Influence of Anthropogenic Activities on Forest Carbon Stocks—A Case Study from Gori Valley, Western Himalaya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Sampling and Data Analysis
2.3. Regeneration Pattern
2.4. Levels of Anthropogenic Disturbance
2.5. Growing Stock, Biomass, and Carbon Stock Estimation
| Tree Species | Volume Equations |
|---|---|
| Hardwood | |
| Acer caesium Wall. ex Brandis | V = −0.162945 + 3.109717 × D |
| Acer cappadocicum Gled. | V = −0.162945 + 3.109717 × D |
| Acer oblongum Wall. ex DC. | V = −0.162945 + 3.109717 × D |
| Aesculus indica Colebr. ex Wall. | V = 0.007602 − 0.033037 × D + 1.868567 × D2 + 4.483454 × D3 |
| Alnus nepalensis D. Don | V = 0.0741 − 1.3603 × D + 10.9229 × D2 |
| Castanopsis tribuloides (Sm.) A.DC. | V = −0.02301 + 0.12721 × D + 2.4127 × D2 + 8.12834 × D3 |
| Lyonia ovalifolia (Wall.) Drude. | V = 0.03468 − 0.56878 × D + 4.72282 × D2 |
| Machilus odoratissima Nees | V = 6.678 × D × D − 0.240 × D − 0.024 |
| Myrica esculenta Buch.—Ham. ex D.Don | V = 0.007602 − 0.033037 × D + 1.868567 × D2 + 4.483454 × D3 |
| Perseaduthiei (King) Kosterm. | V = 6.678 × D × D − 0.240 × D − 0.024 |
| Pyrus pashia Buch. -Ham. ex D.Don | V = 0.046 − 0.646 × D + 4.272 × D2 |
| Quercus floribunda Lindl. ex A. Camus | V = 0.0988 − 1.55471 × D + 10.16317 × D2 |
| Quercus lanuginosa D.Don | V = 0.0988 − 1.55471 × D + 10.16317 × D2 |
| Quercus leucotrichophora A.Cam. ex Bah. | √V = 0.240157 + 3.820069 × D − 1.39452 × √D |
| Quercus semecarpifolia Sm. | V = 0.0988 − 1.55471 × D + 10.16317 × D2 |
| Rhododendron arboreum Sm. | V = 0.06007 − 0.21874√D + 3.63428× D2 |
| Symplocos chinensis (Lour.) Druce | V = −0.212798 + 3.288996 × D + 0.046417 × √D |
| Softwood | |
| Cupressus torulosa D.Don | V = 0.007602 − 0.033037 × D + 1.868567 × D2 + 4.483454 × D3 |
| Pinus roxburghii Sarg. | √V = 0.05131 + 3.9859 × D − 1.0245 × √D |
| Rest of the species | V = 0.007602 − 0.033037 × D + 1.868567 × D2 + 4.483454 × D3 |
2.6. Litter Fall Estimation
2.7. Soil Sampling and Analysis
2.8. Statistical Analysis
3. Results
3.1. Community Structure
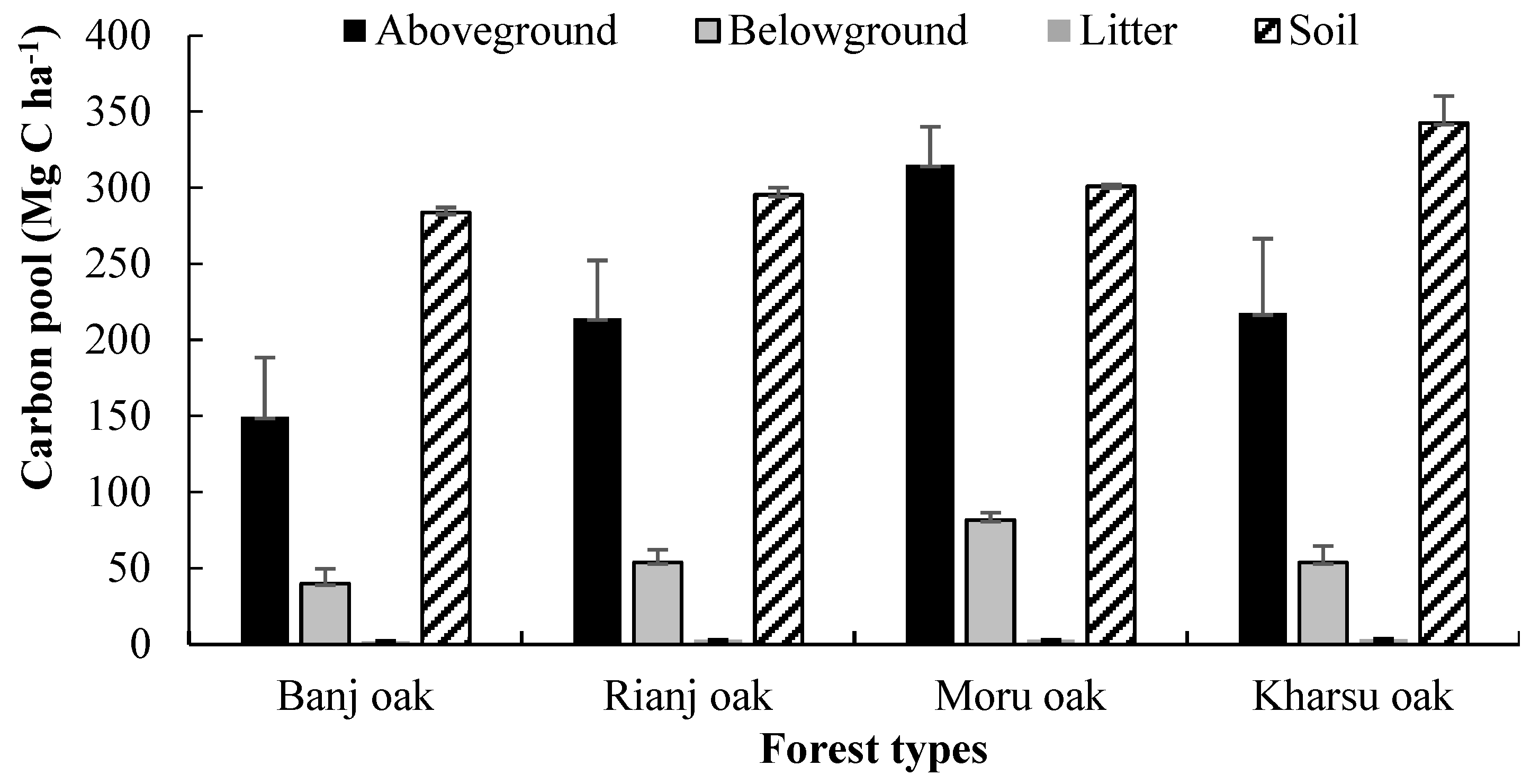
3.2. Biomass and Carbon Stock
3.3. Anthropogenic Disturbance and Regeneration Pattern
3.4. Soil Characteristics
3.5. Relationship between Vegetative Parameters, Soil Physico-Chemical Properties, Altitude and Carbon Stock Variables
4. Discussion
4.1. Influence of Community Structure on Carbon Stock
4.2. Influence of Altitude
4.3. Influence of Regeneration and Disturbance
4.4. Influence of Soil Nutrients
4.5. Comparative Study of Biomass, Carbon Stock and Chemical Properties of Soil
4.6. Sustainable Forest Management Regimes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Information
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Hayes, D. A large and persistent carbon sink in the world’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO-UNEP. The State of the World’s Forests; Forests, Biodiversity and People: Rome, Italy, 2020. [Google Scholar]
- FSI. The State of Forest Report; Forest Survey of India; Ministry of Environment & Forests: Dehradun, India, 2021.
- FSI. The State of Forest Report; Forest Survey of India; Ministry of Environment & Forests: Dehradun, India, 2019.
- Van derwerf, G.R.; Morton, D.C.; DeFries, R.S.; Olivier, J.G.J.; Kasibhatla, P.S.; Jackson, R.B.; Collatz, G.J.; Randerson, J.T. CO2 emissions from forest loss. Nat. Geosci. 2009, 2, 737–738. [Google Scholar] [CrossRef]
