho, S.Y.; Kim, J.M.; Lim, K.; Suh, H.J.; Oh, S. Antioxidant Effect and Functional Properties of Hydrolysates Derived from Egg-White Protein. Korean J. Food Sci. Anim. Resour. 2014, 34, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Deeth, H.; Bansal, N. Whey Proteins: An overview. In Whey Proteins from Milk to Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 1–50. [Google Scholar]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT-Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Neto, Y.A.H.; Rosa, J.C.; Cabral, H. Peptides with antioxidant properties identified from casein, whey, and egg albumin hydrolysates generated by two novel fungal proteases. Prep. Biochem. Biotechnol. 2019, 49, 639–648. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. The effect of chicken skin gelatin and whey protein interactions on rheological and thermal properties. Food Hydrocoll. 2015, 45, 83–92. [Google Scholar]
- Mullen, A.M.; Hayes, M.; Bolton, D.; Di Bernardini, R.; Rai, D.; Hayes, J.; Harnedy, P.; Stan, C. Nutraceutical and Functional Food Bioactive Peptides in Beef, Bovine Offals and Fermented Meat Products; Teagasc: Dublin, Ireland, 2012. [Google Scholar]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar]
- Cheng, F.-Y.; Wan, T.C.; Huang, C.W.; Tominaga, K.; Lin, L.C.; Sakata, R. The Effects of Chicken Leg Bone Extract on Antioxidative Properties under Different Heating Condition. Asian-Australas. J. Anim. Sci. 2008, 21, 1815–1820. [Google Scholar] [CrossRef]
- Saiga, A.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Kitamura, S.; Nishimura, T.; Morimatsu, F. Angiotensin I-Converting Enzyme-Inhibitory Peptides Obtained from Chicken Collagen Hydrolysate. J. Agric. Food Chem. 2008, 56, 9586–9591. [Google Scholar] [CrossRef]
- Nakade, K.; Kamishima, R.; Inoue, Y.; Ahhmed, A.; Kawahara, S.; Nakayama, T.; Maruyama, M.; Numata, M.; Ohta, K.; AOKI, T.; et al. Identification of an antihypertensive peptide derived from chicken bone extract. Anim. Sci. J. 2008, 79, 710–715. [Google Scholar] [CrossRef]
- Fujita, H.; Yokoyama, K.; Yoshikawa, M. Classification and Antihypertensive Activity of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Food Proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar]
- Terashima, M.; Baba, T.; Ikemoto, N.; Katayama, M.; Morimoto, T.; Matsumura, S. Novel angiotensin-converting enzyme (ACE) inhibitory peptides derived from boneless chicken leg meat. J. Agric. Food Chem. 2010, 58, 7432–7436. [Google Scholar] [CrossRef] [PubMed]
- Intorasoot, S. Antimicrobial peptides: The natural proteins and the future application for treatment of infectious diseases. Bull. Chiang Mai Assoc. Med. Sci. 2013, 46, 1–19. [Google Scholar]
- Zanello, P.P.; Sforza, S.; Dossena, A.; Lambertini, F.; Bottesini, C.; Nikolaev, I.V.; Koroleva, O.; Ciociola, Y.; Magliani, W.; Conti, S.; et al. Antimicrobial activity of poultry bone and meat trimmings hydrolyzates in low-sodium turkey food. Food Funct. 2014, 5, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z.; Pietrasik, Z.; Gaudette, N.J.; Betti, M. Poultry protein isolate prepared using an acid solubilization/precipitation extraction influences the microstructure, the functionality and the consumer acceptability of a processed meat product. Food Struct. 2014, 2, 49–60. [Google Scholar] [CrossRef]
- Pramualkijja, T.; Pirak, T.; Euston, S.R. Valorization of chicken slaughterhouse byproducts: Production and properties of chicken trachea hydrolysates using commercial proteases. Int. J. Food Prop. 2021, 24, 1642–1657. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Purification and characterization of antioxidative peptides derived from chicken skin gelatin hydrolysate. Food Hydrocoll. 2018, 85, 311–320. [Google Scholar] [CrossRef]
- Noman, A.; Xu, Y.; AL-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W. Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Chakka, A.K.; Elias, M.; Jini, R.; Sakhare, P.Z.; Bhaskar, N. In-vitro antioxidant and antibacterial properties of fermentatively and enzymatically prepared chicken liver protein hydrolysates. J. Food Sci. Technol. 2015, 52, 8059–8067. [Google Scholar] [CrossRef] [Green Version]
- Binsan, W.; Benjakul, S.; Visessanguan, W.; Roytrakul, S.; Tanaka, M.; Kishimura, H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 2008, 106, 185–193. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.-Y.; Wan, T.C.; Liu, Y.T.; Chen, C.M.; Lin, L.C.; Sakata, R. Determination of angiotensin-I converting enzyme inhibitory peptides in chicken leg bone protein hydrolysate with alcalase. Anim. Sci. J. 2009, 80, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V.; Campbell, L.; Euston, S.R. Effects of sucrose and sodium chloride on foaming properties of egg white proteins. Food Res. Int. 2007, 40, 347–355. [Google Scholar] [CrossRef]
- Davis, J.P.; Foegeding, E.A.; Hansen, F.K. Electrostatic effects on the yield stress of whey protein isolate foams. Coll. Surf. B Biointerfaces 2004, 34, 13–23. [Google Scholar] [CrossRef]
- Shimofuji, S.; Ohtani, K.; Matsui, M. The Role of Ovalbumin in the Stability of Whipped Egg White Prepared in Copper Bowl. J. Cook. Sci. Jpn. 2013, 46, 335–342. [Google Scholar]
