3.1. Chemical Composition of Spent Coffee Grounds
To use spent coffee grounds as a soil improving or plant growth-promoting substance, the concentration of humic substances in the SCG was determined. To perform the analysis, dried SCG with 5.83 ± 0.04 wt% moisture content was used. The study showed that the concentration of humic substances, soluble in 0.1 M sodium hydroxide solution, was equal to 2.00 ± 0.09 wt%. According to the studies presented by the other researchers, low concentrations of humic substances (that are soluble in alkaline solution) could be explained by the fact that during the roasting process of coffee beans, carbon is eliminated as the oxide or fully carbonized to a form that is not decomposable [
42,
43].
During this research, the organic matter content in SCG was determined. The results showed that the total organic matter content in the SCG, determined by the dry burning method, was equal to 98.66 ± 0.48 wt%. Similar results were presented by Cruz-Lopes et al. [
44].
Our previous studies have shown that spent coffee grounds contain primary plant nutrients (N, P, K). The nitrogen concentration in the spent coffee grounds varied in the range of 1.94 ± 0.30 wt%. The concentration of phosphorus (calculated to P2O5) in SCG was insignificant (0.46 ± 0.20 wt%). In addition, it is to be noted that all phosphorus in SCG was found to be water soluble. The concentration of potassium (soluble in mineral acid solution) was equal to 2.32 ± 0.36 wt% (calculated to K2O), and 0.55 ± 0.07 wt% easily absorbed by plants (water soluble).
According to Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019, it could be stated that the concentrations of the primary plant nutrients are high enough for the spent coffee grounds to be classified as organic fertilizers.