- Rawat, P.K.; Tiwari, P.C.; Pant, C.C.; Sharma, A.K.; Pant, P.D. Climate change and its geo-hydrological impacts on mountainous terrain: A case study through remote sensing and GIS modeling. Int. Sci. Res. J. 2011, 3, 51–69. [Google Scholar]
- Awasthi, P.; Bargali, K.; Bargali, S.S.; Jhariya, M.K. Structure and Functioning of Coriaria nepalensis Wall dominated Shrublands in degraded hills of Kumaun Himalaya. I. Dry Matter Dynamics. Land Degrad. Dev. 2022, 33, 1474–1494. [Google Scholar] [CrossRef]
- Sagar, R.; Raghubanshi, A.S.; Singh, J.S. Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. For. Ecol. Manag. 2003, 186, 61–71. [Google Scholar] [CrossRef]
- Singh, J.S.; Singh, S.P. Structure and function of the Central Himalayan Oak forests. Proc. Plant Sci. 1986, 96, 156–189. [Google Scholar] [CrossRef]
- Singh, R.D.; Gumber, S.; Joshi, H.; Singh, S.P. Allocation to tree bark in pine and oak species in fire affected mixed forests across the Northern Hemisphere. For. Ecol. Manag. 2022, 509, 120081. [Google Scholar] [CrossRef]
- Rawat, S.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Thakur, T.K.; Kumar, M.; Cabral-Pinto, M.M. Variation in carbon stock and soil properties in different Quercus leucotrichophora forests of Garhwal Himalaya. Catena 2022, 213, 106210. [Google Scholar] [CrossRef]
- Kalambukattu, J.G.; Singh, R.; Patra, A.K.; Kalaimurthy, A.K. Soil carbon pools and carbon management index under different land use systems in the Central Himalayan region. Soil Plant Sci. 2013, 63, 200–205. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Rana, K.P.S.; Larsem, L.; Sehgal, J. Soils of Himachal Pradesh for Optimizing Land Uses. National Bureau of Soil Survey and Land Use Planning. Bulletin 1997, 57, 73. [Google Scholar]
- Bargali, K.; Manral, V.; Padalia, K.; Bargali, S.S.; Upadhyay, V.P. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan Forest soils, India. Catena 2018, 171, 125–135. [Google Scholar] [CrossRef]
- Sagar, R.; Singh, J.S. Structure, diversity, and regeneration of tropical dry deciduous forest of northern India. Biodivers. Conserv. 2005, 14, 935–959. [Google Scholar] [CrossRef]
- Joshi, A.K.; Joshi, P.K. Forest ecosystem services in the central Himalaya: Local benefits and global relevance. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 785–792. [Google Scholar] [CrossRef]
- Singh, G.; Rawat, G.S. Is the future of oak (Quercus spp.) forests safe in the Western Himalayas? Curr. Sci. 2010, 98, 1420. [Google Scholar]
- Pandey, A.N.; Pathak, P.C.; Singh, J.S. Water, sediment and nutrient movement in forested and non-forested catchments in Kumaun Himalaya. For. Ecol. Manag. 1983, 7, 19–29. [Google Scholar] [CrossRef]
- Singh, V.; Thadani, R.; Tewari, A.; Ram, J. Human influence on Banj oak (Quercus leucotrichophora, A. Camus) forests of Central Himalaya. J. Sustain. For. 2014, 33, 373–386. [Google Scholar] [CrossRef]
- Naudiyal, N.; Schmerbeck, J. Potential distribution of oak forests in the central Himalayas and implications for future ecosystem services supply to rural communities. Ecosyst. Serv. 2021, 50, 101–310. [Google Scholar] [CrossRef]
- Sharma, C.M.; Baduni, N.P.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. For. Ecol. Manag. 2010, 260, 2170–2179. [Google Scholar] [CrossRef]
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Government of India Publications: New Delhi, India, 1968.
