Ubiquitous Occurrence of a Biogenic Sulfonate in Marine Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Determination of Biogeochemistry
2.3. Metabolite Extraction
2.4. Targeted Metabolomics Method
2.5. Statistical Analysis
3. Results and Discussion
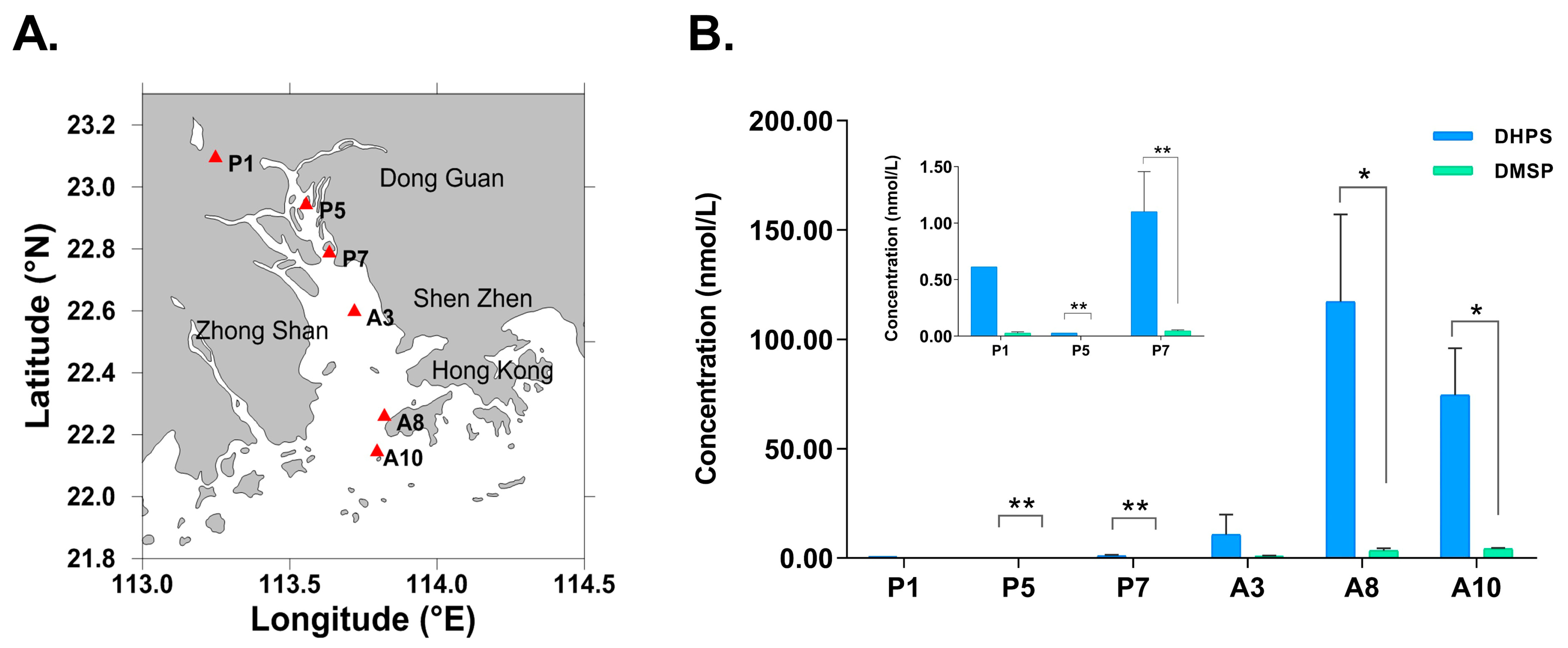
3.1. Distribution of DHPS and DMSP in the Pearl River Estuary
3.2. Dynamics of DHPS and DMSP Concentrations in a Coastal Mesocosm Experiment
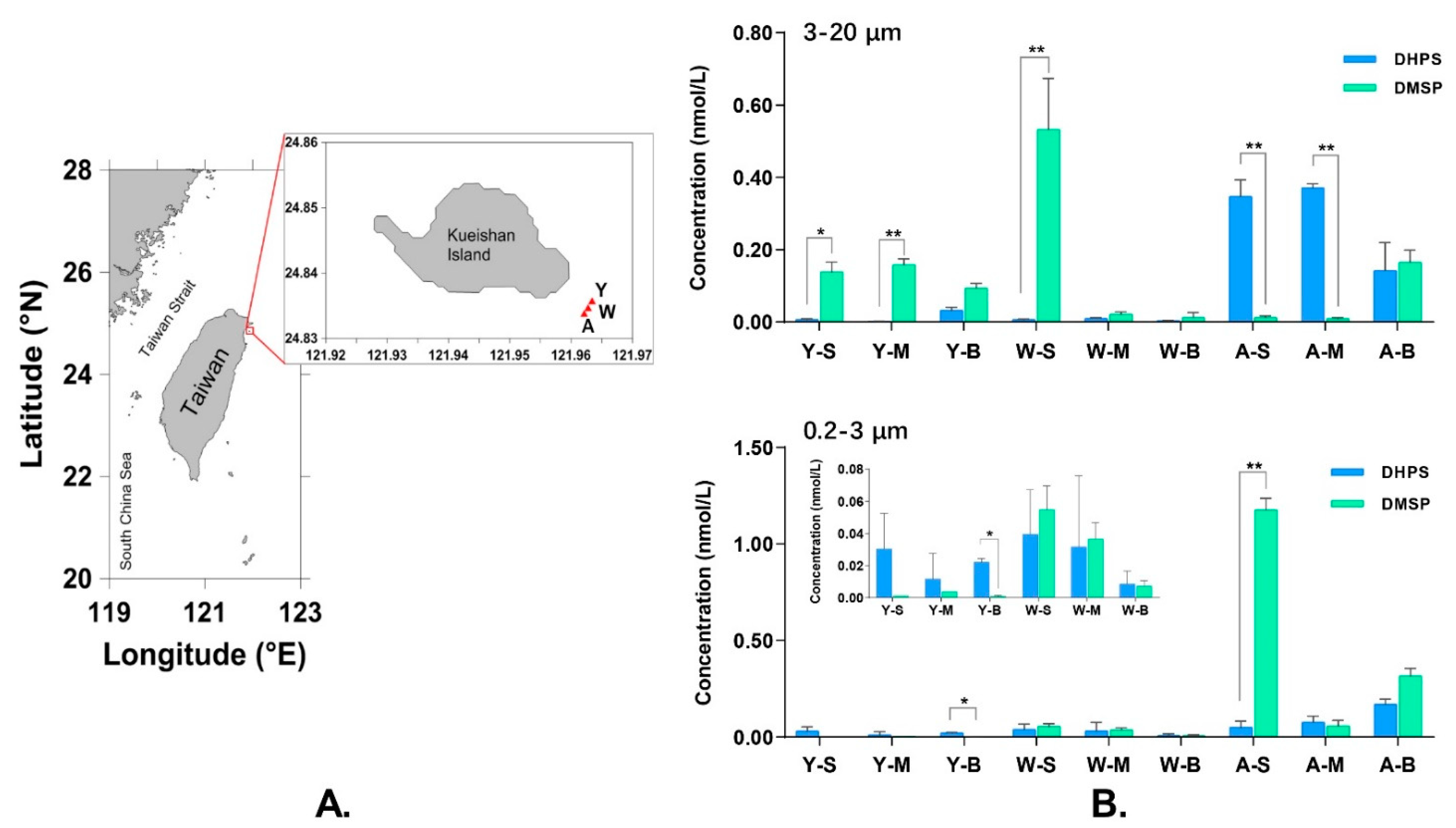
3.3. Occurrence of DHPS and DMSP in a Shallow-Sea Hydrothermal System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, J.; Kroneck, P.M. Microbial sulfite respiration. Adv. Microb. Physiol. 2013, 62, 45–117. [Google Scholar] [PubMed]
- Moran, M.A.; Durham, B.P. Sulfur metabolites in the pelagic ocean. Nat. Rev. Microbiol. 2019, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Tang, K. Chemical diversity and biochemical transformation of biogenic organic sulfur in the ocean. Front. Mar. Sci. 2020, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Durham, B.P.; Boysen, A.K.; Carlson, L.T.; Groussman, R.D.; Heal, K.R.; Cain, K.R.; Morales, R.L.; Coesel, S.N.; Morris, R.M.; Ingalls, A.E.; et al. Sulfonate-based networks between eukaryotic phytoplankton and heterotrophic bacteria in the surface ocean. Nat. Microbiol. 2019, 4, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Durham, B.P.; Dearth, S.P.; Sharma, S.; Amin, S.A.; Smith, C.B.; Campagna, S.R.; Armbrust, E.V.; Moran, M.A. Recognition cascade and metabolite transfer in a marine bacteria-phytoplankton model system. Environ. Microbiol. 2017, 19, 3500–3513. [Google Scholar] [CrossRef]
- Dawson, H.M.; Heal, K.R.; Boysen, A.K.; Carlson, L.T.; Ingalls, A.E.; Young, J.N.; Helmig, D.; Arrigo, K. Potential of temperature- and salinity-driven shifts in diatom compatible solute concentrations to impact biogeochemical cycling within sea ice. Elem.-Sci. Anthrop. 2020, 8, 25. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Gao, X.; Dai, X.; Han, Y.; Chen, Q.; Tang, K. Metabolism of chiral sulfonate compound 2,3-dihydroxypropane-1-sulfonate (DHPS) by Roseobacter bacteria in marine environment. Environ. Int. 2021, 157, 106829. [Google Scholar] [CrossRef]