No less important are the secondary plant nutrients: calcium, magnesium, and sulfur. The concentration of magnesium oxide determined in all tested samples was higher than that of calcium oxide. The magnesium oxide concentration range was 0.55 ± 0.08 wt%. The determined concentration of calcium oxide was two times lower than that of magnesium and resulted in 0.22 ± 0.02 wt%. Along with that, it was discovered that SCG contain micronutrients (MN), such as Fe, Mn, Cu, Cr, and Zn.
3.2. Agronomic Evaluation of Spent Coffee Grounds on Rye Growth
For this in vitro study, topsoil samples were collected in Kaunas, Lithuania (arable layer, 0–20 cm). The distribution of the used soil particles by diameter is presented in
Table 1.
Before the study, the physical and chemical properties of the collected topsoil were determined, and also chemical composition analysis was performed. The results showed that the moisture of air-dried soil samples was 2.26 ± 0.15 wt%, and the pH value of aqueous 10%
v/
v solutions resulted in 6.90 ± 0.00 and 7.62 ± 0.06 in 1 M KCl. Chemical composition analysis showed that the collected soil sample was low in primary nutrients (0.29 ± 0.05 wt% N, 0.02 ± 0.003 wt% P
2O
5, 0.03 ± 0.002 wt% K
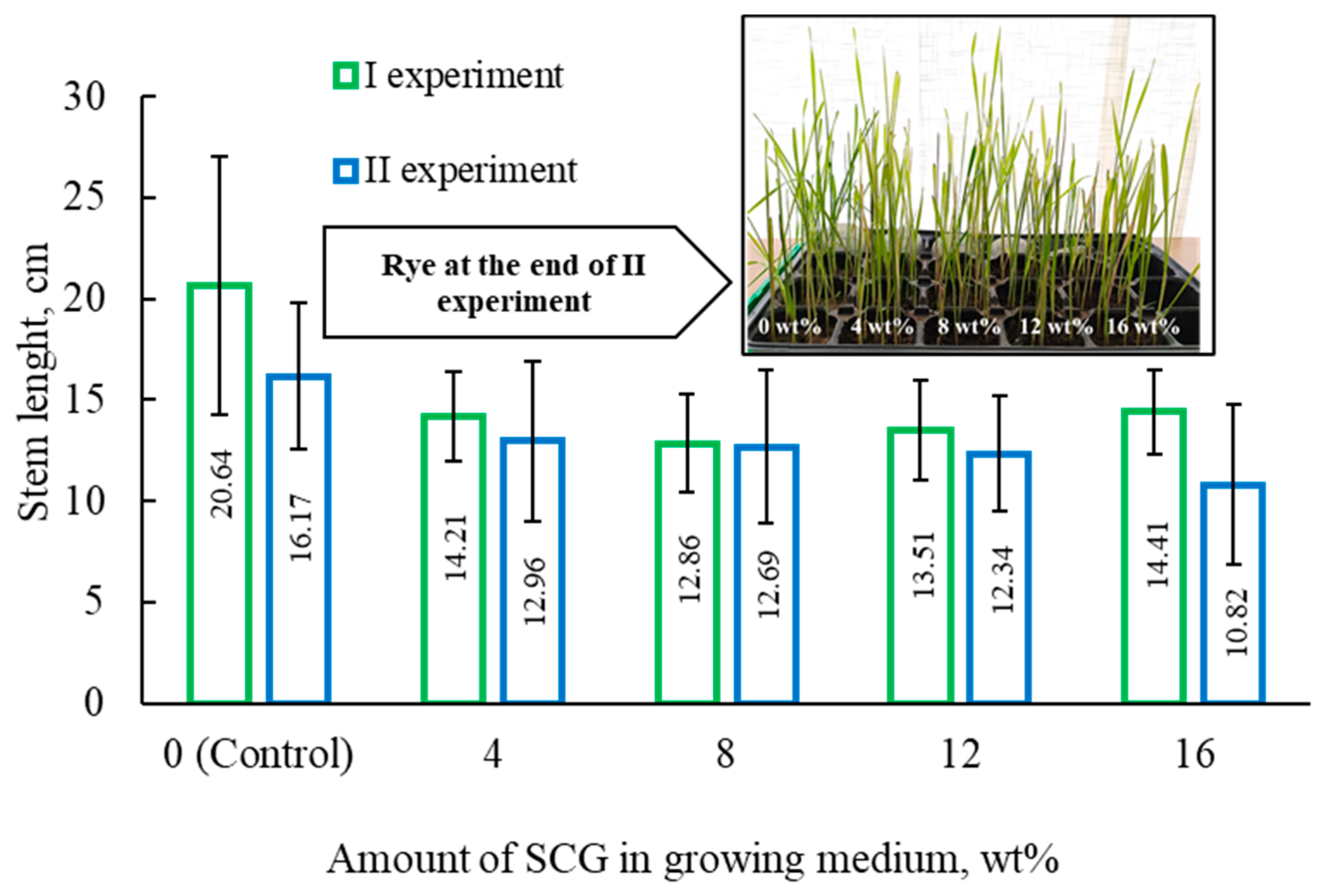
2O). It is to be mentioned that the low concentrations of nutrients in the topsoil allowed us to determine the impact of SCG on rye growth more accurately. After 12 days, the grown rye was cut. To determine the impact of the SCG additive, each rye plant was weighed, and the length of each stem was measured (
Figure 1). For complete determination, the number of leaves on each stem was counted. To determine the ash content, rye plants were burned in a muffle furnace.
As can be seen from the results (
Table 2), the addition of spent coffee grounds had a negative impact on rye growth. The planned comparison of two independent experiments revealed that the stem weight, number of leaves, and stem length of the control samples were higher than those grown in a medium that contained SCG.
