Using Trophy Hunting to Save Wildlife Foraging Resources: A Case Study from Moyowosi-Kigosi Game Reserves, Tanzania
Abstract
1. Introduction
2. Material and Methods
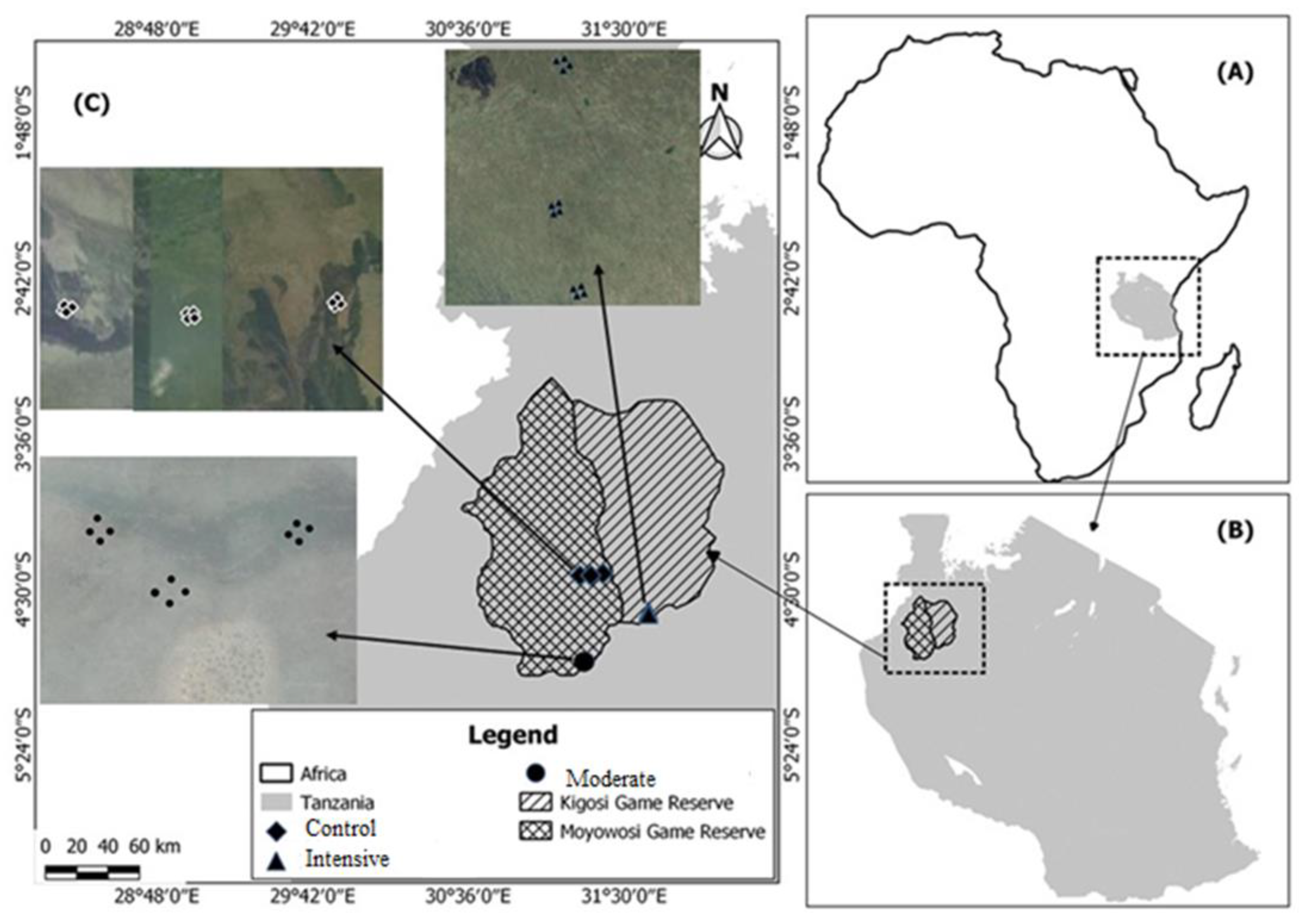
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Illegal Grazing Lowers Grass Biomass and Grass Cover in Non-Operational Wildlife Hunting Blocks
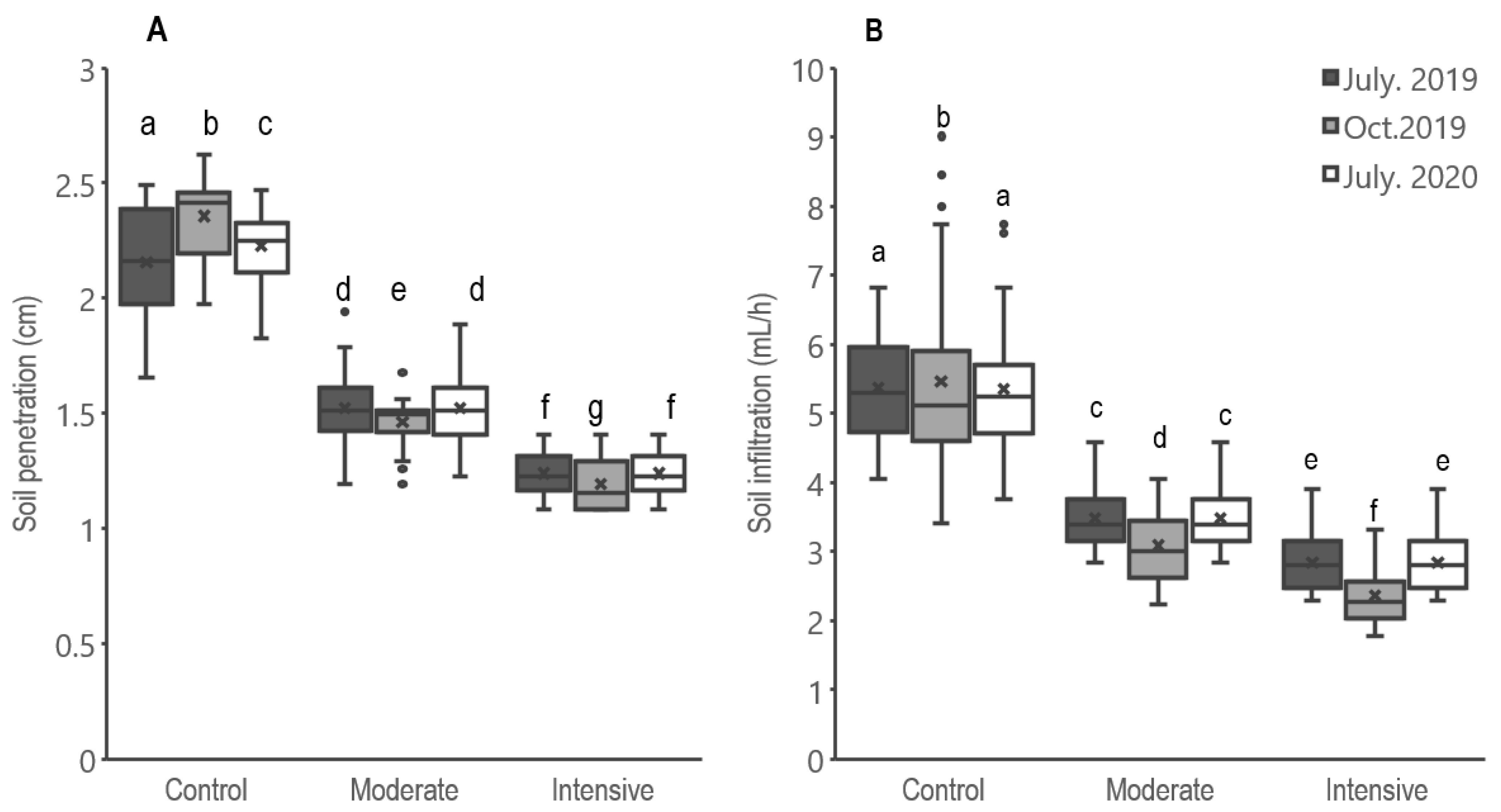
3.2. Illegal Grazing Reduces Soil Penetration and Infiltration in Non-Operational Wildlife Hunting Blocks
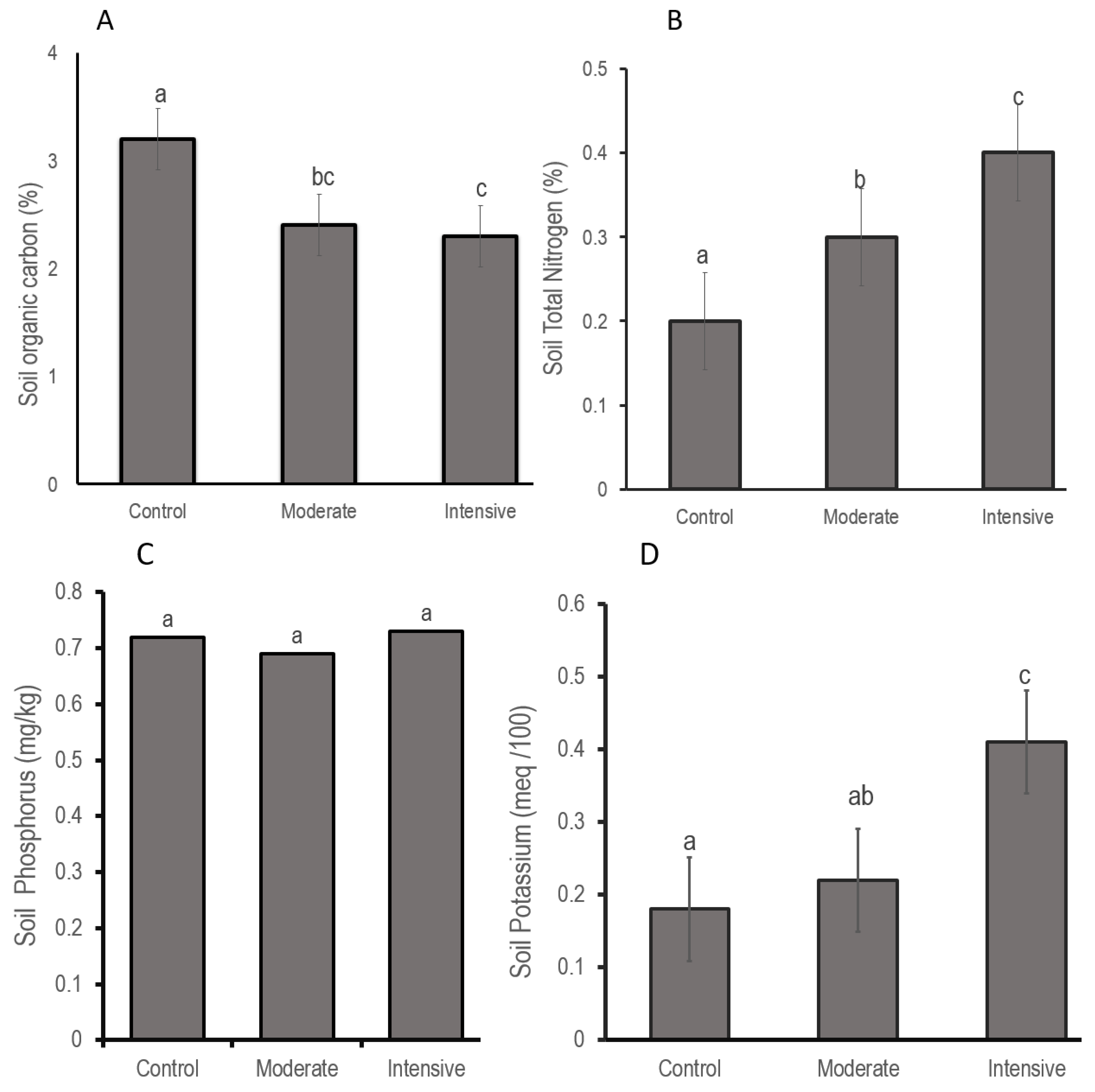
3.3. Illegal Grazing Affects SOC and N-P-K in Non-Operational Wildlife Hunting Blocks
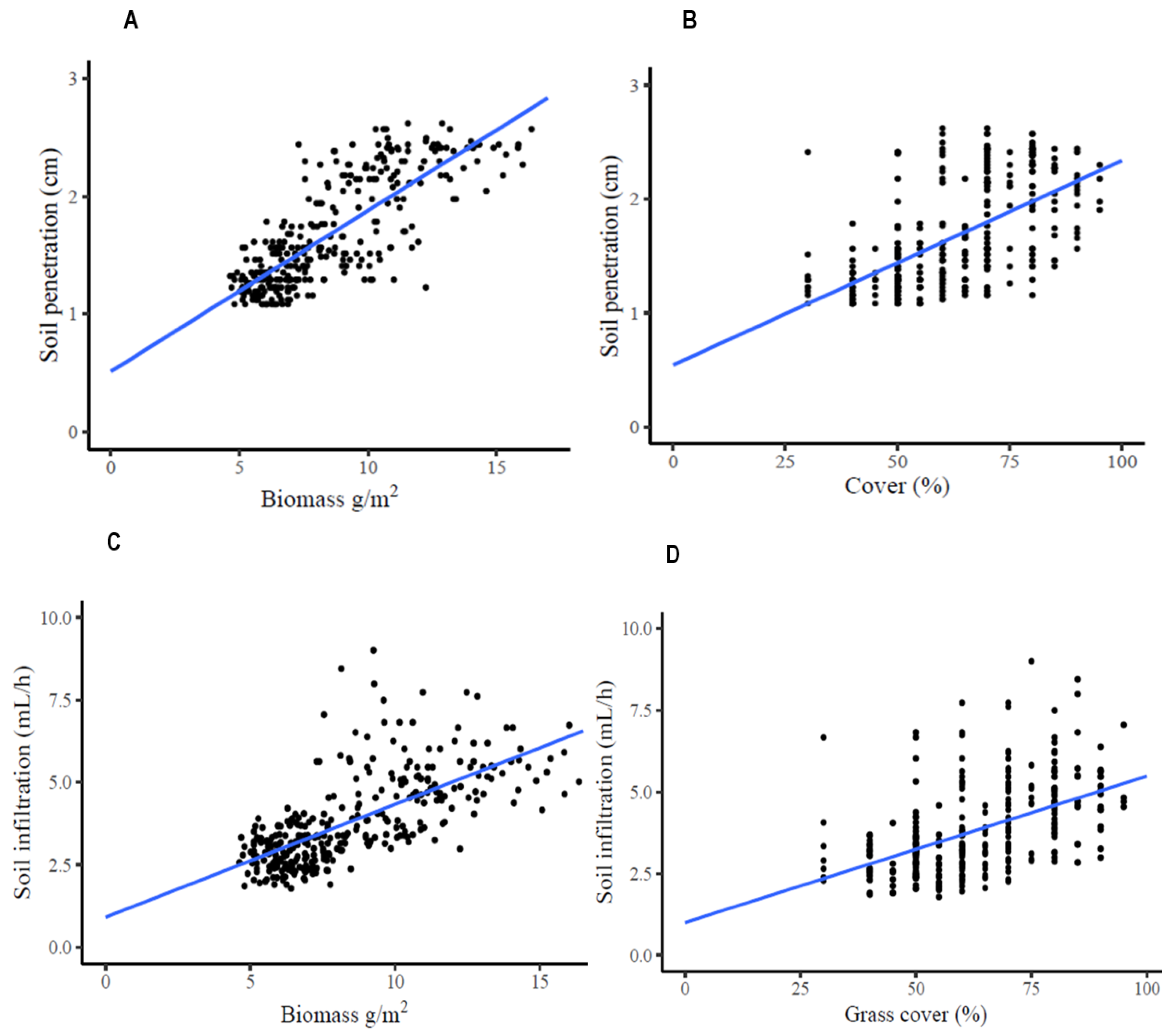
