Synthesis of Urchin-Shaped Gold Nanoparticles Utilizing Green Reducing and Capping Agents at Different Preparation Conditions: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Gold Nanoparticles Preparation
2.3. Characterization
2.3.1. UV–Visible Spectrophotometer Measuring
2.3.2. Transmission Electron Microscopy (TEM) imaging
2.3.3. Particle Size Analysis
2.3.4. X-ray Diffraction (XRD) Analysis
2.3.5. Fourier Transform-Spectroscopy (FT-IR) Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized Gold Nanoparticles: Synthesis, Structure and Colloid Stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold Nanoparticles: Past, Present, and Future. Langmuir 2009, 25, 13840–13851. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and Cellular Uptake of Gold Nanoparticles: What We Have Learned so Far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Fernández, J.; Pérez-Juste, J.; García De Abajo, F.J.; Liz-Marzán, L.M. Seeded Growth of Submicron Au Colloids with Quadrupole Plasmon Resonance Modes. Langmuir 2006, 22, 7007–7010. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud Univ. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional Gold Nanorods: Synthesis, Self-Assembly, and Sensing Applications. Adv. Mater. 2012, 24, 4811–4841. [Google Scholar] [CrossRef]
- Pellas, V.; Hu, D.; Mazouzi, Y.; Mimoun, Y.; Blanchard, J.; Guibert, C.; Salmain, M.; Boujday, S. Gold Nanorods for LSPR Biosensing: Synthesis, Coating by Silica, and Bioanalytical Applications. Biosensors 2020, 10, 146. [Google Scholar] [CrossRef]
- Kim, F.; Sohn, K.; Wu, J.; Huang, J. Chemical Synthesis of Gold Nanowires in Acidic Solutions. J. Am. Chem. Soc. 2008, 130, 14442–14443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qiu, X.; Fu, G.; Sun, J.; Huang, Z.; Sun, D.; Xu, L.; Zhou, J.; Tang, Y. Highly Simple and Rapid Synthesis of Ultrathin Gold Nanowires with (111)-Dominant Facets and Enhanced Electrocatalytic Properties. J. Mater. Chem. A 2018, 6, 17682–17687. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Pastoriza-Santos, I.; Rodríguez-González, B.; Javier García De Abajo, F.; Liz-Marzán, L.M. High-Yield Synthesis and Optical Response of Gold Nanostars. Nanotechnology 2008, 19, 15606. [Google Scholar] [CrossRef]
