Novel Application of Tagua Shell (Phytelephas aequatorialis) as Adsorbent Material for the Removal of Pb(II) Ions: Kinetics, Equilibrium, and Thermodynamics of the Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbent and Adsorbate
2.2. Characterization
2.3. Experimental Conditions of the Adsorption Process
2.4. Adsorption Kinetics and Equilibrium
2.5. Adsorption Thermodynamics
3. Results and Discussion
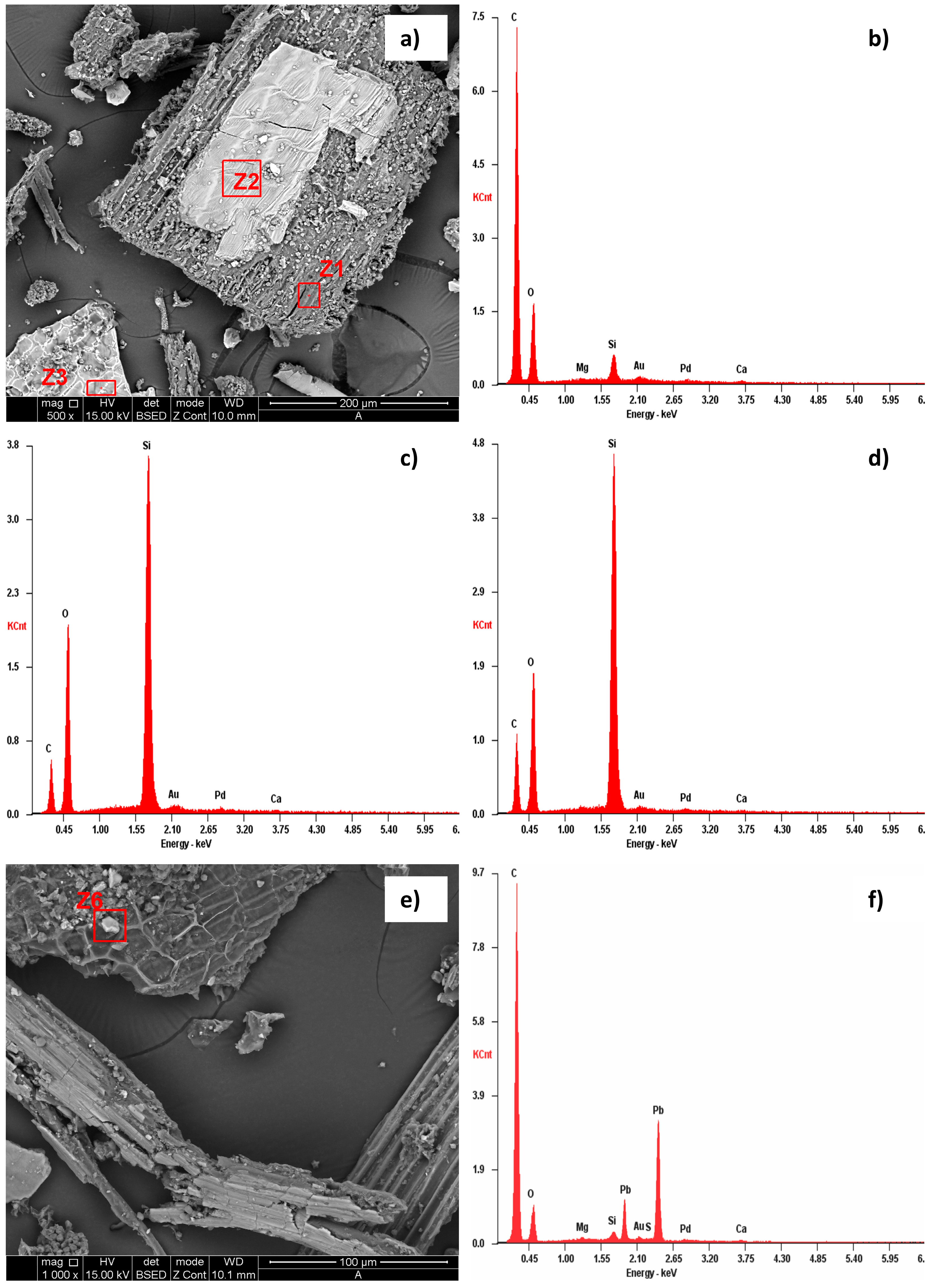
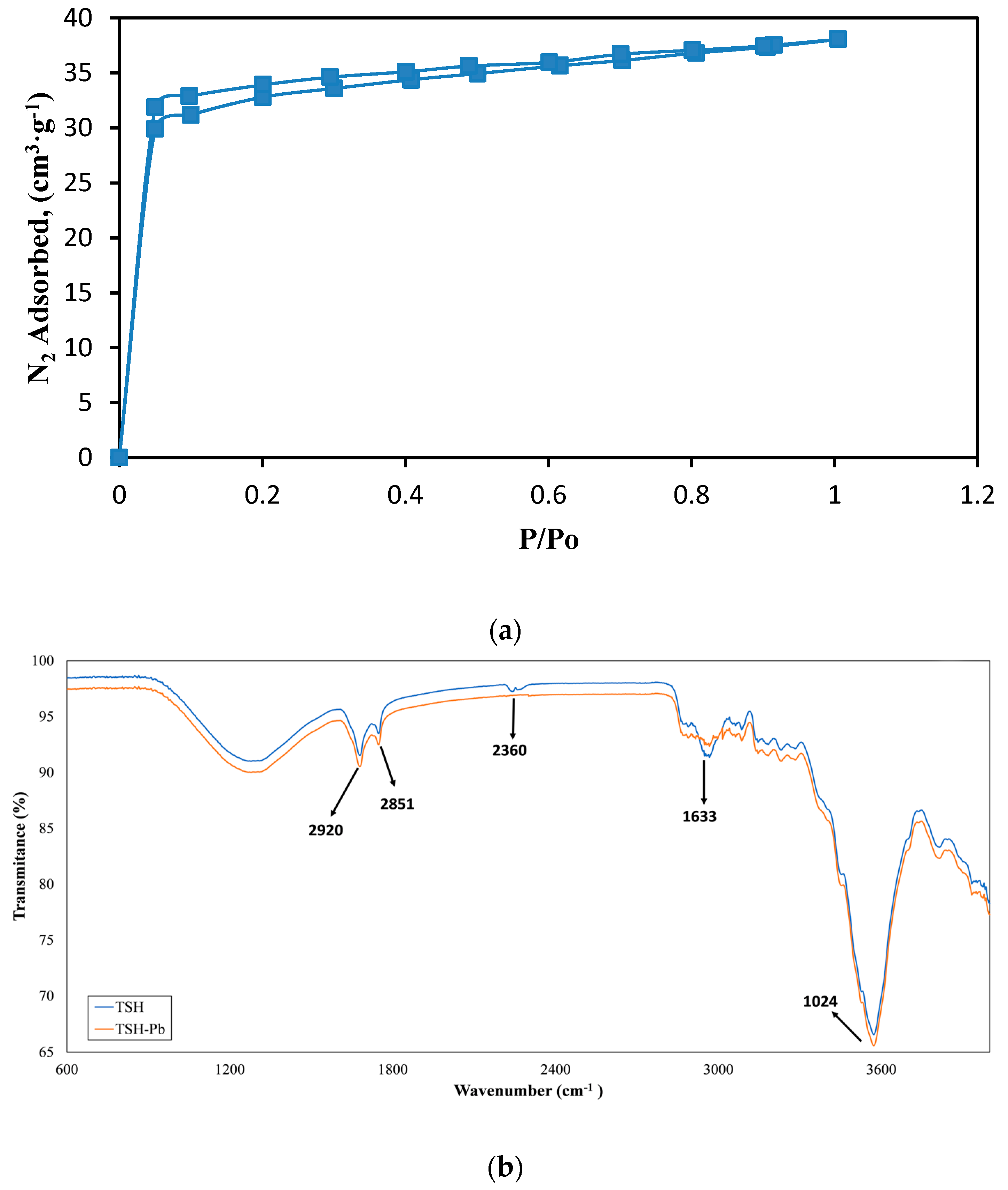
3.1. TSH Characterization
- The surface charge is positive when the pH of the solution is below seven, which favors anion adsorption;
- The surface charge is negative when the solution pH is above seven, which favors cation adsorption, and;
- The net charge of the adsorbents is zero when the pH is close to pHpzc.