3.4. Soil Penetration and Infiltration Rate Increases with Increasing Grass Biomass and Cover
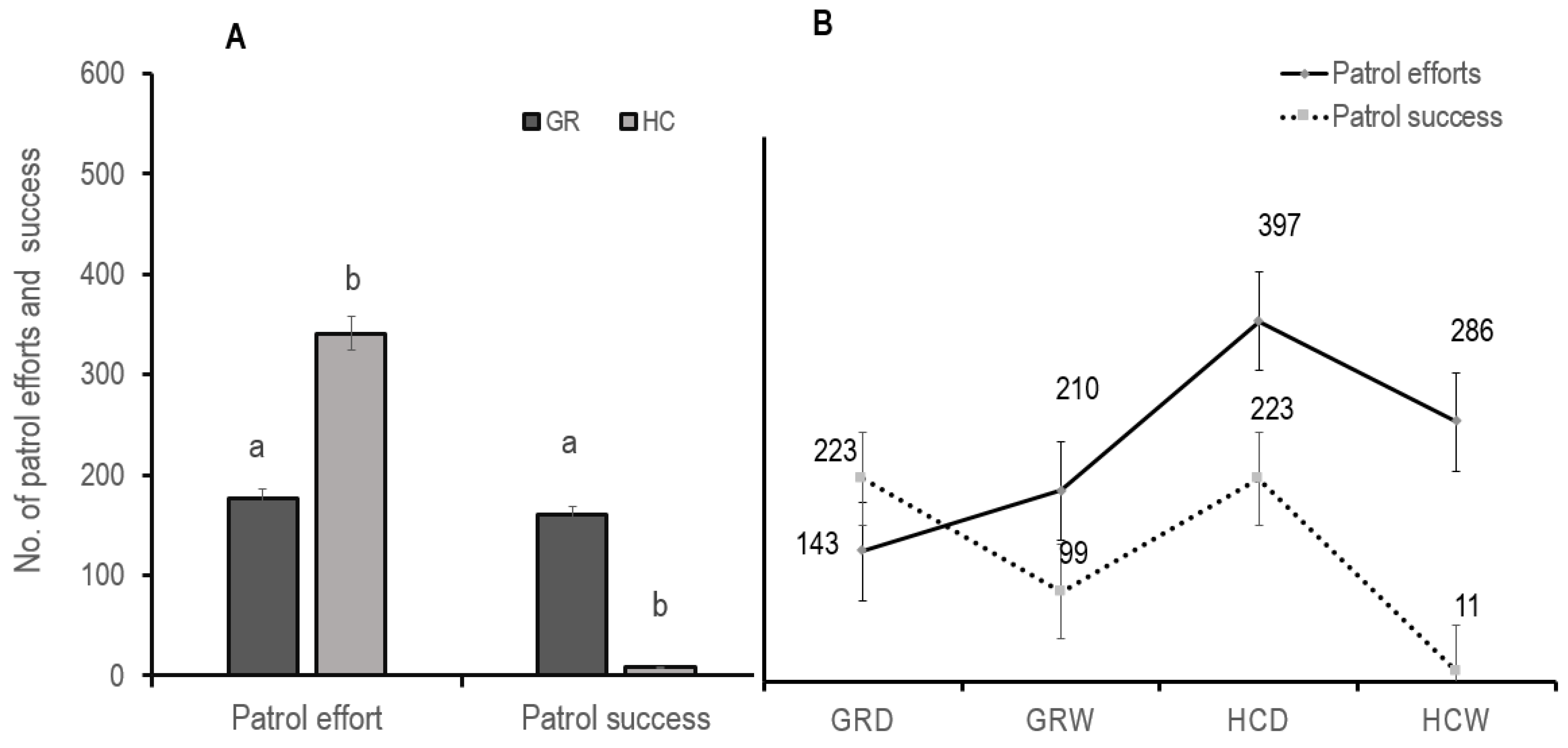
3.5. Hunting Company Complements Anti-Poaching Efforts of the Game Reserve
4. Discussion
4.1. Low Grass Biomass and Cover in Non-Operational Wildlife Hunting Blocks
4.2. Reduced Soil Infiltration and Penetration in Non-Operational Wildlife Hunting Blocks
4.3. Mixed Results on SOC and N-P-K in Non-Operational Wildlife Hunting Blocks
4.4. Soil Penetration and Infiltration Rate Increase with Increasing Grass Cover and Grass Biomass
4.5. Hunting Company Complements Patrol Efforts in the Game Reserve
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, R. The contribution of wildlife to the economies of Sub Saharan Africa; Institute of Development Studies: Brighton, UK, 2017. [Google Scholar]