- Andreiuk, B.; Nicolson, F.; Clark, L.M.; Panikkanvalappil, S.R.; Kenry; Rashidian, M.; Harmsen, S.; Kircher, M.F. Design and Synthesis of Gold Nanostars-Based SERS Nanotags for Bioimaging Applications. Nanotheranostics 2022, 6, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Selbach, F.; Langolf, L.; Schlücker, S. Ideal Dimers of Gold Nanospheres for Precision Plasmonics: Synthesis and Characterization at the Single-Particle Level for Identification of Higher Order Modes. Small 2018, 14, 1702754. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Zhang, R.Y.; Zhao, D.H.; Zhang, X.S.; An, J.; Cheng, K.; Hou, X.L.; Song, X.L.; Zhao, Y.D.; Yang, X.Q. Ultrafast Synthesis of Gold Nanosphere Cluster Coated by Graphene Quantum Dot for Active Targeting PA/CT Imaging and near-Infrared Laser/PH-Triggered Chemo-Photothermal Synergistic Tumor Therapy. Chem. Eng. J. 2019, 369, 87–99. [Google Scholar] [CrossRef]
- Scarabelli, L.; Coronado-Puchau, M.; Giner-Casares, J.J.; Langer, J.; Liz-Marzán, L.M. Monodisperse Gold Nanotriangles: Size Control, Large-Scale Self-Assembly, and Performance in Surface-Enhanced Raman Scattering. ACS Nano 2014, 8, 5833–5842. [Google Scholar] [CrossRef]
- Koetz, J. The Effect of Surface Modification of Gold Nanotriangles for Surface-Enhanced Raman Scattering Performance. Nanomaterials 2020, 10, 2187. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C. Supported Gold Nanoparticles as Catalysts for the Oxidation of Alcohols and Alkanes. Front. Chem. 2019, 7, 702. [Google Scholar] [CrossRef] [Green Version]
- Anjana, P.M.; Bindhu, M.R.; Rakhi, R.B. Green Synthesized Gold Nanoparticle Dispersed Porous Carbon Composites for Electrochemical Energy Storage. Mater. Sci. Energy Technol. 2019, 2, 389–395. [Google Scholar] [CrossRef]