- Landa, M.; Burns, A.S.; Durham, B.P.; Esson, K.; Nowinski, B.; Sharma, S.; Vorobev, A.; Nielsen, T.; Kiene, R.P.; Moran, M.A. Sulfur metabolites that facilitate oceanic phytoplankton-bacteria carbon flux. ISME J. 2019, 13, 2536–2550. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, J.; Liu, J.; Yang, G.; Xue, C.X.; Curson, A.R.J.; Todd, J.D. Biogenic production of DMSP and its degradation to DMS—Their roles in the global sulfur cycle. Sci. China Life Sci. 2019, 62, 1296–1319. [Google Scholar] [CrossRef]
- Longnecker, K.; Sievert, S.M.; Sylva, S.P.; Seewald, J.S.; Kujawinski, E.B. Dissolved organic carbon compounds in deep-sea hydrothermal vent fluids from the east pacific rise at 9°50′N. Org. Geochem. 2018, 125, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Gotz, F.; Longnecker, K.; Kido Soule, M.C.; Becker, K.W.; McNichol, J.; Kujawinski, E.B.; Sievert, S.M. Targeted metabolomics reveals proline as a major osmolyte in the chemolithoautotroph Sulfurimonas denitrificans. MicrobiologyOpen 2018, 7, e00586. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.G.; Gebruk, A.V.; Mironov, A.N.; Moskalev, L.I. Deep-sea and shallow-water hydrothermal vent communities: Two different phenomena? Chem. Geol. 2005, 224, 5–39. [Google Scholar] [CrossRef]
- Tarasov, V.G. Effects of shallow-water hydrothermal venting on biological communities of coastal marine ecosystems of the western Pacific. Adv. Mar. Biol. 2006, 50, 267–421. [Google Scholar]
- Dai, M.; Lifang, W.; Guo, X.; Zhai, W.; Li, Q.; He, B.; Kao, S.-J.I. Nitrification and inorganic nitrogen distribution in a large perturbed river/estuarine system: The Pearl River Estuary, China. Biogeosciences 2008, 5, 1227–1244. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.S.; Lin, H.T.; Wang, B.S.; Huang, W.J.; Lin, L.H.; Tsai, A.Y. Intense but variable autotrophic activity in a rapidly flushed shallow-water hydrothermal plume (Kueishantao islet, Taiwan). Geobiology 2020, 19, 87–101. [Google Scholar] [CrossRef]
- Dittmar, T.; Koch, B.; Hertkorn, N.; Kattner, G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater: SPE-DOM from seawater. Limnol. Oceanogr Methods 2008, 6, 230–235. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef]
- Chen, M.; Jia, H.; Su, W.; Zhang, S.; Zhao, K. Structural characteristics and associated factors influencing phytoplankton abundance and species composition in Huangmaohai bay, Pearl River Estuary. J. Coast. Res. 2019, 35, 72–81. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Wang, Y.-S.; Cheng, H.; Sun, C.-C.; Wu, M.-L. Variation of phytoplankton community structure from the Pearl River Estuary to South China Sea. Ecotoxicology 2015, 24, 1442–1449. [Google Scholar] [CrossRef]