- Samant, S.S.; Dhar, U.; Rawal, R.S. Assessment of fuel resource diversity and utilization patterns in Askot Wildlife Sanctuary in Kumaun Himalaya, India, for conservation and management. Environ. Conserv. 2000, 27, 5–13. [Google Scholar] [CrossRef]
- Samant, S.S.; Rawal, R.S.; Dhar, U. Diversity, extraction and status of fodder species in Askot Wildlife Sanctuary, West Himalaya, India. Int. J. Biodivers. Sci. Manag. 2006, 2, 29–42. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. The interrelations of certain analytical and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Misra, R. Ecological Work Book; Oxford and IBH Publishing Company: Calcutta, India, 1968. [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, E. Aims and Methods of Vegetation Ecology; John Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- Saxena, A.K.; Singh, J.S. A phytosociological analysis of woody species in forest communities of a part of Kumaun Himalaya. Vegetatio 1982, 50, 3–22. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Ralhan, P.K.; Saxena, A.K.; Singh, J.S. Analysis of forest vegetation at and around Nainital in Kumaun Himalaya. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1982, 48, 121–137. [Google Scholar]
- Bhuyan, P.; Khan, M.L.; Tripathi, R.S. Tree diversity and population structure in undisturbed and human-impacted stands of tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India. Biodivers. Conserv. 2003, 12, 1753–1773. [Google Scholar] [CrossRef]
- Rawat, B.; Gairola, S.; Sekar, K.C.; Rawal, R.S. Community structure, regeneration potential and future dynamics of natural forest site in part of Nanda Devi Biosphere Reserve, Uttarakhand, India. Afr. J. Plant Sci. 2014, 8, 380–391. [Google Scholar] [CrossRef] [Green Version]
- Shankar, U. A case of high tree diversity in a Sal (Shorea robusta)-dominated lowland forest of Eastern Himalaya: Floristic composition, regeneration and conservation. Curr. Sci. 2001, 81, 776–786. [Google Scholar]
- FSI. Volume Equations for Forests of India, Nepal and Bhutan; Forest Survey of India, Ministry of Environment and Forests; Government of India: Dehradun, India, 1996.
- Dimri, S.; Baluni, P.; Sharma, C.M. biomass production and carbon storage potential of selected old-growth temperate forests in Garhwal Himalaya, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 1327–1333. [Google Scholar] [CrossRef]
- Brown, S.L.; Schroeder, P.; Kern, J.S. Spatial distribution of biomass in forests of the eastern USA. For. Ecol. Manag. 1999, 123, 81–90. [Google Scholar] [CrossRef]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Manhas, R.K.; Negi, J.D.S.; Kumar, R.; Chauhan, P.S. Temporal assessment of growing stock, biomass and carbon stock of Indian forests. Clim. Chang. 2006, 74, 191–221. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: Englewood Clift, NJ, USA, 1958. [Google Scholar]
- Piper, C. Soil and Plant Analysis; Adelaide University, Hassell Press: Adelaide, Australia, 1950. [Google Scholar]
- Walkley, A.; Black, C.A. An examination of Degtjareff methods for determining soil organic matter and a proposed modification of the chronic acid titration methods. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Peach, K.; Tracey, M. Modern Methods of Plant Analysis; Springer: Adelaide, Australia, 1956. [Google Scholar]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture Circular; U.S. Department of Agriculture: Washington, DC, USA, 1954.