- Vasam, M.; Punagoti, R.A.; Mourya, R. Biomedical Applications of Gold Nanoparticles. Nanotechnol. Life Sci. 2021, 15, 41–59. [Google Scholar] [CrossRef]
- Nawaz, A.; Ali, S.M.; Rana, N.F.; Tanweer, T.; Batool, A.; Webster, T.J.; Menaa, F.; Riaz, S.; Rehman, Z.; Batool, F.; et al. Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment. Nanomaterials 2021, 11, 3152. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Fatima Rana, N.; Hussain, I.; Tanweer, T.; Nawaz, A.; Menaa, F.; Janjua, H.A.; Alam, T.; Batool, A.; Naeem, A.; et al. Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs. Nanomaterials 2020, 10, 1769. [Google Scholar] [CrossRef] [PubMed]
- Budaszewski, D.; Chychłowski, M.; Budaszewska, A.; Bartosewicz, B.; Jankiewicz, B.; Woliński, T.R. Enhanced Efficiency of Electric Field Tunability in Photonic Liquid Crystal Fibers Doped with Gold Nanoparticles. Opt. Express 2019, 27, 14260–14269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold Nanoparticle-Based Biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.L.; Hryniewicz, B.M.; Deller, A.E.; Volpe, J.; Marchesi, L.F.; Souto, D.E.P.; Vidotti, M. Electrodes Based on PEDOT Nanotubes Decorated with Gold Nanoparticles for Biosensing and Energy Storage. ACS Appl. Nano Mater. 2021, 4, 9945–9956. [Google Scholar] [CrossRef]
- Teng, Y.; Shi, J.; Pong, P.W.T. Sensitive and Specific Colorimetric Detection of Cancer Cells Based on Folate-Conjugated Gold-Iron-Oxide Composite Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 7421–7431. [Google Scholar] [CrossRef]
- Homik, Z.; Kopniczky, J.; Smausz, T.; Berkesi, D.; Hopp, B. Formation of Gold/Silver Composite Nanoparticles by Pulsed Laser Ablation of Gold–Silver Layered Films in Liquid. Appl. Phys. A Mater. Sci. Process. 2022, 128, 797. [Google Scholar] [CrossRef]