After analyzing the data obtained, it could be stated that increasing the amount of SCG in the mixture (from 4 wt% to 16 wt%) changed the results very insignificantly. During the experiment, rye was reduced to ash. From the obtained results, it is clear that SCG interfered and did not allow for the accumulation of nutrients. The mineral content in the control samples was higher compared to that of the samples grown in mediums containing SCG. The higher concentration of SCG resulted in less mineral content in the tested samples. These results tie well with the findings reported by Gomez et al. Researchers performed an evaluation study on the impact of fresh and composted coffee grounds on lettuce (
Lactuca sativa L.) growth [
45]. The conducted experiment demonstrated that a small amount of fresh SCG improved lettuce growth compared to the control sample (plain soil). The same result was observed with composted SCG. Optimal results were achieved when fresh SCG accounted for 2.5–5.0%
v/
v in the growing mixture. Higher concentrations of spent coffee grounds had a notable negative impact on lettuce growth, due to increased concentrations of phytotoxic elements and reduced nutrient uptake. In the research paper by Ronga et al., evaluation studies of composted spent coffee grounds as an alternative component of a growing medium were conducted [
46]. The presented results suggested that a moderate amount of composted SCG in a growing medium enhanced plant (basil and tomatoes) growth. Summarizing the results of the in vitro study, it could be concluded that the addition of SCG (under the studied conditions) had a negative impact on the growth of
Secale cereale L. Nevertheless, it is very important to highlight that during the experiment, it was noticed that water needed for irrigation was inversely proportional to the amount of SCG in the mixture. Mixtures with higher quantity of SCG required less frequent watering.
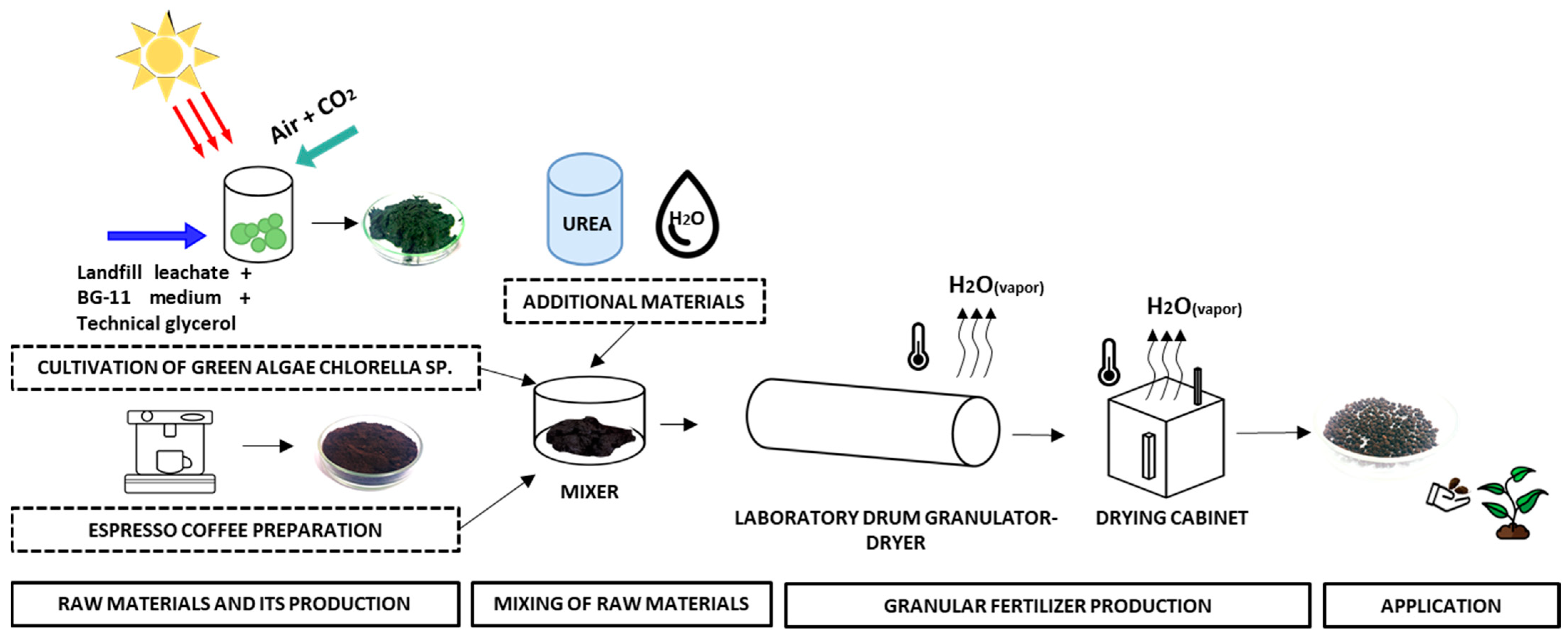
3.6. Granulation of Spent Coffee Grounds with Green Algae Chlorella sp. Biomass and Urea
Spent coffee grounds were mixed with green algae Chlorella sp. biomass, a renewable and environmentally-friendly material, to obtain environmentally-friendly organic bulk fertilizers. The direct application of a mixture with such a composition is very complicated, due to high moisture content of raw materials. Initial moisture of GACh was equal to 84.58 ± 1.60 wt%, and for SCG it resulted in 53.95 ± 1.27 wt%. After a short period of time, under ambient temperature and humidity conditions, mixed components start to rot, and because of this, that kind of mixture cannot be stored for a long time and used when needed. Another limiting factor is the uniform distribution of the fertilizing mixture on the soil surface.