- Zong, Y.; Kemp, A.C.; Yu, F.; Lloyd, J.M.; Huang, G.; Yim, W.W.S. Diatoms from the Pearl River Estuary, China and their suitability as water salinity indicators for coastal environments. Mar. Micropaleontol. 2010, 75, 38–49. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U.; Czerny, J.; von Bröckel, K.; Boxhammer, T.; Büdenbender, J.; Deckelnick, M.; Fischer, M.; Hoffmann, D.; Krug, S.A.; Lentz, U.; et al. Technical note: A mobile sea-going mesocosm system—New opportunities for ocean change research. Biogeosciences 2013, 10, 1835–1847. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Sun, J.; Yang, Y.; Jiang, X.; Wang, Z.; Song, X.; Wang, T.; Zhang, D.; Li, H.; Yi, X.; et al. Elevated pCO2 impedes succession of phytoplankton community from diatoms to dinoflagellates along with increased abundance of viruses and bacteria. Front. Mar. Sci. 2021, 8, 642208. [Google Scholar] [CrossRef]
- Spilling, K.; Olli, K.; Lehtoranta, J.; Kremp, A.; Tedesco, L.; Tamelander, T.; Klais, R.; Peltonen, H.; Tamminen, T. Shifting diatom—Dinoflagellate dominance during spring bloom in the Baltic Sea and its potential effects on biogeochemical cycling. Front. Mar. Sci. 2018, 5, 327. [Google Scholar] [CrossRef] [Green Version]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef] [Green Version]
- Nowinski, B.; Motard-Cote, J.; Landa, M.; Preston, C.M.; Scholin, C.A.; Birch, J.M.; Kiene, R.P.; Moran, M.A. Microdiversity and temporal dynamics of marine bacterial dimethylsulfoniopropionate genes. Environ. Microbiol. 2019, 21, 1687–1701. [Google Scholar] [CrossRef]
- Kiene, R.P.; Nowinski, B.; Esson, K.; Preston, C.; Marin, R.; Birch, J.; Scholin, C.; Ryan, J.; Moran, M.A. Unprecedented DMSP concentrations in a massive dinoflagellate bloom in Monterey Bay, CA. Geophys. Res. Lett. 2019, 46, 12279–12288. [Google Scholar] [CrossRef]
- Han, Y.; Jiao, N.; Zhang, Y.; Zhang, F.; He, C.; Liang, X.; Cai, R.; Shi, Q.; Tang, K. Opportunistic bacteria with reduced genomes are effective competitors for organic nitrogen compounds in coastal dinoflagellate blooms. Microbiome 2021, 9, 71. [Google Scholar] [CrossRef]