- Nghiem, T.H.L.; Le, T.N.; Do, T.H.; Vu, T.T.D.; Do, Q.H.; Tran, H.N. Preparation and Characterization of Silica-Gold Core-Shell Nanoparticles. J. Nanoparticle Res. 2013, 15, 2091. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [Green Version]
- Zecchina, A.; Groppo, E.; Bordiga, S. Selective Catalysis and Nanoscience: An Inseparable Pair. Chem.-A Eur. J. 2007, 13, 2440–2460. [Google Scholar] [CrossRef] [PubMed]
- Nehl, C.L.; Hafner, J.H. Shape-Dependent Plasmon Resonances of Gold Nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419. [Google Scholar] [CrossRef] [Green Version]
- Rajapantulu, A.; Bandyopadhyaya, R. Formation of Gold Nanoparticles in Water-in-Oil Microemulsions: Experiment, Mechanism, and Simulation. Langmuir 2021, 37, 6623–6631. [Google Scholar] [CrossRef] [PubMed]
- Revina, A.A.; Chernyshova, K.F.; Tabachkova, N.Y.; Parkhomenko, Y.N. Gold Nanoparticles in Reverse Micellar Solutions: Preparation, Optical Properties, and Dimensional Characteristics. Russ. Chem. Bull. 2019, 68, 1164–1170. [Google Scholar] [CrossRef]
- Yahaya, M.L.; Zakaria, N.D.; Noordin, R.; Abdul Razak, K. Synthesis of Large and Stable Colloidal Gold Nanoparticles (AuNPs) by Seeding-Growth Method. Mater. Today Proc. 2022, 66, 2943–2947. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S. Recent Advances in Inorganic Nanomaterials Synthesis Using Sonochemistry: A Comprehensive Review on Iron Oxide, Gold and Iron Oxide Coated Gold Nanoparticles. Molecules 2021, 26, 2453. [Google Scholar] [CrossRef]
- Bianchi, P.; Petit, G.; Monbaliu, J.C.M. Scalable and Robust Photochemical Flow Process towards Small Spherical Gold Nanoparticles. React. Chem. Eng. 2020, 5, 1224–1236. [Google Scholar] [CrossRef]
- Bondaz, L.; Fontaine, P.; Muller, F.; Pantoustier, N.; Perrin, P.; Morfin, I.; Goldmann, M.; Cousin, F. Controlled Synthesis of Gold Nanoparticles in Copolymers Nanomolds by X-ray Radiolysis. Langmuir 2020, 36, 6132–6144. [Google Scholar] [CrossRef]