- Gayo, L.; Nahonyo, C.L.; Masao, C.A. Will the sustainability of lion tourist hunting be certain amidst non compliance to conservation legal frameworks? A case of Selous Game. Tanzan. J. Sociol. 2020, 6, 26. [Google Scholar]
- Dickson, B.; Hutton, J.; Adams, W.M. (Eds.) Recreational Hunting, Conservation Conservation Science and Practice Series; Blackwell Publishing Ltd.: Oxford, UK, 2009; ISBN 9781405167857. [Google Scholar]
- McNamara, T.; Claasen, C.; Irena, D. Trophy Hunting in Namibia: Controversial but Sustainable? A Case Study of “Hunters Namibia Safaris”; ESC Rennes School of Business: Rennes, France, 2015. [Google Scholar]
- Cooney, R.; Freese, C.; Dublin, H.; Roe, D.; Mallon, D.; Knight, M.; Emslie, R.; Pani, M.; Booth, V.; Mahoney, S.; et al. The baby and the bathwater: Trophy hunting, conservation and rural livelihoods. Unasylva 2017, 68, 3–16. [Google Scholar]
- Gamborg, C.; Jensen, F.S. Attitudes towards recreational hunting: A quantitative survey of the general public in Denmark. J. Outdoor Recreat. Tour. 2017, 17, 20–28. [Google Scholar] [CrossRef]
- IUCN Informing Decisions on Trophy Hunting: A Briefing Paper Regarding Issues to Be Taken into Account When Considering Restriction of Imports of Hunting Trophies; IUCN Briefing Paper; IUCN: Gland, Switzerland, 2016; p. 19.