- Denger, K.; Weiss, M.; Felux, A.K.; Schneider, A.; Mayer, C.; Spiteller, D.; Huhn, T.; Cook, A.M.; Schleheck, D. Sulphoglycolysis in Escherichia coli k-12 closes a gap in the biogeochemical sulphur cycle. Nature 2014, 507, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Chivers, W.J.; Walne, A.W.; Hays, G.C. Mismatch between marine plankton range movements and the velocity of climate change. Nat. Commun. 2017, 8, 14434. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.Y.; Cheung, S.; Mak, J.; Liu, K.; Xia, X.; Zhang, X.; Yung, Y.; Liu, H. Distinct interaction effects of warming and anthropogenic input on diatoms and dinoflagellates in an urbanized estuarine ecosystem. Glob. Chang. Biol. 2021, 27, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.A.; Zeng, Z.; Kuo, F.-W.; Yang, T.F.; Wang, B.-J.; Tu, Y.-Y. Tide-influenced acidic hydrothermal system offshore NE Taiwan. Chem. Geol. 2005, 224, 69–81. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, C.-T.A.; Yin, X.; Zhang, X.; Wang, X.; Zhang, G.; Wang, X.; Chen, D. Origin of native sulfur ball from the Kueishantao hydrothermal field offshore northeast Taiwan: Evidence from trace and rare earth element composition. J. Asian Earth Sci. 2011, 40, 661–671. [Google Scholar] [CrossRef]
- Price, R.E.; Giovannelli, D. Marine shallow-water hydrothermal vents: Geochemistry. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, England, 2019; Volume 4, pp. 346–352. [Google Scholar]
- Tang, K.; Zhang, Y.; Lin, D.; Han, Y.; Chen, C.A.; Wang, D.; Lin, Y.S.; Sun, J.; Zheng, Q.; Jiao, N. Cultivation-independent and cultivation-dependent analysis of microbes in the shallow-sea hydrothermal system off Kueishantao island, Taiwan: Unmasking heterotrophic bacterial diversity and functional capacity. Front. Microbiol. 2018, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Teeling, H.; Fuchs, B.M.; Becher, D.; Klockow, C.; Gardebrecht, A.; Bennke, C.M.; Kassabgy, M.; Huang, S.; Mann, A.J.; Waldmann, J.; et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 2012, 336, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Liu, K.; Jiao, N.; Zhang, Y.; Chen, C.T. Functional metagenomic investigations of microbial communities in a shallow-sea hydrothermal system. PLoS ONE 2013, 8, e72958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, Z.; Chen, C.T.; Tang, K.; Su, J.; Jiao, N. Sulfur metabolizing microbes dominate microbial communities in andesite-hosted shallow-sea hydrothermal systems. PLoS ONE 2012, 7, e44593. [Google Scholar] [CrossRef] [Green Version]