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the Methodologies Used in the Synthesis Gold Nanoparticles by Chemical Reduction. J. Alloy. Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Rawat, P.; Rajput, Y.S.; Bharti, M.K.; Sharma, R. A Method for Synthesis of Gold Nanoparticles Using 1-Amino-2-Naphthol-4-Sulphonic Acid as Reducing Agent. Curr. Sci. 2016, 110, 2297–2300. [Google Scholar] [CrossRef]
- Johan, M.R.; Chong, L.C.; Hamizi, N.A. Preparation and Stabilization of Monodisperse Colloidal Gold by Reduction with Monosodium Glutamate and Poly (Methyl Methacrylate). Int. J. Electrochem. Sci. 2012, 7, 4567–4573. [Google Scholar]
- Yang, S.K.; Kim, Y. Nanogold Particles Produced by NaBH4 Reduction of Gold Salt in the Presence of Laponite Sol. Bull. Korean Chem. Soc. 2013, 34, 363–364. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.D.S.; Blanchard, G.J. Formation of Gold Nanoparticles Using Amine Reducing Agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Singh, T.; Hussain, J.I.; Hashmi, A.A. Au(III)-CTAB Reduction by Ascorbic Acid: Preparation and Characterization of Gold Nanoparticles. Colloids Surf. B Biointerfaces 2013, 104, 11–17. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Sushma, N.J.; Deva Prasad Raju, B. Novel, Fast, Bio-Derivatized Sonochemical Synthesis of Gold Nanoparticles by Using Piper Betle Leaf Broth as a Reducing and Capping Agent. In Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2013; Volume 143, pp. 41–49. ISBN 9783642342158. [Google Scholar]
- Boruah, S.K.; Boruah, P.K.; Sarma, P.; Medhi, C.; Medhi, O.K. A Study on the Electrospinning Behaviour and Nanofibre Morphology of Anionically Charged Lignin. Adv. Mater. Lett. 2012, 3, 481–486. [Google Scholar] [CrossRef]
- Wu, S.; Yan, S.; Qi, W.; Huang, R.; Cui, J.; Su, R.; He, Z. Green Synthesis of Gold Nanoparticles Using Aspartame and Their Catalytic Activity for P-Nitrophenol Reduction. Nanoscale Res. Lett. 2015, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Das, A.R.; Guha, A.K. Gold Nanoparticles: Microbial Synthesis and Application in Water Hygiene Management. Langmuir 2009, 25, 8192–8199. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Verma, H.N.; Singh, P.; Chavan, R.M. Gold Nanoparticle: Synthesis and Characterization. Vet. World 2014, 7, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Schulz, F.; Homolka, T.; Bastús, N.G.; Puntes, V.; Weller, H.; Vossmeyer, T. Little Adjustments Significantly Improve the Turkevich Synthesis of Gold Nanoparticles. Langmuir 2014, 30, 10779–10784. [Google Scholar] [CrossRef]
- Dozol, H.; Mériguet, G.; Ancian, B.; Cabuil, V.; Xu, H.; Wang, D.; Abou-Hassan, A. On the Synthesis of Au Nanoparticles Using EDTA as a Reducing Agent. J. Phys. Chem. C 2013, 117, 20958–20966. [Google Scholar] [CrossRef]
- Chae, S.Y.; Son, S.; Lee, M.; Jang, M.K.; Nah, J.W. Deoxycholic Acid-Conjugated Chitosan Oligosaccharide Nanoparticles for Efficient Gene Carrier. J. Control. Release 2005, 109, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Borges, J.; Lopes, C.; Pereira, R.M.S.; Vasilevskiy, M.I.; Vaz, F. Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies. Appl. Sci. 2021, 11, 5388. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Fatima Rana, N.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Britto Hurtado, R.; Cortez-Valadez, M.; Ramírez-Rodríguez, L.P.; Larios-Rodriguez, E.; Alvarez, R.A.B.; Rocha-Rocha, O.; Delgado-Beleño, Y.; Martinez-Nuñez, C.E.; Arizpe-Chávez, H.; Hernández-Martínez, A.R.; et al. Instant Synthesis of Gold Nanoparticles at Room Temperature and SERS Applications. Phys. Lett. A 2016, 380, 2658–2663. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, D.J.; So, M.G.; Kim, K.S. Experimental Measurements of Gold Nanoparticle Nucleation and Growth by Citrate Reduction of HAuCl4. Adv. Powder Technol. 2010, 21, 111–118. [Google Scholar] [CrossRef]
- Neupane, M.P.; Lee, S.J.; Park, I.S.; Lee, M.H.; Bae, T.S.; Kuboki, Y.; Uo, M.; Watari, F. Synthesis of Gelatin-Capped Gold Nanoparticles with Variable Gelatin Concentration. J. Nanoparticle Res. 2011, 13, 491–498. [Google Scholar] [CrossRef]