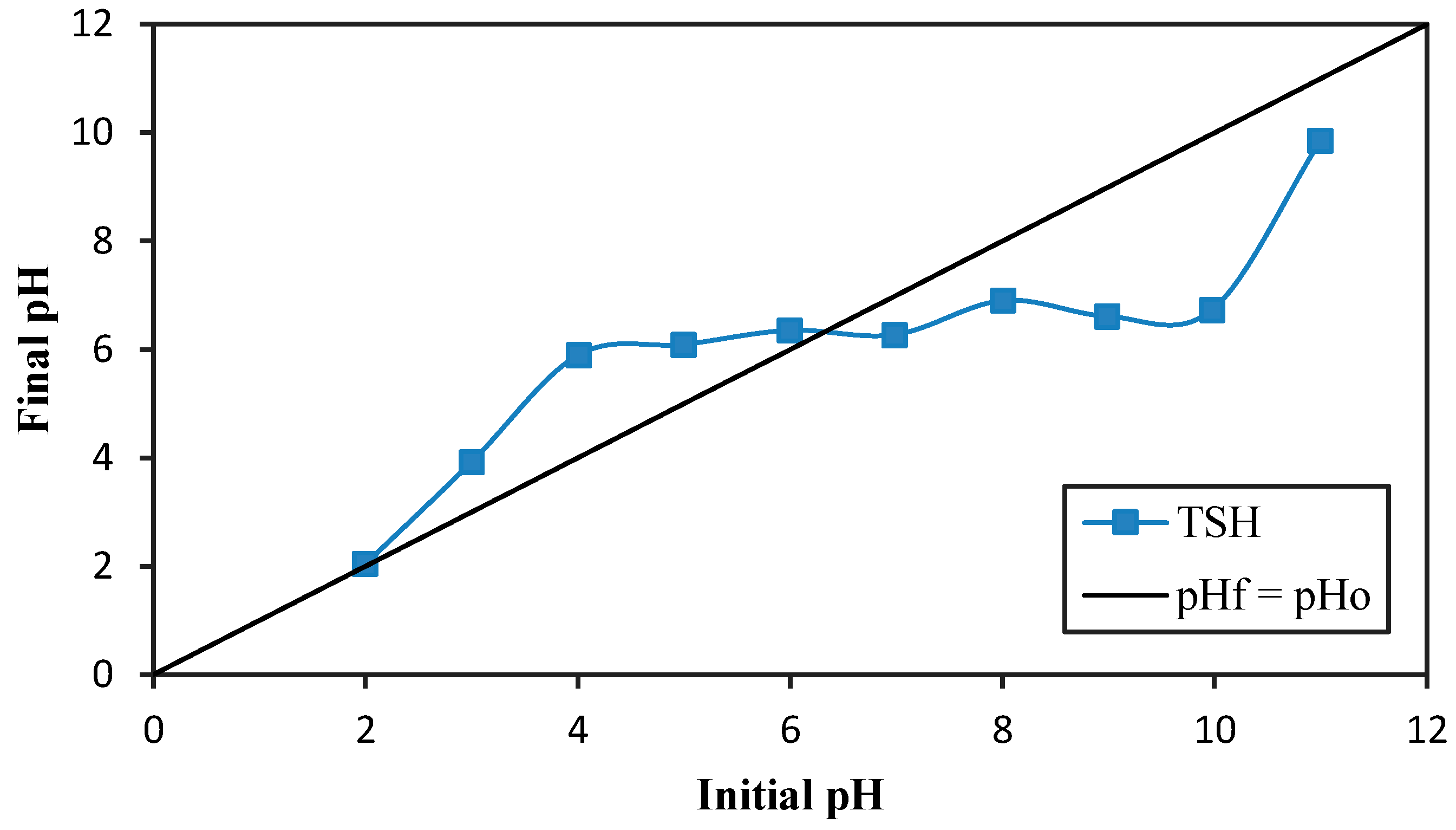
3.2. Effect of pH in Solution