- Heffelfinger, J.R.; Geist, V.; Wishart, W. The role of hunting in North American wildlife conservation. Int. J. Environ. Stud. 2013, 70, 399–413. [Google Scholar] [CrossRef]
- Mallon, D. Trophy Hunting of CITES—Listed Species in Central Asia; CITES Secretariat: Geneva, Switzerland, 2013. [Google Scholar]
- Booth, V.R. Economic Assessment of the Value of Wildlife to the Tanzania Hunting Industry; Tanzania’s Ministry of Natural Resources and Tourism: Dar es Salaam, Tanzania, 2017.
- URT. A Perfomance Audit Report on the Management of Wildlife in Game Reserves and Game Controlled Areas; URT: Dar es Salaam, Tanzania, 2013. [Google Scholar]
- Kideghesho, J.R. Reversing the trend of wildlife crime in Tanzania: Challenges and opportunities. Biodivers. Conserv. 2016, 25, 427–449. [Google Scholar] [CrossRef]
- Deere, N.J. Exploitation or Conservation? Can the hunting tourism industry in Africa be sustainable? Environ. Sci. Policy Sustain. Dev. 2011, 53, 20–32. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y.; et al. Grazing enhances plant photosynthetic capacity by altering soil nitrogen in alpine grasslands on the Qinghai-Tibetan plateau. Agric. Ecosyst. Environ. 2019, 280, 161–168. [Google Scholar] [CrossRef]
- Rahmanian, S.; Hejda, M.; Ejtehadi, H.; Farzam, M.; Memariani, F.; Pyšek, P. Effects of livestock grazing on soil, plant functional diversity, and ecological traits vary between regions with different climates in northeastern Iran. Ecol. Evol. 2019, 9, 8225–8237. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V.; Anderson, S.H.; Eivazi, F.; Zaibon, S. In situ infiltration as influenced by cover crop and tillage management. J. Soil Water Conserv. 2018, 73, 164–172. [Google Scholar] [CrossRef]
- Butt, B.; Turner, M.D. Clarifying competition: The case of wildlife and pastoral livestock in East Africa. Res. Policy Pract. 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Musika, N.V.; Wakibara, J.V.; Ndakidemi, P.A.; Treydte, A.C. Spatio-Temporal Patterns of Increasing Illegal Livestock Grazing over Three Decades at Moyowosi Kigosi Game. Land 2021, 10, 1325. [Google Scholar] [CrossRef]
- Rottstock, T.; Göttert, T.; Zeller, U. Relatively undisturbed African savannas—An important reference for assessing wildlife responses to livestock grazing systems in European rangelands Relatively undisturbed African savannas—An important reference for assessing wildlife responses to live. Glob. Ecol. Conserv. 2020, 23, e01124. [Google Scholar] [CrossRef]
- Lindsey, P..; Roulet, P.A.; Romañach, S. Economic and conservation significance of the trophy hunting industry in sub-Saharan Africa. Biol. Conserv. 2007, 134, 455–469. [Google Scholar] [CrossRef]
- Lindsey, P.A.; Havemann, C.P.; Lines, R.M.; Price, A.E.; Retief, T.A.; Rhebergen, T.; Van Der Waal, C.; Romañach, S.S. Benefits of wildlife-based land uses on private lands in Namibia and limitations affecting their development. Oryx 2013, 47, 41–53. [Google Scholar] [CrossRef]
- Kubiak, B.B.; Galiano, D.; De Freitas, T.R.O. Sharing the space: Distribution, habitat segregation and delimitation of a new sympatric area of subterranean rodents. PLoS ONE 2015, 10, e0123220. [Google Scholar] [CrossRef]
- TAWIRI. Aerial Census of Large Animals in the Selous-Mikumi Ecosystem: Population Status of African Elephant, Dry Season, 2013; TAWIRI: Arusha, Tanzania, 2013. [Google Scholar]
- The World Bank. Project Information Document/Integrated Safeguards Data Sheet (PID/ISDS); The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- IUCN. State of Protected and Conserved Areas in Eastern and Southern Africa; IUCN: Nairobi, Kenya, 2020. [Google Scholar]
- TAWA. Taarifa ya Matumizi Endelevu ya Wanyamapori; TAWA: Morogoro, Tanzania, 2017. [Google Scholar]
- TAWIRI. Aerial Census in Malagarasi -Muyovozi Ecosystem, Tanzania; TAWIRI: Arusha, Tanzania, 2015. [Google Scholar]
- Li, G.; Zhang, Z.; Shi, L.; Zhou, Y.; Yang, M.; Cao, J.; Wu, S.; Lei, G. Effects of different grazing intensities on soil c, n, and p in an alpine meadow on the qinghai—tibetan plateau, china. Int. J. Environ. Res. Public Health 2018, 15, 2584. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Fang, J.Y.; Pan, Y.D.; Ji, C.J. Aboveground biomass in Tibetan grasslands. J. Arid Environ. 2009, 73, 91–95. [Google Scholar] [CrossRef]
- Guarino, S.; Conrad, S.E.; Papini, M.R. Frustrative nonreward: Chemogenetic inactivation of the central amygdala abolishes the effect of reward downshift without affecting alcohol intake. Neurobiol. Learn. Mem. 2020, 169, 107173. [Google Scholar] [CrossRef] [PubMed]