- Nellore, J.; Pauline, P.C.; Amarnath, K. Biogenic Synthesis by Sphearanthus Amaranthoids; towards the Efficient Production of the Biocompatible Gold Nanoparticles. Dig. J. Nanomater. Biostructures 2012, 7, 123–133. [Google Scholar]
- Robb, D.T.; Privman, V. Model of Nanocrystal Formation in Solution by Burst Nucleation and Diffusional Growth. Langmuir 2008, 24, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Mountrichas, G.; Pispas, S.; Kamitsos, E.I. Effect of Temperature on the Direct Synthesis of Gold Nanoparticles Mediated by Poly(Dimethylaminoethyl Methacrylate) Homopolymer. J. Phys. Chem. C 2014, 118, 22754–22759. [Google Scholar] [CrossRef]
- Lim, S.; Gunasekaran, S.; Imm, J.Y. Gelatin-Templated Gold Nanoparticles as Novel Time-Temperature Indicator. J. Food Sci. 2012, 77, N45–N49. [Google Scholar] [CrossRef] [PubMed]
- Mock, A.J.; Kang, J. Comment on Degradation of Ascorbic Acid in Ethanolic Solutions. J. Agric. Food Chem. 2013, 61, 2580–2582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Mohanty, B.; Bohidar, H.B. Systematic of Alcohol-Induced Simple Coacervation in Aqueous Gelatin Solutions. Biomacromolecules 2003, 4, 1080–1086. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Soritau, O.; Maniu, D.; Astilean, S. One-Pot, Green Synthesis of Gold Nanoparticles by Gelatin and Investigation of Their Biological Effects on Osteoblast Cells. Colloids Surf. B Biointerfaces 2015, 132, 122–131. [Google Scholar] [CrossRef]
- Allouche, J.; Soulé, S.; Dupin, J.C.; Masse, S.; Coradin, T.; Martinez, H. Design of Gold Nanoshells via a Gelatin-Mediated Self-Assembly of Gold Nanoparticles on Silica Cores. RSC Adv. 2014, 4, 63234–63237. [Google Scholar] [CrossRef]



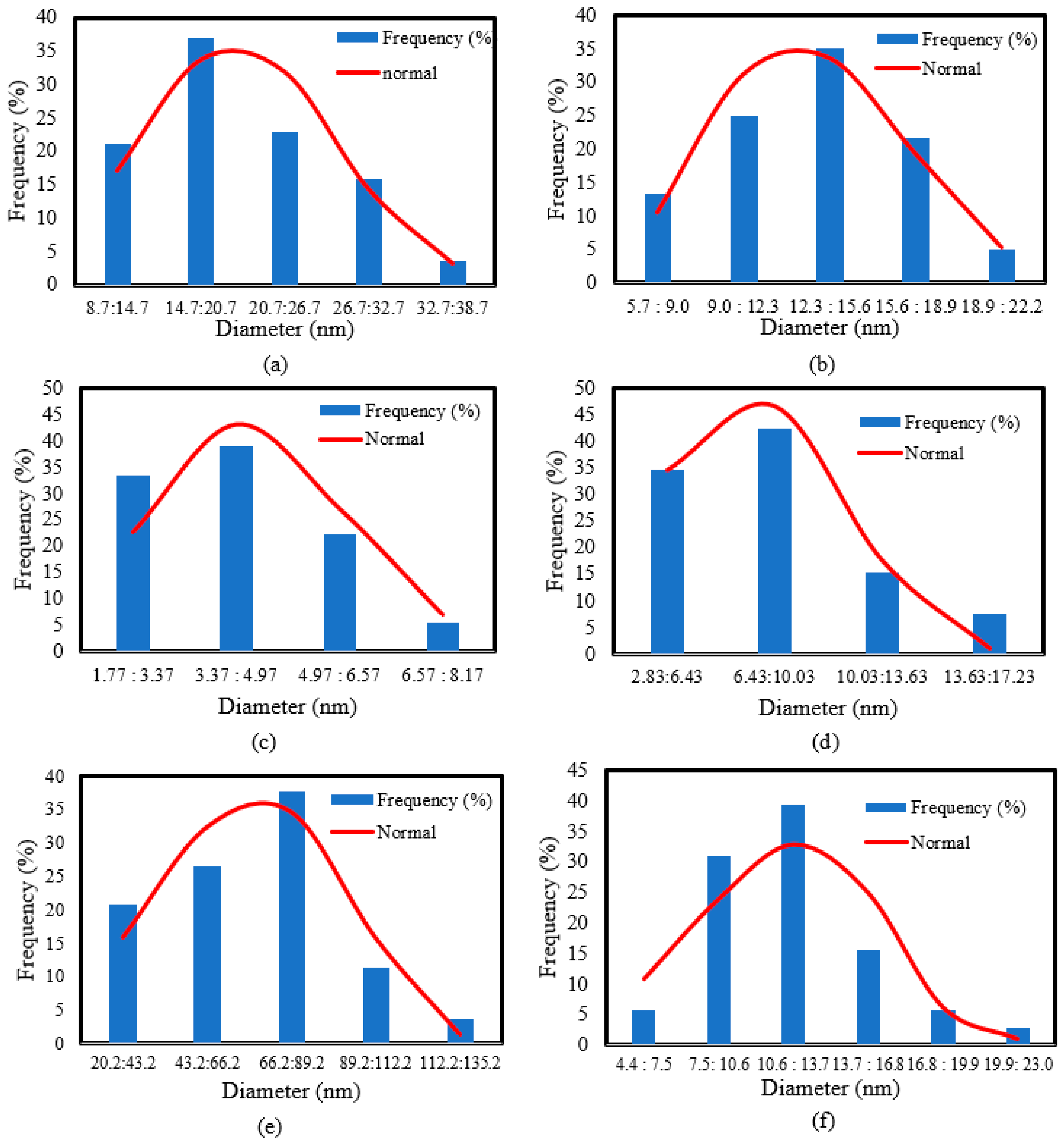
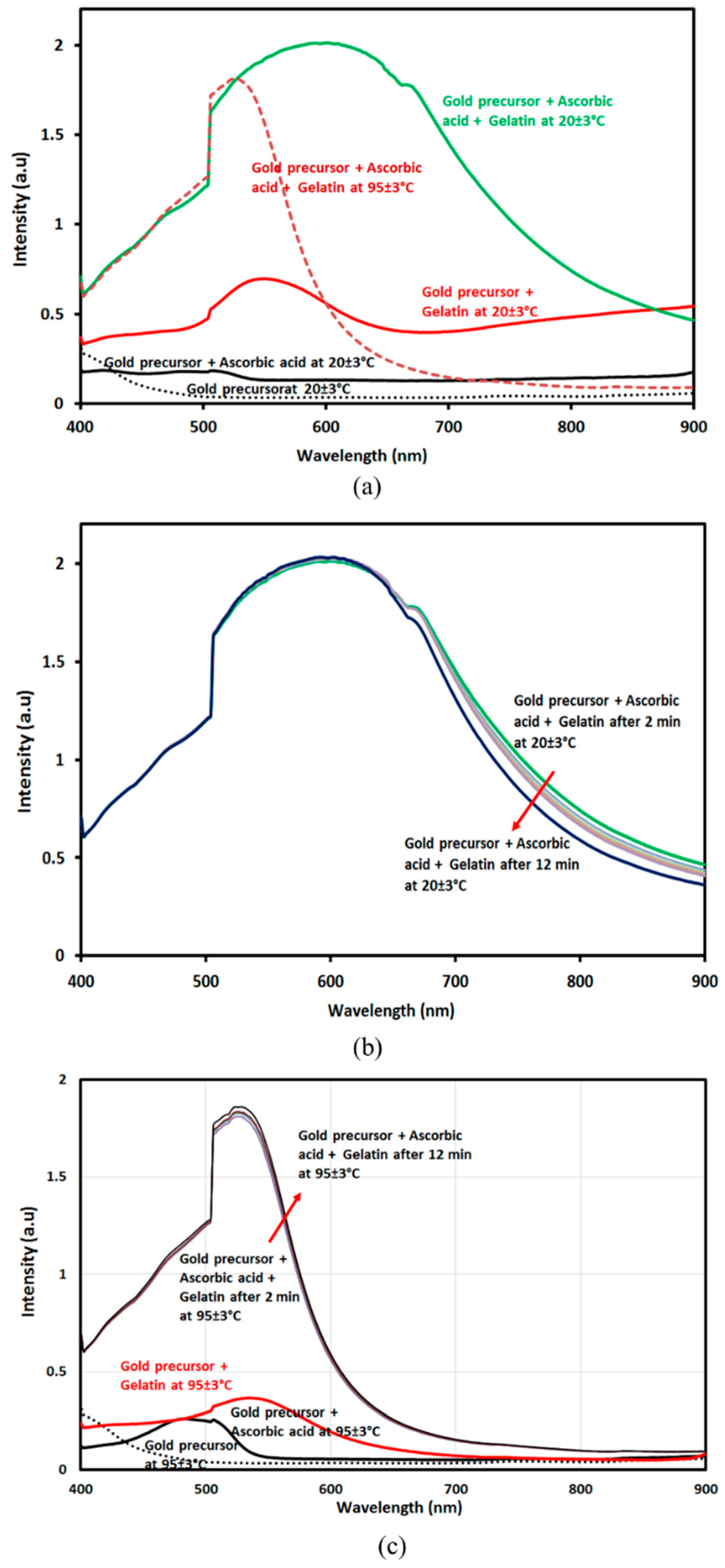
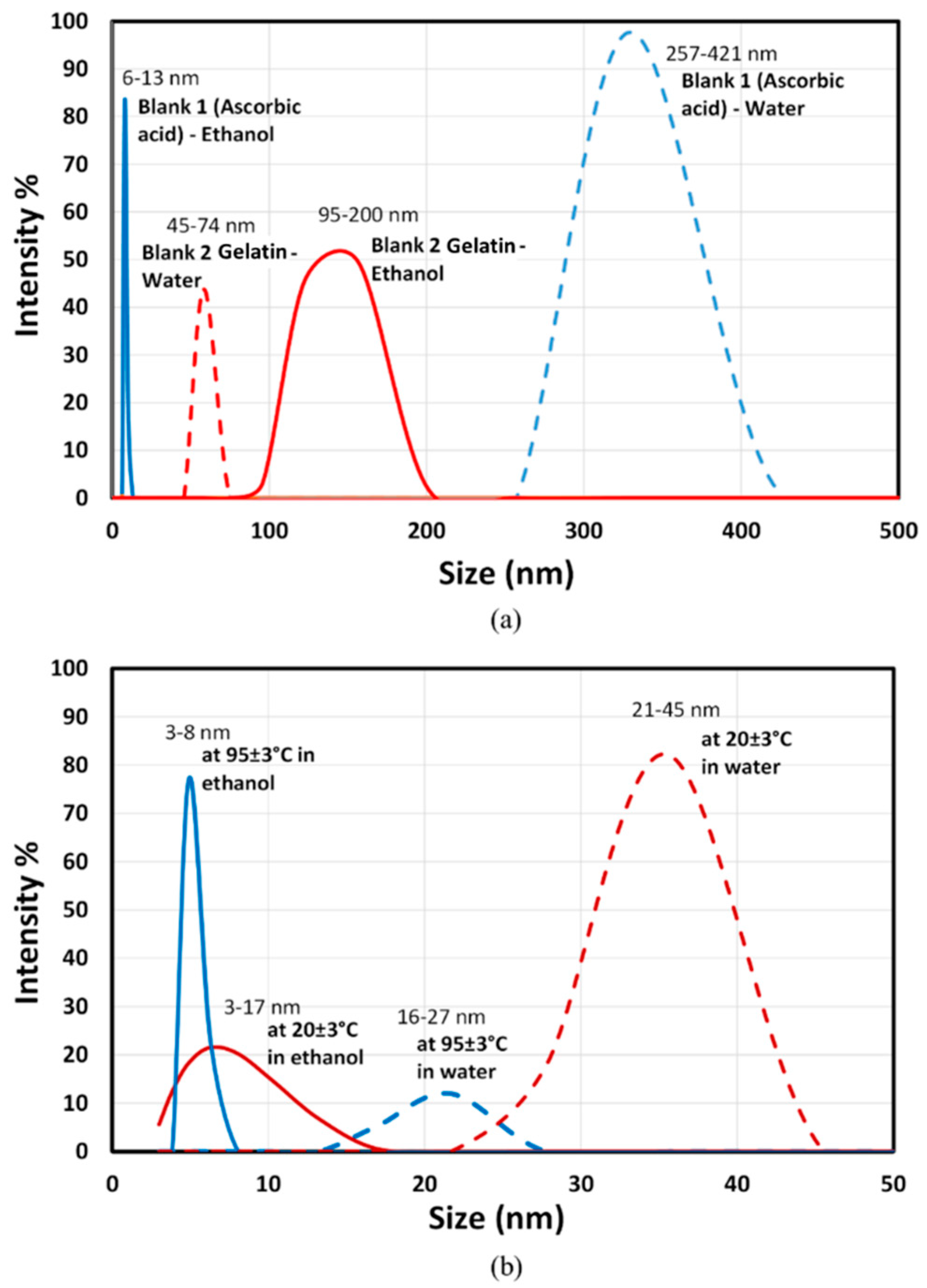
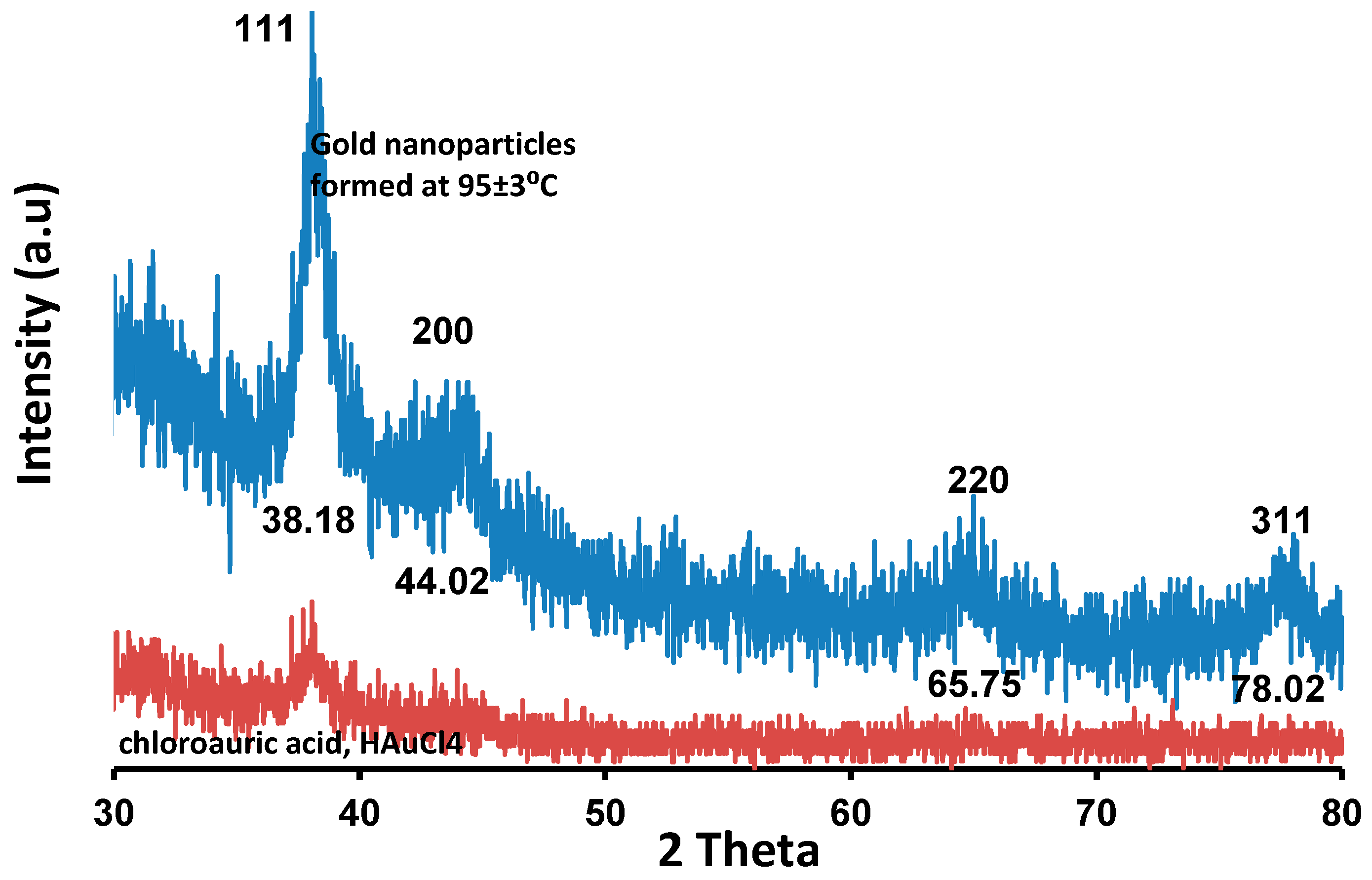

| Sample | Mean Value (nm) | Standard Deviation (nm) |