- Kaushal, S.; Baishya, R. Stand structure and species diversity regulate biomass carbon stock under major Central Himalayan Forest types of India. Ecol. Process. 2021, 10, 14. [Google Scholar] [CrossRef]
- Sharma, C.M.; Tiwari, O.P.; Rana, Y.S.; Krishan, R.; Mishra, A.K. Elevational behaviour on dominance–diversity, regeneration, biomass and carbon storage in ridge forests of Garhwal Himalaya, India. For. Ecol. Manag. 2018, 424, 105–120. [Google Scholar] [CrossRef]
- Chhabra, A. Growing stock-based forest biomass estimate for India. Biomass Bioenergy 2002, 22, 187–194. [Google Scholar] [CrossRef]
- Gairola, S.; Sharma, C.M.; Ghildiyal, S.K.; Suyal, S. Live tree biomass and carbon variation along an altitudinal gradient in moist temperate valley slopes of the Garhwal Himalaya (India). Curr. Sci. 2011, 100, 1862–1870. [Google Scholar]
- Gandhi, D.S.; Sundarapandian, S. Large-scale carbon stock assessment of woody vegetation in tropical dry deciduous forest of Sathanur reserve forest, Eastern Ghats, India. Environ. Monit. Assess. 2017, 189, 187. [Google Scholar] [CrossRef]
- Sagar, R.; Verma, P. Effects of soil physical characteristics and biotic interference on the herbaceous community composition and species diversity on the campus of Banaras Hindu University, India. Environmentalist 2010, 30, 289–298. [Google Scholar] [CrossRef]
- Donkor, N.T.; Gedir, J.V.; Hudson, R.J.; Bork, E.W.; Chanasky, D.S.; Naeth, M.A. Impacts of grazing system on soil compaction and pasture production in Alberta. Can. J. Soil Sci. 2002, 82, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wang, Y.; Li, Y.; Cheng, H. Influences of alpine ecosystem responses to climatic change on soil properties on the Qinghai-Tibet Plateau, China. Catena 2007, 70, 506–514. [Google Scholar] [CrossRef]
- Gairola, S.; Sharma, C.M.; Ghildiyal, S.K.; Suyal, S. Chemical properties of soils in relation to forest composition in moist temperate valley slopes of Garhwal Himalaya, India. Environmentalist 2012, 32, 512–523. [Google Scholar] [CrossRef]
- Tomlinson, G.H.; Tomlinson, F.L. Effects of Acid Decomposition on the Forests of Europe and North America; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Hanawalt, R.B.; Whittaker, R.H. Altitudinally coordinated patterns of soils and vegetation in-the San Jacinto Mountains, California. Soil Sci. 1976, 121, 114–124. [Google Scholar] [CrossRef]
- Singh, S.P.; Adhikari, B.S.; Zobel, D.B. Biomass, productivity, leaf longevity, and forest structure in the Central Himalaya. Ecol. Monogr. 1994, 64, 401–421. [Google Scholar] [CrossRef]
- Tripathi, B.P. Review of acid soil and its management in Nepal; Lumle Seminar Paper; Pokhara: Kathamndu, Nepal, 1999; Volume 99, pp. 1–11. [Google Scholar]
- Hughes, R.F.; Kauffman, J.B.; Jaramillo, V.J. Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of Mexico. Ecology 1999, 80, 1892–1907. [Google Scholar]
- Thadani, R.; Ashton, P.M.S. Regeneration of banj oak (Quercus leucotrichophora A. Camus) in the central Himalaya. For. Ecol. Manag. 1995, 78, 217–224. [Google Scholar] [CrossRef]
- Thakur, U.; Bisht, N.S.; Kumar, A.; Kumar, M.; Sahoo, U.K. Regeneration potential of forest vegetation of Churdhar wildlife sanctuary of India: Implication of forest management. Water Air Soil Pollut. 2021, 232, 373. [Google Scholar] [CrossRef]