For this reason, to prolong the storage time of the fertilizing mixture and to avoid the limitations mentioned, the mixture was granulated with a laboratory drum granulator. Granulation is the key process in the fertilizer industry. Granulated fertilizers are easier to store, transfer, and handle. Moreover, they can be stored for a long period of time under ambient conditions. The principal scheme of laboratory fertilizer production using the described raw materials is presented in
Figure 3.
Two mixtures with different compositions were granulated during this research. To obtain the first, SCG were mixed with GACh (ratio 2:1
w/
w). The mixture of the second composition was prepared by adding a 10 wt% urea additive to the previous one. To determine the grade of fertilizers with such composition, a chemical analysis was performed (
Table 5). The most abundant primary nutrient in the SCG and GACh mixture was nitrogen, and its concentration was equal to 2.82 ± 0.09 wt%. By adding urea, the concentration increased to 12.85 ± 0.09 wt% (N). Chemical analysis showed that the SCG and GACh mixture contained 0.70 ± 0.02 wt% phosphorus and 0.63 ± 0.02 wt% potassium. By adding urea, the concentration of these primary nutrients decreased to 0.49 ± 0.05 wt% and 0.39 ± 0.00 wt%, respectively. After testing the chemical composition of both mixtures, it was possible to determine the grade of fertilizers. For mixture with the first composition, it was set to 3–1–1+MN and 13–1–0+MN for the second one (
Table 5).
Prepared mixtures of raw materials were granulated using a laboratory drum granulator. The raw material granulation was performed at a low temperature range (62.1 ± 1.4–74.1 ± 2.0 °C) due to the thermal stability of urea. After granulation, granules were dried in the drying oven until a constant weight was reached. Once the finished product was dried, the granulometric composition was determined (
Figure 4). The initial moisture content of the SCG and GACh mixture was equal to 61.3 wt%, and small diameter granules (less than 2 mm) were dominant in the product. Moisture content in the following mixtures was increased by adding water. Four mixtures of different moisture contents were prepared, namely 61.8 wt%, 62.9 wt%, 63.6 wt%, and 66.9 wt%, respectively. In this way, the influence of the moisture content on the diameter of the granules was determined.