- Amary, N.M. Assessing the Quality of Forage for Livetsock in a Semi-Arid Pastoral System in South Africa. Master’s Thesis, University of Western Cape, Cape Town, South Africa, 2016. [Google Scholar]
- Smith, G. Sasol First Field Guide to Grasses of Southern Africa; Penguin Random House South Africa: Johanenesburg, South Africa, 2012; ISBN 9781868729524. [Google Scholar]
- Cui, Z.; Wu, G.; Huang, Z.; Liu, Y. Fine roots determine soil in fi ltration potential than soil water content in semi-arid grassland soils. J. Hydrol. 2019, 578, 124023. [Google Scholar] [CrossRef]
- Ogogo, A.U.; Asuk, S.A.; Ikpeme, R.V. Evaluation of the Anti-Poaching Programme of the Cross River National Park Okwango Division, Nigeria from 2002 to 2013. Open J. For. 2014, 4, 507–511. [Google Scholar]
- Nahonyo, C. Assessment of anti-poaching effort in Ruaha National Park, Tanzania. Tanzan. J. Sci. 2005, 31, 9. [Google Scholar] [CrossRef][Green Version]
- Wato, Y.A.; Wahungu, G.M.; Okello, M.M. Correlates of wildlife snaring patterns in Tsavo West National Park, Kenya. Biol. Conserv. 2006, 132, 500–509. [Google Scholar] [CrossRef]
- Tarimo, C.O. The Effectiveness of Law Enforcement in Wildlife and Forest Resources Management: A Case Study of JUHIBU and JUHIBEKO, Tanzania. Ph.D. Thesis, Sokoine University of Agriculture, Morogoro, Tanzania, 2016. [Google Scholar]
- Zeyede, A. Optimization of the analytical method for the determination of organic matter. J. Soil Sci. Environ. Manag. 2020, 11, 1–5. [Google Scholar] [CrossRef][Green Version]
- Stafilov, T.; Špirić, Z.; Glad, M.; Barandovski, L.; Bačeva Andonovska, K.; Šajn, R.; Antonić, O. Study of nitrogen pollution in the Republic of North Macedonia by moss biomonitoring and Kjeldahl method. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Onawumi, O.A.; Oluwaponle, A.I.; Isienyi, N.C. Influence of Composted Substrate Types on the Growth Performance of Sour Sop (Annona muricata). J. Exp. Agric. Int. 2020, 42, 243–249. [Google Scholar] [CrossRef]
- Lee, H. Foundations of Applied Statistical Methods; Springer: Cham, Switzerland, 2014; ISBN 9783319024011. [Google Scholar]
- The Jamovi Project Jamovi. (Version 1.2) [Computer Software]; Jamovi: Sydney, Australia, 2020.
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Zainelabdeen, Y.M.; Yan, R.; Xin, X.; Yan, Y.; Ahmed, A.I.; Hou, L.; Zhang, Y. The impact of grazing on the grass composition in Temperate Grassland. Agronomy 2020, 10, 1230. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, X.; Zhou, X.; Lv, P.; Lian, J.; Yue, X. Long-term grazing effects on vegetation characteristics and soil properties in a semiarid grassland, northern China. Environ. Monit. Assess. 2017, 189, 216. [Google Scholar] [CrossRef]
- TMA. Statement on the Status of the Global Climate in 2020; TMA: Morogoro, Tanzania, 2020. [Google Scholar]
- Okach, D.O. Effects of Livestock Grazing and Rainfall Variability on the Structure and Function of the Herbaceous Layer Community of a Humid Savanna Ecosystem in Lambwe Valley-Kenya. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2019. [Google Scholar]
- Koerner, S.E.; Collins, S.L.; Blair, J.M.; Knapp, A.K.; Smith, M.D. Rainfall variability has minimal effects on grassland recovery from repeated grazing. J. Veg. Sci. 2014, 25, 36–44. [Google Scholar] [CrossRef]
- Kawo, S.T. Estimating Grass Productivity under Different Clipping Frequencies and Rainfall Amount: Implications for Rangeland Responses to Climate Change. Ph.D. Thesis, University of Hohenheim, Stuttgart, Germany, 2017. [Google Scholar]
- Liang, M.; Liang, C. Grazing effect on grasslands escalated by abnormal precipitations in Inner Mongolia. Ecol. Evol. 2018, 8187–8196. [Google Scholar] [CrossRef]
- Co du Toit, J.; Ramaswiela, T.; Pauw, M.J.; O’Connor, T.G. Interactions of grazing and rainfall on vegetation at Grootfontein in the eastern Karoo. Afr. J. Range Forage Sci. 2018, 35, 267–276. [Google Scholar] [CrossRef]
- Bilotta, G.S.; Brazier, R.E.; Haygarth, P.M. The Impacts of Grazing Animals on the Quality of Soils, Vegetation, and Surface Waters in Intensively Managed Grasslands. Adv. Agron. 2007, 94, 237–280. [Google Scholar] [CrossRef]
- Menneer, J.C.; Ledgard, S.F.; McLay, C.D.A.; Silvester, W.B. The effects of treading by dairy cows during wet soil conditions on white clover productivity, growth and morphology in a white clover-perennial ryegrass pasture. Grass Forage Sci. 2005, 60, 46–58. [Google Scholar] [CrossRef]