- Paudel, S.; Sah, J. Physicochemical characters of soil in tropical soil (Shorea robusta Gaertn.) forests in eastern Nepal. Himalayan J. Sci. 2003, 1, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Bargali, S.S.; Singh, R.P.; Joshi, M. Changes in soil characteristics in eucalypt plantations replacing natural broadleaved forests. J. Veg. Sci. 1993, 4, 25–28. [Google Scholar] [CrossRef]
- Bargali, S.S.; Singh, S.P.; Singh, R.P. Patterns of weight loss and nutrient release from decomposing leaf litter in an age series of Eucalypt plantations. Soil Biol. Biochem. 1993, 25, 1731–1738. [Google Scholar] [CrossRef]
- Baumler, R. Soils. In Nepal: An Introduction to the Natural History, Ecology and Human Environment in the Himalayas—A Companion to the Flora of Nepal; Miehe, S., Pendry, C.A., Eds.; The Royal Botanical Garden Edinburgh: Edinburgh, UK, 2015. [Google Scholar]
- Manral, V.; Bargali, K.; Bargali, S.S.; Shahi, C. Changes in soil biochemical properties following replacement of Banj oak forest with Chir pine in Central Himalaya, India. Ecol. Process. 2020, 9, 30. [Google Scholar] [CrossRef]
- Tanner, E.V.J.; Vitousek, P.A.; Cuevas, E. Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 1998, 79, 10–22. [Google Scholar] [CrossRef]
- Duan, Z.; Xiao, H.; Li, X.; Dong, Z.; Wang, G. Evolution of soil properties on stabilized sands in the Tengger Desert, China. Geomorphology 2004, 59, 237–246. [Google Scholar]
- Farley, K.A.; Kelly, E.F. Effects of afforestation of a Paramo grassland on soil nutrient status. For. Ecol. Manag. 2004, 195, 281–290. [Google Scholar] [CrossRef]
- Padalia, K.; Bargali, S.S.; Bargali, K.; Khulbe, K. Microbial biomass carbon and nitrogen in relation to cropping systems in Central Himalaya, India. Curr. Sci. 2018, 115, 1741–1750. [Google Scholar] [CrossRef]
- Gupta, M.K.; Sharma, S.D. Effect of tree plantation on soil properties, profile morphology and productivity index I. Poplar in Uttarakhand. Ann. For. 2009, 16, 209–224. [Google Scholar]
- Kumar, S.; Ghotekar, Y.S.; Dadhwal, V.K. C-equivalent correction factor for soil organic carbon inventory by wet oxidation, dry combustion and loss on ignition methods in Himalayan region. J. Earth Syst. Sci. 2019, 128, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.J.; Liu, S.L.; Ma, K.M.; Zhu, Y.G. Relationship between soil characteristics, topography and plant diversity in a heterogeneous deciduous broad-leaved forest near Beijing, China. Plant Soil 2004, 261, 47–54. [Google Scholar] [CrossRef]
- Wilde, S.A. Forest Soils and Forest Growth; Periodical Experts Book Agency: New Delhi, India, 1946. [Google Scholar]
- Martin, D.; Lal, T.; Sachdev, C.B.; Sharma, J.P. Soil organic carbon storage changes with climate change, landform and land use conditions in Garhwal hills of the Indian Himalayan mountains. Agric. Ecosyst. Environ. 2010, 138, 64–73. [Google Scholar] [CrossRef]
- Rawat, Y.S.; Singh, J.S. Structure and Function of Oak Forests in Central Himalaya. I. Dry Matter Dynamics. Ann. Bot. 1988, 62, 397–411. [Google Scholar] [CrossRef]
- Singh, P. Climate Change Response on Tree Phenology of Major Tree Species in the Central Himalaya. Ph.D. thesis, Kumaun University, Nainital, Uttarakhand, India, 2019.
- Joshi, V.C.; Negi, V.S.; Bisht, D.; Sundriyal, R.C.; Arya, D. Tree biomass and carbon stock assessment of subtropical and temperate forests in the Central Himalaya, India. Trees For. People 2021, 6, 100–147. [Google Scholar] [CrossRef]
- Rana, B.S.; Singh, R.P.; Singh, S.P. Carbon and energy dynamics of seven Central Himalayan forests. Trop. Ecol. 1989, 30, 253–269. [Google Scholar]