- Johnson, W.M.; Kido Soule, M.C.; Kujawinski, E.B. Extraction efficiency and quantification of dissolved metabolites in targeted marine metabolomics. Limnol. Oceanogr. Methods 2017, 15, 417–428. [Google Scholar] [CrossRef] [Green Version]

| Factors | DHPS | DMSP |
|---|---|---|
| Chl a | 0.143 | 0.086 |
| Temperature | −0.093 | −0.309 |
| pH | 0.886 * | 0.943 ** |
| DO | 0.943 ** | 0.886 * |
| Salinity | 0.886 * | 0.943 ** |
| NO2-N | −0.886 * | −0.943 ** |
| NO3-N | −0.943 ** | −1.000 ** |
| NH4-N | −0.600 | −0.657 |
| DIN | −0.886 * | −0.943 ** |
| DSi | −0.886 * | −0.943 ** |
| TSM | −0.657 | −0.600 |
| Factors | P1 | P5 | P7 | A3 | A8 | A10 |
|---|---|---|---|---|---|---|
| Chl a (μg/L) | 42.99 | 2.61 | 2.05 | 2.19 | 6.12 | 4.98 |
| Temperature (°C) | 20.4 | 20.3 | 20.1 | 20.1 | 20.4 | 20.1 |
| pH | 6.98 | 7.11 | 7.37 | 7.74 | 8.24 | 8.30 |
| DO (%) | 25.0 | 44.0 | 62.0 | 81.4 | 103.8 | 101.4 |
| Salinity (psu) | 0.2 | 2.4 | 7.0 | 15.3 | 28.3 | 30.7 |
| NO2-N (μM) | 35.26 | 14.76 | 13.64 | 7.74 | 1.30 | 0.44 |
| NO3-N (μM) | 175.53 | 204.39 | 135.22 | 88.23 | 16.61 | 3.21 |
| NH4-N (μM) | 417.38 | 22.68 | 36.55 | 26.44 | 4.63 | 1.85 |
| DIN (μM) | 628.17 | 241.82 | 185.41 | 122.41 | 22.53 | 5.50 |
| DSi (μM) | 194.45 | 177.98 | 164.87 | 101.93 | 12.72 | 3.12 |
| TSM (mg/L) | 42.3 | 44.6 | 21.2 | 41.4 | 24.5 | 28.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Han, Y.; Chen, Q.; Lin, H.; Lin, S.; Wang, D.; Tang, K. Ubiquitous Occurrence of a Biogenic Sulfonate in Marine Environment. Sustainability 2022, 14, 1240. https://doi.org/10.3390/su14031240
Chen X, Han Y, Chen Q, Lin H, Lin S, Wang D, Tang K. Ubiquitous Occurrence of a Biogenic Sulfonate in Marine Environment. Sustainability. 2022; 14(3):1240. https://doi.org/10.3390/su14031240
Chicago/Turabian StyleChen, Xiaofeng, Yu Han, Quanrui Chen, Huaying Lin, Shanshan Lin, Deli Wang, and Kai Tang. 2022. "Ubiquitous Occurrence of a Biogenic Sulfonate in Marine Environment" Sustainability 14, no. 3: 1240. https://doi.org/10.3390/su14031240
APA StyleChen, X., Han, Y., Chen, Q., Lin, H., Lin, S., Wang, D., & Tang, K. (2022). Ubiquitous Occurrence of a Biogenic Sulfonate in Marine Environment. Sustainability, 14(3), 1240. https://doi.org/10.3390/su14031240