- Erftemeijer, P.L.A. Status of Vegetation, Disturbances and Threats to Habitats in the Malagarasi- Muyovozi Ramsar Site (Tanzania)—Results from an Aerial Survey; University of Dar es Salaam: Dar es Salaam, Tanzania, 2001. [Google Scholar]
- Hashmie, N.; Gunagi, P.; Belagal, P.; Manoharan, S. Utilisation of Recycled Glass Powder for the Stabilization of Black Cotton soil by Alkali Activation. IOP Conf. Ser. Mater. Sci. Eng. 2020, 955, 012063. [Google Scholar] [CrossRef]
- Tuohy, P.; Fenton, O.; Holden, N.M.; Humphreys, J. The effects of treading by two breeds of dairy cow with different live weights on soil physical properties, poaching damage and herbage production on a poorly drained clay-loam soil. J. Agric. Sci. 2015, 153, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Jebari, A.; Álvaro-Fuentesorge, J.; Pardo, G.; Almagro, M.; del Prado, A. Simulating soil organic C dynamics in managed grasslands under humid temperate climatic conditions. SOIL Discuss. 2020, 1–40. [Google Scholar] [CrossRef]
- Munjonji, L.; Ayisi, K.K.; Mudongo, E.I.; Mafeo, T.P.; Behn, K.; Mokoka, M.V.; Linstädter, A.; Carlyle, C. Disentangling Drought and Grazing Effects on Soil Carbon Stocks and CO 2 Fluxes in a Semi-Arid African Savanna. Front. Environ. Sci. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Sumiya, V.; David, J.E.; Samantha, K.T.; James, V.; Ian, O. Microsite and grazing intensity drive infiltration in a semi-arid woodland. Ecohydrology 2017, 10, 27. [Google Scholar] [CrossRef]
- Arcoverde, G.B.; Andersen, A.N.; Setterfield, S.A. Is livestock grazing compatible with biodiversity conservation? Impacts on savanna ant communities in the Australian seasonal tropics. Biodivers. Conserv. 2016, 26, 883–897. [Google Scholar] [CrossRef]
- Teague, W.R.; Duke, S.E.; Waggoner, J.A.; Dowhower, S.L.; Gerrard, S.A. Rangeland vegetation and soil response to summer patch fires under continuous grazing. Arid Land Res. Manag. 2008, 22, 228–241. [Google Scholar] [CrossRef]
- Hao, Y.; He, Z. Effects of grazing patterns on grassland biomass and soil environments in China: A meta-analysis. PLoS ONE 2019, 14, e0215223. [Google Scholar] [CrossRef]
- Jamsranjav, C. Effects of Grazing and Community-Based Management on Rangeland of Somalia. Ph.D. Thesis, Colorado State Universit, Fort Collins, CO, USA, 2015. [Google Scholar]
- Hou, D.; Guo, K.; Liu, C. Asymmetric effects of grazing intensity on macroelements and microelements in grassland soil and plants in Inner Mongolia Grazing alters nutrient dynamics of grasslands. Ecol. Evol. 2020, 8916–8926. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Usman, M.; Nangere, M.G. Study of Cattle Trampling and Its Effect on Soil Properties and Sorghum Productivity in Parts of Yobe State, Nigeria. Asian Plant Res. J. 2020, 4, 34–40. [Google Scholar] [CrossRef]
- Lu, X.; Kelsey, K.C.; Yan, Y.; Sun, J.; Wang, X.; Cheng, G.; Neff, J.C. Effects of grazing on ecosystem structure and function of alpine grasslands in Qinghai-Tibetan Plateau: A synthesis. Ecosphere 2017, 8, e01656. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Xie, F.; Wang, K. Response of vegetation and soil carbon and nitrogen storage to grazing intensity in semi-arid grasslands in the agro-pastoral zone of northern China. PLoS ONE 2014, 9, e96604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Peth, S.; Krümmelbein, J.; Horn, R.; Wang, Z.; Steffens, M.; Hoffmann, C.; Peng, X. Spatial variability of soil properties affected by grazing intensity in Inner Mongolia grassland. Ecol. Model. 2007, 205, 241–254. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Song, X.; Chang, Q.; Frank, D.A.; Wang, D.; Li, J.; Lin, H.; Du, F. Towards a mechanistic understanding of the effect that different species of large grazers have on grassland soil N availability. J. Ecol. 2018, 106, 357–366. [Google Scholar] [CrossRef]
- Musa, A.; Ogbadoyi, O.E. Effects of excessive nitrogen on leaves.pdf. Asian J. Crop Sci. 2012, 4, 11. [Google Scholar]
- Brooks, M.L. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave Desert. J. Appl. Ecol. 2003, 40, 344–353. [Google Scholar] [CrossRef]