- Adhikari, B.S.; Rawat, Y.S.; Singh, S.P. Structure and function of high-altitude forest of Central Himalaya I. Dry Matter Dynamics. Ann. Bot. 1995, 75, 237–248. [Google Scholar] [CrossRef]
- Sharma, C.M.; Baduni, N.P.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Effects of slope aspects on forest compositions, community structures and soil properties in natural temperate forests of Garhwal Himalaya. J. For. Res. 2010, 21, 331–337. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, C.M.; Rajwar, G.S. Physico-chemical properties of forest soil along altitudinal gradient in Garhwal Himalaya. J. Hill Res. 2004, 17, 60–64. [Google Scholar]
- Nazir, T. Estimation of Site Quality of Important Temperate Forest Cover on the Basis of Soil Nutrient and Growing Stock in Garhwal Himalaya. Ph.D. Thesis, HNB Garhwal University, Srinagar (Garhwal), Uttarakhand, India, 2009. [Google Scholar]
- Semwal, S. Studies on Phytosociology, Diversity Patterns and Competition along an Altitudinal Gradient in a Part of Lesser Himalaya in Garhwal, Uttaranchal. Ph.D. Thesis, HNB Garhwal University, Srinagar, India, 2006. [Google Scholar]
- Chavan, S.B.; Kumar, N.; Uthappa, A.R.; Keerthika, A.; Handa, A.K.; Sridhar, K.B.; Singh, M.; Kumar, D.; Ram, N. Tree Management Practices in Agroforestry. In Forests, Climate Change and Biodiversity; ICAR: New Delhi, India, 2017; pp. 87–101. [Google Scholar]










| Forest Types * | Altitude (m) | Dominant Tree Species (IVI) | Area (km2) |
|---|---|---|---|
| Banj oak (12/C1a) | 1700–2100 | Q. leucotrichophora (179.3), R. arboreum (52.5), others (68.2) | 46.3 |
| Rianj oak | 1900–2200 | Q. lanuginosa (140.2), L. ovalifolia (64.2), others (95.6) | 19.6 |
| Moru oak (12/C1b) | 2300–2600 | Q. floribunda (101.3), P. duthiei (48.9), others (149.8) | 61.9 |
| Kharsu oak (12/C2a) | 2600–3000 | Q. semecarpifolia (195.1), R. arboreum (58.9), others (46.0) | 59.5 |
| Forest Type | Sites | Altitude (m) | Basal Area (m2 ha−1) | Density (indi. ha−1) | Diversity (H) | Richness (R) | Regeneration | Disturbance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | T | Sa | Se | T | Sa | Se | T | Sa | Se | |||||
| Banj oak | B1 | 1700 | 34.2 | 620 | 400 | 80 | 0.51 | 1.19 | 0.00 | 5 | 4 | 1 | No | High |
| B2 | 1800 | 46.7 | 610 | 400 | 280 | 1.36 | 0.90 | 0.60 | 8 | 3 | 2 | Poor | Moderate | |
| B3 | 2100 | 21.6 | 650 | 480 | 160 | 1.20 | 1.24 | 0.00 | 4 | 4 | 1 | No | Moderate | |
| Rianj oak | R1 | 1900 | 26.2 | 910 | 760 | 720 | 1.34 | 1.59 | 0.45 | 4 | 5 | 2 | Poor | High |
| R2 | 2100 | 56.0 | 450 | 400 | 80 | 1.24 | 1.28 | 0.00 | 4 | 4 | 1 | Poor | High | |
| R3 | 2200 | 46.2 | 1000 | 480 | 1840 | 1.25 | 1.29 | 1.53 | 5 | 4 | 5 | Fair | Moderate | |
| Moru oak | M1 | 2300 | 40.1 | 610 | 640 | 400 | 1.86 | 1.55 | 0.00 | 10 | 6 | 1 | Poor | Moderate |
| M2 | 2400 | 74.5 | 660 | 680 | 640 | 1.39 | 1.18 | 0.00 | 7 | 4 | 1 | Poor | Moderate | |
| M3 | 2600 | 60.2 | 700 | 360 | 240 | 1.50 | 1.27 | 0.00 | 8 | 4 | 1 | Poor | Moderate | |
| Kharsu oak | K1 | 2600 | 21.9 | 540 | 520 | 40 | 0.52 | 1.33 | 0.00 | 4 | 4 | 1 | Poor | Moderate |
| K2 | 2700 | 71.7 | 460 | 800 | 360 | 0.86 | 1.31 | 0.00 | 3 | 4 | 1 | Poor | High | |
| K3 | 3000 | 73.3 | 460 | 960 | 40 | 0.98 | 1.05 | 0.00 | 3 | 3 | 1 | Poor | Moderate | |
| Forest Type | Sites | GSV (m³ ha−1) | AGB (Mg ha−1) | BGB (Mg ha−1) | TB (Mg ha−1) | LF (Mg ha−1 year−1) | AGC (Mg C ha−1) | BGC (Mg ha−1) | TC (Mg C ha−1) |
|---|---|---|---|---|---|---|---|---|---|
| Banj oak | B1 | 227.6 | 294.2 | 77.2 | 371.4 | 2.96 | 132.4 | 34.7 | 167.1 |