- Dickinson, E. Mixed biopolymers at interfaces: Competitive adsorption and multilayer structures. Food Hydrocoll. 2011, 25, 1966–1983. [Google Scholar] [CrossRef]
- Duygu, A.; Doğan, M. The influence of ultrasound on the stability of dairy-based, emulsifier-free emulsions: Rheological and morphological aspect. Eur. Food Res. Technol. 2018, 244, 409–421. [Google Scholar]
- Patel, A.; Longmore, N.; Mohanan, A.; Ghosh, S. Salt and pH-Induced Attractive Interactions on the Rheology of Food Protein-Stabilized Nanoemulsions. ACS Omega 2019, 4, 11791–11800. [Google Scholar] [CrossRef] [Green Version]
- Pramualkijja, T.; Pirak, T.; Kerdsup, P. Effect of salt, rice bran oil and malva nut gum on chemical, physical and physico-chemical properties of beef salt e Soluble protein and its application in low fat salami. Food Hydrocoll. 2016, 53, 303–310. [Google Scholar] [CrossRef]
- Raikos, V.; Campbell, L.; Euston, S.R. Rheology and texture of hen’s egg protein heat-set gels as affected by pH and the addition of sugar and/or salt. Food Hydrocoll. 2007, 21, 237–244. [Google Scholar] [CrossRef]
- Tang, Q.; Munro, P.A.; Mccathy, O.J. Rheology of whey protein concentrate solutions as a function of concentration, temperature, pH and salt concentration. J. Dairy Res. 1993, 60, 349–361. [Google Scholar] [CrossRef]
- El-Shibiny, S.; Farrag, A.F.; El-Garawany, G.; Assem, F.M. Rheological and Functional Properties of Whey Protein Concentrate and β-Lactoglobulin and α-Lactalbumin Rich Fractions. Int. J. Dairy Sci. 2007, 2, 196–206. [Google Scholar]
- Holt, D.L.; Watson, M.A.; Dill, C.W.; Alford, E.S.; Edwards, R.L.; Diehl, K.C.; Gardner, F.A. Correlation of the Rheological Behavior of Egg Albumen to Temperature, pH, and NaCl Concentration. J. Food Sci. 1984, 49, 137–141. [Google Scholar] [CrossRef]
- Loveday, S.M.; Sawyer, L. β-Lactoglobulin: Nomenclature, Structure, and Function. In Reference Module in Food Science; SciTech Connect: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Yousr, M.; Howell, N. Antioxidant and ACE Inhibitory Bioactive Peptides Purified from Egg Yolk Proteins. Int. J. Mol. Sci. 2015, 16, 29161–29178. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Tummala, J.; Diwan, P.D. Nutritional composition, ACE-inhibitory, and metal chelating properties of rohu (Labeo rohita) egg protein hydrolysate produced by Alcalase. Int. Food Res. J. 2016, 23, 1017–1026. [Google Scholar]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014, 150, 366–373. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. Secretory Peptide Hormones Are Biochemical Antioxidants: Structure-Activity Relationship. Mol. Pharm. 2002, 61, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Tunieva, E.K.; Kotenkova, E.A. The Study on Effect of Sodium Chloride on the Antioxidant Activity of Meet. Foods Raw Mater. 2017, 5, 105–111. [Google Scholar] [CrossRef]
- Chen, X.; Tume, R.K.; Xu, X.; Zhou, G. Solubilization of myofibrillar proteins in water or low ionic strength media: Classical techniques, basic principles, and novel functionalities. Crit. Rev. Food Sci. Nutr. 2017, 57, 3260–3280. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hosseini, E. Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J. Funct. Foods 2015, 19, 259–268. [Google Scholar] [CrossRef]
- Wu, Q.; Du, J.; Jia, J.; Kuang, C. Production of ACE inhibitory peptides from sweet sorghum grain protein using alcalase: Hydrolysis kinetic, purification and molecular docking study. Food Chem. 2016, 199, 140–149. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Chicken Protein Hydrolysate (%) | Sodium Chloride (%) |
|---|---|---|
| E | 0 | 0 |
| E/0.25 H | 0.25 | 0 |
| E/0.50 H | 0.50 | 0 |
| E/1.5 S | 0 | 1.5 |
| E/0.25 H/1.5 S | 0.25 | 1.5 |
| E/0.50 H/1.5 S | 0.50 | 1.5 |
| W | 0 | 0 |
| W/0.25 H | 0.25 | 0 |
| W/0.50 H | 0.50 | 0 |
| W/1.5 S | 0 | 1.5 |
| W/0.25 H/1.5 S | 0.25 | 1.5 |
| W/0.50 H/1.5 S | 0.50 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pramualkijja, T.; Pirak, T.; Euston, S.R. Functional Properties of Egg White Protein and Whey Protein in the Presence of Bioactive Chicken Trachea Hydrolysate and Sodium Chloride. Sustainability 2022, 14, 16782. https://doi.org/10.3390/su142416782
Pramualkijja T, Pirak T, Euston SR. Functional Properties of Egg White Protein and Whey Protein in the Presence of Bioactive Chicken Trachea Hydrolysate and Sodium Chloride. Sustainability. 2022; 14(24):16782. https://doi.org/10.3390/su142416782
Chicago/Turabian StylePramualkijja, Teeda, Tantawan Pirak, and Stephen Robert Euston. 2022. "Functional Properties of Egg White Protein and Whey Protein in the Presence of Bioactive Chicken Trachea Hydrolysate and Sodium Chloride" Sustainability 14, no. 24: 16782. https://doi.org/10.3390/su142416782
APA StylePramualkijja, T., Pirak, T., & Euston, S. R. (2022). Functional Properties of Egg White Protein and Whey Protein in the Presence of Bioactive Chicken Trachea Hydrolysate and Sodium Chloride. Sustainability, 14(24), 16782. https://doi.org/10.3390/su142416782