- Elhanafi, L.; Houhou, M.; Rais, C.; Mansouri, I.; Elghadraoui, L.; Greche, H. Impact of Excessive Nitrogen Fertilization on the Biochemical Quality, Phenolic Compounds, and Antioxidant Power of Sesamum indicum L Seeds. J. Food Qual. 2019, 2019, 6. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Jean, D.B. Human Health Effects of Exposure to Nitrate, Nitrite, and Nitrogen Dioxide. In Just Enough Nitrogen; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Ji, L.; Qin, Y.; Jimoh, S.O.; Hou, X.; Zhang, N.; Gan, Y.; Luo, Y. Impacts of livestock grazing on vegetation characteristics and soil chemical properties of alpine meadows in the eastern Qinghai-Tibetan Plateau. Écoscience 2020, 27, 107–118. [Google Scholar] [CrossRef]
- Larson, D.L.; Hernández, D.L.; Larson, J.L.; Leone, J.B.; Pennarola, N. Management of remnant tallgrass prairie by grazing or fire: Effects on plant communities and soil properties. Ecosphere 2020, 11, e03213. [Google Scholar] [CrossRef]
- Chai, J.; Yu, X.; Xu, C.; Xiao, H.; Zhang, J.; Yang, H.; Pan, T. Effects of yak and Tibetan sheep trampling on soil properties in the northeastern Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2019, 144, 147–154. [Google Scholar] [CrossRef]
- Wells, K.L.; Dougherty, C.T. Soil management for intensive grazing. Soil Sci. News Views 1997, 18, 18–23. [Google Scholar]
- Koch, B.; Homburger, H.; Edwards, P.J.; Schneider, M.K. Phosphorus redistribution by dairy cattle on a heterogeneous subalpine pasture, quantified using GPS tracking. Agric. Ecosyst. Environ. 2018, 257, 183–192. [Google Scholar] [CrossRef]
- Zarekia, S.; Agricultural, Y.; Jafari, M.; Arzani, H.; Jafari, A.A. Grazing effects on some of the physical and chemical properties of soil. World Appl. Sci. J. 2012, 20, 205–212. [Google Scholar] [CrossRef]
- Faghihinia, M.; Zou, Y.; Chen, Z.; Bai, Y.; Li, W.; Marrs, R.; Staddon, P.L. Environmental drivers of grazing effects on arbuscular mycorrhizal fungi in grasslands. Appl. Soil Ecol. 2020, 153, 103591. [Google Scholar] [CrossRef]
- Gholinejad, B.; Jaffari, H.J. Effect of environmental traits and grazing intensities on plant community distribution (case study: Saral Rangelands, Iran). J. Rangel. Sci. 2020, 10, 162–171. [Google Scholar]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. J. Environ. Qual. 2018, 47, 758–765. [Google Scholar] [CrossRef]
- Li, C.; de Jong, R.; Schmid, B.; Wulf, H.; Schaepman, M.E. Spatial variation of human influences on grassland biomass on the Qinghai-Tibetan plateau. Sci. Total Environ. 2019, 665, 678–689. [Google Scholar] [CrossRef]
- Baral, A.N. Impacts of Wildlife Tourism on Poaching of Greater One-horned Rhinoceros (Rhinoceros unicornis) in Chitwan National Park, Nepal. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2013. [Google Scholar]
- Njerekai, C.; Mabika, P. A Review of the Global Trophy Hunting Procedures and Processes with Illustrations from Zimbabwe. Afr. J. Hosp. Tour. Leis. 2016, 5, 1–15. [Google Scholar]
- Kyando, M.; Ikanda, D.; Røskaft, E. Hotspot elephant-poaching areas in the Eastern Selous Game Reserve, Tanzania. Afr. J. Ecol. 2017, 55, 365–371. [Google Scholar] [CrossRef]
- Snyder, K.D. Predicting the risk of illegal activity and evaluating law enforcement interventions in the western Serengeti. Conserv. Sci. Pract. 2019, 1, e81. [Google Scholar] [CrossRef]

| Years | Control | Moderate | Intensive | |||
|---|---|---|---|---|---|---|
| Number | Density | Number | Density | Number | Density | |
| 2015 | 100 | 0.0006 | 6087 | 0.02 | 13,117 | 0.07 |
| 2016 | 120 | 0.0007 | 6980 | 0.02 | 13,397 | 0.07 |
| 2017 | 90 | 0.0005 | 5830 | 0.02 | 19,397 | 0.10 |
| 2018 | 0 | 0.0000 | 5767 | 0.02 | 12,716 | 0.06 |
| 2019 | 0 | 0.0000 | 5127 | 0.02 | 10,731 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musika, N.V.; Wakibara, J.V.; Ndakidemi, P.A.; Treydte, A.C. Using Trophy Hunting to Save Wildlife Foraging Resources: A Case Study from Moyowosi-Kigosi Game Reserves, Tanzania. Sustainability 2022, 14, 1288. https://doi.org/10.3390/su14031288
Musika NV, Wakibara JV, Ndakidemi PA, Treydte AC. Using Trophy Hunting to Save Wildlife Foraging Resources: A Case Study from Moyowosi-Kigosi Game Reserves, Tanzania. Sustainability. 2022; 14(3):1288. https://doi.org/10.3390/su14031288
Chicago/Turabian StyleMusika, Nyangabo V., James V. Wakibara, Patrick A. Ndakidemi, and Anna C. Treydte. 2022. "Using Trophy Hunting to Save Wildlife Foraging Resources: A Case Study from Moyowosi-Kigosi Game Reserves, Tanzania" Sustainability 14, no. 3: 1288. https://doi.org/10.3390/su14031288
APA StyleMusika, N. V., Wakibara, J. V., Ndakidemi, P. A., & Treydte, A. C. (2022). Using Trophy Hunting to Save Wildlife Foraging Resources: A Case Study from Moyowosi-Kigosi Game Reserves, Tanzania. Sustainability, 14(3), 1288. https://doi.org/10.3390/su14031288