The results showed that to obtain the maximum quantity of commercial fraction (composed of 2.0–3.15 mm and 3.15–4.0 mm diameter granules), the optimal moisture content of the raw material mixture was equal to 66.9 wt%. By further increasing the moisture content in the mixture, the diameter of the formed granules was greater than 5 mm. During the granulation process of the SCG+GACh+urea mixture, the same process parameters were maintained. Five different mixtures of SCG, GACh, and urea were prepared with a moisture content of 56.6 wt%, 57.1 wt%, 57.3 wt%, 57.6 wt%, and 58.0 wt%, respectively. The moisture content of the mixture with no added water was equal to 56.6 wt%. After granulating the mixture of such initial moisture, fine particles (less than 2.0 mm) were predominant in the resulting product. A slight increase of the added water amount resulted in larger diameter of granules. From the obtained results it could be claimed that the maximum quantity of commercial fraction was obtained by granulating the mixture with 57.6 wt% moisture. Granulation of a 58.0 wt% moisture mixture resulted in granules larger than 5 mm. Images of the granular fertilizers obtained are presented in
Figure 5. As can be seen from the figure, granules of both compositions were similar to each other, and the form was close to a sphere shape. The shape of the granules has a huge impact on crushing strength and loose bulk density. Moreover, shape influences storage, transportation, and distribution of the fertilizers. Granules containing urea were paler, due to recrystallization of urea on the surfaces of the granules (
Figure 5g). There were no significant differences in the inside of the granules, since almost all urea was recrystallized on the surface (
Figure 5d,h).
To summarize the data of granulometric composition, it could be stated that by increasing the moisture content in the mixture of raw materials, the diameter of granules also increased. The obtained results highlight that even a small change in the moisture content of the raw materials has a huge impact on the granulometric composition of the finished product. Furthermore, it was observed that by adding urea, less moisture was needed for granules to form. This last statement fully complies with one of the key principles of granulation, which states that using such hygroscopic salts (i.e., urea) makes it easier for crystallization bridges to form and facilitates the agglomeration process of particles. During this study, other physical and chemical properties, such as moisture content, loose bulk density, pH value, and the crushing strength of the finished product were determined. All measurements of the parameters listed were performed for 2.0–3.15 mm and 3.15–4.0 mm diameter granules (
Table 6).
Since the raw materials used in this study were high in moisture, the moisture content of the granules was quite high even after drying. Residual moisture values ranged from 5.44 wt% to 6.26 wt% for the product composed of SCG and GACh. It is clear from the results that the urea additive not only decreased the amount of moisture needed for granule formation, but also the moisture content of the dried product was lower (3.97–5.53 wt%) compared to the fertilizer granules of 3–1–1+MN grade.
The loose bulk density of granules composed of SCG, GACh, and urea was slightly higher than of those without urea. The maximum loose bulk density value of granules composed of SCG and GACh biomass was 370.9 kg/m
3, while the maximum value of loose bulk density of granules with urea additive was 423.1 kg/m
3. In conclusion, the obtained results indicate that by adding urea, the loose bulk density of the finished product increased. Before the granulation process, the pH values of raw materials were determined. The measured pH value of the GACh suspension was equal to 8.53 ± 0.04, and for the aqueous solution of spent coffee grounds with 10 wt% concentration it was 5.6 ± 0.02. The aqueous 10 wt% urea solution accounted for a pH of 6.97 ± 0.14. Solutions of the same concentration were prepared to determine the pH value of obtained granular fertilizers. As could be concluded from
Table 6, pH values of 3–1–1 grade fertilizer ranged from 6.28 to 6.43, while the pH values of second grade granules resulted in 6.88–7.05. Granules composed of SCG, green algae
Chlorella sp., and urea were almost neutral, due to the decreased amount of spent coffee grounds in the mixture and the addition of alkaline properties containing urea. The crushing strength of the obtained granules was measured for the commercial fraction. The present study confirmed that the urea additive also improved the crushing strength of the obtained granular fertilizers. The maximum force to crush the granule of SCG, algae, and urea was almost 1.5 times greater than without the urea additive. Although the urea additive improved some physical and chemical properties of the finished product, it is important to note that the presence of urea had a negative impact on hygroscopicity of the granules. The results confirmed that urea is a highly hygroscopic substance, and during the experiment, granules containing urea absorbed much more moisture, regardless of the environment in the desiccator. As can be seen from
Figure 6a, after spending 8 days in the water vapor environment, granules released water. The absorbed water content was 72.0 wt%. In the environment of the saturated NaNO
2 solution, absorbance was lower (9.7 wt%). A slight fluctuation in the amount of water absorbed during the experiment could be explained by changes in temperature and humidity in the desiccator.