| B2 | 388.1 | 497.2 | 130.2 | 627.5 | 3.94 | 223.8 | 58.6 | 282.4 | |
| B3 | 100.1 | 204.6 | 58.4 | 263.0 | 5.39 | 92.1 | 26.3 | 118.3 | |
| Rianj oak | R1 | 193.6 | 310.7 | 84.3 | 395.0 | 5.29 | 139.8 | 37.9 | 177.8 |
| R2 | 647.1 | 592.9 | 143.3 | 736.2 | 6.40 | 266.8 | 64.5 | 331.3 | |
| R3 | 487.0 | 523.7 | 131.8 | 655.5 | 5.54 | 235.7 | 59.3 | 295.0 | |
| Moru oak | M1 | 335.3 | 597.4 | 165.4 | 762.8 | 4.44 | 268.8 | 74.5 | 343.3 |
| M2 | 731.0 | 715.4 | 177.0 | 892.4 | 6.10 | 321.9 | 79.6 | 401.6 | |
| M3 | 649.2 | 787.3 | 201.8 | 989.1 | 6.28 | 354.3 | 90.8 | 445.1 | |
| Kharsu oak | K1 | 188.5 | 267.5 | 71.5 | 339.0 | 5.09 | 120.4 | 32.2 | 152.5 |
| K2 | 683.7 | 616.5 | 147.4 | 763.9 | 6.98 | 277.4 | 66.3 | 343.8 | |
| K3 | 526.2 | 565.9 | 139.4 | 705.2 | 7.28 | 254.7 | 62.7 | 317.4 |
| FT | Sites | Mo | WHC | BD | pH | OC | N | P | K | SOM | SCS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (g cm−3) | (%) | (%) | (Kg ha−1) | (Kg ha−1) | (%) | (Mg C ha−1) | |||
| Banj oak | B1 | 30.5 ± 1.6 | 41.0 ± 1.8 | 0.68 ± 0.06 | 6.01 ± 0.07 | 2.62 ± 0.1 | 0.24 ± 0.03 | 12.3 ± 1.2 | 103.6 ± 17.9 | 3.0 ± 0.3 | 17.7 ± 1.0 |
| B2 | 29.7 ± 2.2 | 37.7 ± 3.0 | 0.64 ± 0.05 | 5.76 ± 0.17 | 2.92 ± 0.1 | 0.22 ± 0.02 | 18.1 ± 1.3 | 110.1 ± 26.4 | 3.6 ± 0.3 | 18.5 ± 1.3 | |
| B3 | 26.6 ± 1.3 | 34.5 ± 1.5 | 0.59 ± 0.08 | 6.41 ± 0.07 | 3.12 ± 0.1 | 0.19 ± 0.01 | 13.9 ± 1.5 | 112.0 ± 20.1 | 3.7 ± 0.2 | 18.3 ± 1.8 | |
| Rianj oak | R1 | 29.3 ± 1.4 | 38.5 ± 1.3 | 0.66 ± 0.06 | 6.03 ± 0.21 | 2.70 ± 0.1 | 0.25 ± 0.02 | 12.5 ± 1.2 | 110.0 ± 9.5 | 4.7 ± 0.1 | 19.5 ± 0.5 |
| R2 | 27.6 ± 2.7 | 38.0 ± 1.1 | 0.63 ± 0.06 | 5.70 ± 0.17 | 2.95 ± 0.1 | 0.26 ± 0.02 | 18.4 ± 1.9 | 132.5 ± 14.8 | 5.1 ± 0.1 | 19.1 ± 1.5 | |
| R3 | 28.0 ± 1.5 | 35.6 ± 2.3 | 0.42 ± 0.06 | 5.68 ± 0.19 | 2.87 ± 0.1 | 0.28 ± 0.02 | 19.0 ± 0.9 | 112.0 ± 11.6 | 4.9 ± 0.2 | 18.3 ± 1.1 | |
| Moru oak | M1 | 23.8 ± 1.3 | 36.3 ± 2.3 | 0.68 ± 0.03 | 6.53 ± 0.10 | 2.85 ± 0.1 | 0.25 ± 0.02 | 11.0 ± 1.6 | 146.5 ± 19.7 | 3.2 ± 0.2 | 19.2 ± 0.5 |
| M2 | 22.9 ± 1.5 | 33.6 ± 2.0 | 0.66 ± 0.04 | 5.89 ± 0.09 | 2.97 ± 0.1 | 0.27 ± 0.02 | 12.5 ± 1.7 | 180.0 ± 17.8 | 3.5 ± 0.2 | 19.4 ± 0.3 | |
| M3 | 20.2 ± 1.2 | 24.0 ± 2.3 | 0.62 ± 0.05 | 6.24 ± 0.17 | 3.11 ± 0.2 | 0.30 ± 0.03 | 14.4 ± 1.9 | 203.7 ± 22.0 | 3.7 ± 0.4 | 19.1 ± 0.4 | |
| Kharsu oak | K1 | 21.6 ± 0.9 | 40.6 ± 4.9 | 0.71 ± 0.04 | 6.31 ± 0.07 | 2.83 ± 0.1 | 0.28 ± 0.02 | 10.5 ± 0.4 | 219.8 ± 16.4 | 3.2 ± 0.2 | 20.0 ± 0.4 |
| K2 | 20.3 ± 1.1 | 40.2 ± 0.9 | 0.68 ± 0.05 | 6.20 ± 0.06 | 3.51 ± 0.4 | 0.31 ± 0.02 | 12.2 ± 1.1 | 246.8 ± 29.4 | 4.4 ± 0.7 | 23.6 ± 1.4 | |
| K3 | 17.4 ± 1.9 | 39.1 ± 4.9 | 0.63 ± 0.05 | 6.15 ± 0.13 | 3.52 ± 0.3 | 0.34 ± 0.01 | 10.6 ± 1.4 | 324.6 ± 21.8 | 4.4 ± 0.5 | 21.9 ± 1.0 |
| Alt | TD | TBA | TH | Mo | WHC | pH | OC | N | P | K | SOM | D | TC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alt | ||||||||||||||
| TD | ||||||||||||||
| TBA | ||||||||||||||
| TH | ||||||||||||||
| Mo | −0.61 * | |||||||||||||
| WHC | −0.60 * | |||||||||||||
| pH | ||||||||||||||
| OC | 0.77 * | 0.66 * | ||||||||||||
| N | 0.81 * | −0.60 * | 0.60 * | 0.65 * | ||||||||||
| P | −0.61 * | |||||||||||||
| K | 0.94 ** | 0.59 * | −0.67 * | 0.75 * | 0.77 * | |||||||||
| SOM | 0.66* | −0.59 * | 0.68 * | 0.97 ** | 0.67 * | |||||||||
| D | −0.58 * | −0.59 * | ||||||||||||
| TC | 0.84 ** | 0.40 * | −0.65 * |
| Forest Type | Altitude (m) | TB (Mg ha−1) | TC (Mg C ha−1) | Reference |
|---|---|---|---|---|
| Banj oak | 1200–2300 | 391–433 | 176.0–194.9 * | [9] |
| 1950 | 387.3 | 174.3 * | [74] | |