|---|---|---|
| B1,RT | 20.3 | 6.6 |
| B1,BT | 14.0 | 3.6 |
| B2,RT | 4.3 | 1.5 |
| B2,BT | 7.2 | 3.2 |
| M,RT | 65.6 | 25.1 |
| M,BT | 11.8 | 3.6 |
| Preparation Temperature | Property | Ascorbic Acid (B1) pH 3.6 ± 0.02 | Gelatin (B2) pH 3.1 ± 0.01 | Ascorbic Acid and Gelatin (M) pH 3.3 ± 0.02 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RT (20 ± 3 °C) | Determined Size (nm) | TEM (water) | Particle size | TEM (water) | Particle size | TEM (water) | Particle size | |||
| Water | Eth. | water | Eth. | water | Eth. | |||||
| 9–34 | - | - | 2–8 | - | - | 20–120 | 21–45 | 3–17 | ||
| Morphology | Irregular | Spherical/Urchin mix | Urchin | |||||||
| Mixing time | 30 min | 30 min | 15 min | |||||||
| BT (95 ± 3 °C) | Determined Size (nm) | TEM (water) | Particle size | TEM (water) | Particle size | TEM (water) | Particle size | |||
| Water | Eth. | water | Eth. | water | Eth. | |||||
| 5–20 | 257–421 | 6–13 | 3–14 | 45–74 | 95–200 | 5–22 | 16–27 | 3–8 | ||
| Morphology | Spherical | |||||||||
| Mixing time | 10 min | 20 min | 2 min | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, M.S.; Elmarghany, M.R.; Salem, N.; Nady, N. Synthesis of Urchin-Shaped Gold Nanoparticles Utilizing Green Reducing and Capping Agents at Different Preparation Conditions: An In Vitro Study. Sustainability 2022, 14, 16838. https://doi.org/10.3390/su142416838
Salem MS, Elmarghany MR, Salem N, Nady N. Synthesis of Urchin-Shaped Gold Nanoparticles Utilizing Green Reducing and Capping Agents at Different Preparation Conditions: An In Vitro Study. Sustainability. 2022; 14(24):16838. https://doi.org/10.3390/su142416838
Chicago/Turabian StyleSalem, Mohamed S., Mohamed R. Elmarghany, Noha Salem, and Norhan Nady. 2022. "Synthesis of Urchin-Shaped Gold Nanoparticles Utilizing Green Reducing and Capping Agents at Different Preparation Conditions: An In Vitro Study" Sustainability 14, no. 24: 16838. https://doi.org/10.3390/su142416838