Clearly, better results were observed during the absorbance study of the SCG and algae biomass granules. After seven days, in water vapor environment granules started to mold (
Figure 6a). By the eighth day, absorbed water content was 24.9 wt% and the study was completed. The results of the hygroscopicity study in the environment of saturated sodium nitrite solution were equal to those previously described (weight of the granules increased by 3.6 wt%). Thus, the results demonstrated that the urea additive improved the chemical and physical properties of the obtained granules.
3.8. Instrumental Analysis of the Raw Materials and Finished Product
To determine the thermal stability of the obtained granules, TGA and DSC analyses were performed. For granules of 3–1–1+MN grade, the continuous weight loss up to 3 wt% was observed at the temperature up to 250 °C. As shown by previous studies, granules of such composition contain 5.44 wt% to 6.26 wt% moisture, and this weight loss is related to the water evaporation from the granules. Water evaporation was also indicated by the small endothermic effect obtained by the DSC analysis (
Figure 8).
The second weight loss in the thermal decomposition profile occurred due to the volatilization of organic compounds. An intensive peak at around 325 °C was attributed to the fragmentation of hydrocarbon chains of fatty acids in algae biomass. A similar pattern of results was obtained by other researchers [
53,
54]. The second stage of weight loss could also be attributed to the SCG devolatilization, resulting in the formation of CO and CO
2. The DSC curve indicates decomposition of hemicellulose at around 360 °C. The following step is decomposition of cellulose in the 400–500 °C temperature range. The third stage is due to lignin, and it starts at 450 °C until the test sample completely burns down at 800 °C, resulting in 94% of total weight loss [
55]. A similar pattern of results was obtained with 13–1–0 grade fertilizers. However, slight changes in the thermal decomposition profile were observed due to the added urea. A low intensity peak of heat absorption was observed at 116 °C when urea starts to melt; then it was followed by a more intensive peak in the temperature range of 150–250 °C. This process leads to emission of gases because of the urea decomposition. During this stage, 20% of the total mass of test sample was lost [
56]. The following stages in the thermal decomposition profile are the same as previously described for the mixture of SCG and algae biomass.
To compare the finished products with the raw materials, FTIR analysis was performed on SCG and GACh separately, along with granules of both grades (
Figure 9). The FTIR transmittance spectra (
Figure 9) were recorded in the range of 400–4000 cm
−1. To perform this analysis, GACh was dried to constant weight and then crushed into fine particles of less than 200 µm diameter.
An intensive spectral peak at 3677–3000 cm
−1 belongs to the stretching vibration of the O–H group, indicating the presence of phenols, carboxylic acid, and absorbed water in b, c, and d test samples [
57]. A sharp peak in a range of 2920 cm
−1 to 2850 cm
−1 indicates an asymmetric and symmetric C–H stretching of cellulose components [
58]. Elongation of the carbonyl bond in esters is clearly seen from the peak at 1750 cm
−1 in the FTIR transmittance spectra of spent coffee grounds. The absorption of the C–N stretching could be indicated by a less intensive peak at 1500 cm
−1. A spectrum of a different pattern was obtained for the GACh biomass. The presence of proteins in algae biomass is indicated by vibrations of N–H at 1400–1600 cm
−1. Aromatic and aliphatic amines could be determined from the peaks of C–N stretching in the 1000–1300 cm
−1 range. An intensive peak at 3500–3200 cm
–1 represents symmetric and asymmetric stretching of the N–H bonds [
59]. The results demonstrate that the FTIR transmittance spectra of SCG and obtained granules is very similar, because granular product is mainly composed of SCG (ratio of SCG and GACh is 2:1). Moreover, GACh biomass with an initial moisture of 84.58 ± 1.60 wt% was used during the granulation process. For this reason, no significant peaks of amine functional groups were observed in FTIR spectra of the finished product. In addition, no additional chemical compounds formed by mixing SCG with GACh biomass. Nevertheless, it is very important to highlight that the green algae biomass improved granulation process, and significant changes in the strength of the formed granules were observed.