| 1600–2100 | 200.1 | 92.0 | [21] | |
| 1500–1650 | 215.5 | 107.8 | [47] | |
| 1800 | 317–319 | 149.0 | [75] | |
| 1750–1950 | 230.12 | 109.3 | [76] | |
| 1750–2200 | 420.6 ± 108.1 | 189.3 ± 48.6 | Present study | |
| Rianj oak | 1800–2400 | 294–562 | 132.3–252.9 * | [9] |
| 2240 | 285.3 | 128.4 * | [74] | |
| 2150 | 557 | 261.8 | [77] | |
| 2050–2250 | 227.23 | 107.9 | [76] | |
| 1900–2200 | 595.6 ± 103.0 | 268.0 ± 46.3 | Present study | |
| Moru oak | 2100–2700 | 467–787 | 210.2–354.2 * | [9] |
| 2194 | 458.5 | 206.3 * | [74] | |
| 2200 | 782.0 | 367.5 | [77] | |
| 2300–2600 | 292.4 | 134.5 | [21] | |
| 2550–2650 | 429.7 | 214.8 | [47] | |
| 2100–2750 | 588.5 | 276.6 | [35] | |
| 2300–2500 | 881.4 ± 65.6 | 396.6 ± 29.5 | Present study | |
| Kharsu oak | 2650 | 590.2 | 265.5 * | [78] |
| 2500–3000 | 279.31 | 128.5 | [21] | |
| 2650–2850 | 389.5 | 194.7 | [47] | |
| 2100–2750 | 522.34 | 245.5 | [35] | |
| 2600–3000 | 602.7 ± 132.9 | 271.2 ± 59.8 | Present study.; |
| FT | Altitude (m) | pH | OC (%) | N (%) | P (Kg ha−1) | K (Kg ha−1) | SOM (%) |
|---|---|---|---|---|---|---|---|
| Banj oak | 1500–1650 a | 5.50 | 2.44 | 0.17 | 5.75 * | 40.67 * | 4.12 |
| 1600–2100 b | 5.81–6.37 | 0.42–2.31 | 0.07–0.25 | 4.11–6.53 * | 66.89–139.59 * | 0.72–3.99 | |
| 1600–2100 c | 5.5–6.2 | 1.9–2.5 | 0.16–0.21 | 11.5–31.9 | 86.1–603.8 | - | |
| 1700–1850 d | 5.9–6.3 | 0.87–1.01 | 0.08–0.09 | 13.6–15.5 | 180.9–215.7 | - | |
| 1900–2400 e | 5.4–5.7 | 1.3–1.9 | 0.10–0.20 | 9.3–12.0 | 153.2–408.8 | - | |
| Montane f | - | 1.88–4.00 | 0.17–0.30 | - | - | - | |
| Up to 2000 g | 5.37–6.63 | 2.21–3.58 | 0.12–0.30 | 0.02–0.07 ** | - | 2.93–4.74 | |
| 1700–2100 p | 5.76–6.41 | 2.62–3.12 | 0.19–0.24 | 12.3–18.1 | 103.6–112.0 | 2.97–3.71 | |
| Rianj oak | 1900–2200 p | 5.68–6.03 | 2.70–2.95 | 0.25–0.28 | 12.5–19.0 | 110.0–132.5 | 4.70–5.10 |
| Moru oak | 2300–2600 c | 5.9–6.1 | 1.6–2.2 | 0.14–0.17 | 13.8–23.2 | 356.7–712.0 | - |
| 2550–2650 a | 6.13 | 2.70 | 0.26 | 5.30 * | 129.17 * | 4.65 | |
| 2300–2600 p | 5.89–6.53 | 2.85–3.11 | 0.25–0.30 | 11.0–14.4 | 146.5–203.7 | 3.24–3.68 | |
| Kharsu oak | 2500–3000 c | 5.8–6.7 | 2.3–2.6 | 0.19–0.22 | 7.2–14.3 | 72.7–135.1 | - |
| 2650–2850 a | 6.67 | 3.56 | 0.34 | 8.33 * | 261.17 * | 6.15 | |
| 2600–3000 p | 6.15–6.31 | 2.83–3.52 | 0.28–0.34 | 10.5–12.2 | 219.8–324.6 | 3.20–4.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisht, S.; Bargali, S.S.; Bargali, K.; Rawat, G.S.; Rawat, Y.S.; Fartyal, A. Influence of Anthropogenic Activities on Forest Carbon Stocks—A Case Study from Gori Valley, Western Himalaya. Sustainability 2022, 14, 16918. https://doi.org/10.3390/su142416918
Bisht S, Bargali SS, Bargali K, Rawat GS, Rawat YS, Fartyal A. Influence of Anthropogenic Activities on Forest Carbon Stocks—A Case Study from Gori Valley, Western Himalaya. Sustainability. 2022; 14(24):16918. https://doi.org/10.3390/su142416918
Chicago/Turabian StyleBisht, Soni, Surendra Singh Bargali, Kiran Bargali, Gopal Singh Rawat, Yashwant Singh Rawat, and Archana Fartyal. 2022. "Influence of Anthropogenic Activities on Forest Carbon Stocks—A Case Study from Gori Valley, Western Himalaya" Sustainability 14, no. 24: 16918. https://doi.org/10.3390/su142416918
APA StyleBisht, S., Bargali, S. S., Bargali, K., Rawat, G. S., Rawat, Y. S., & Fartyal, A. (2022). Influence of Anthropogenic Activities on Forest Carbon Stocks—A Case Study from Gori Valley, Western Himalaya. Sustainability, 14(24), 16918. https://doi.org/10.3390/su142416918