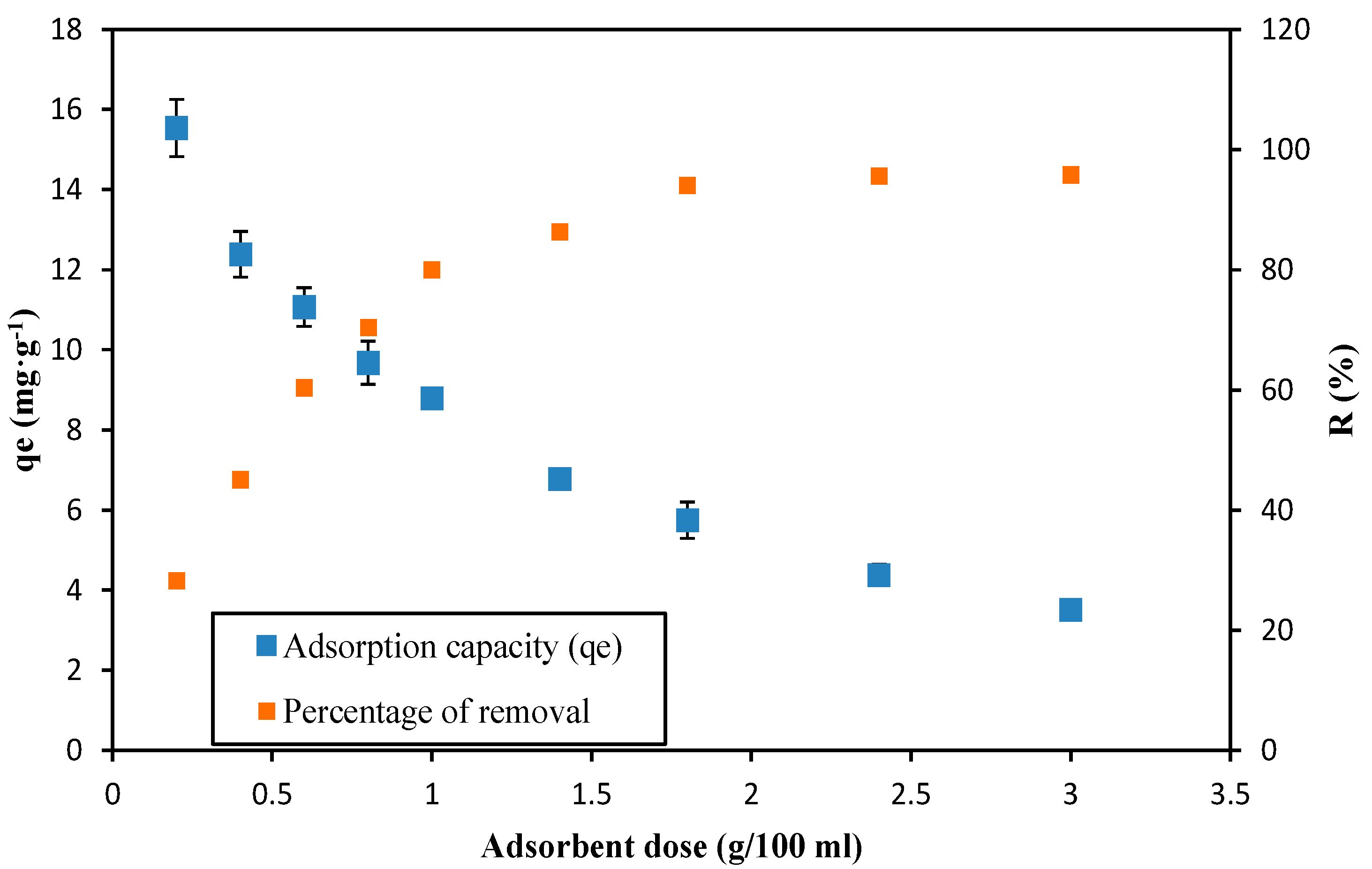
3.3. Adsorbent Dose
3.4. Contact Time
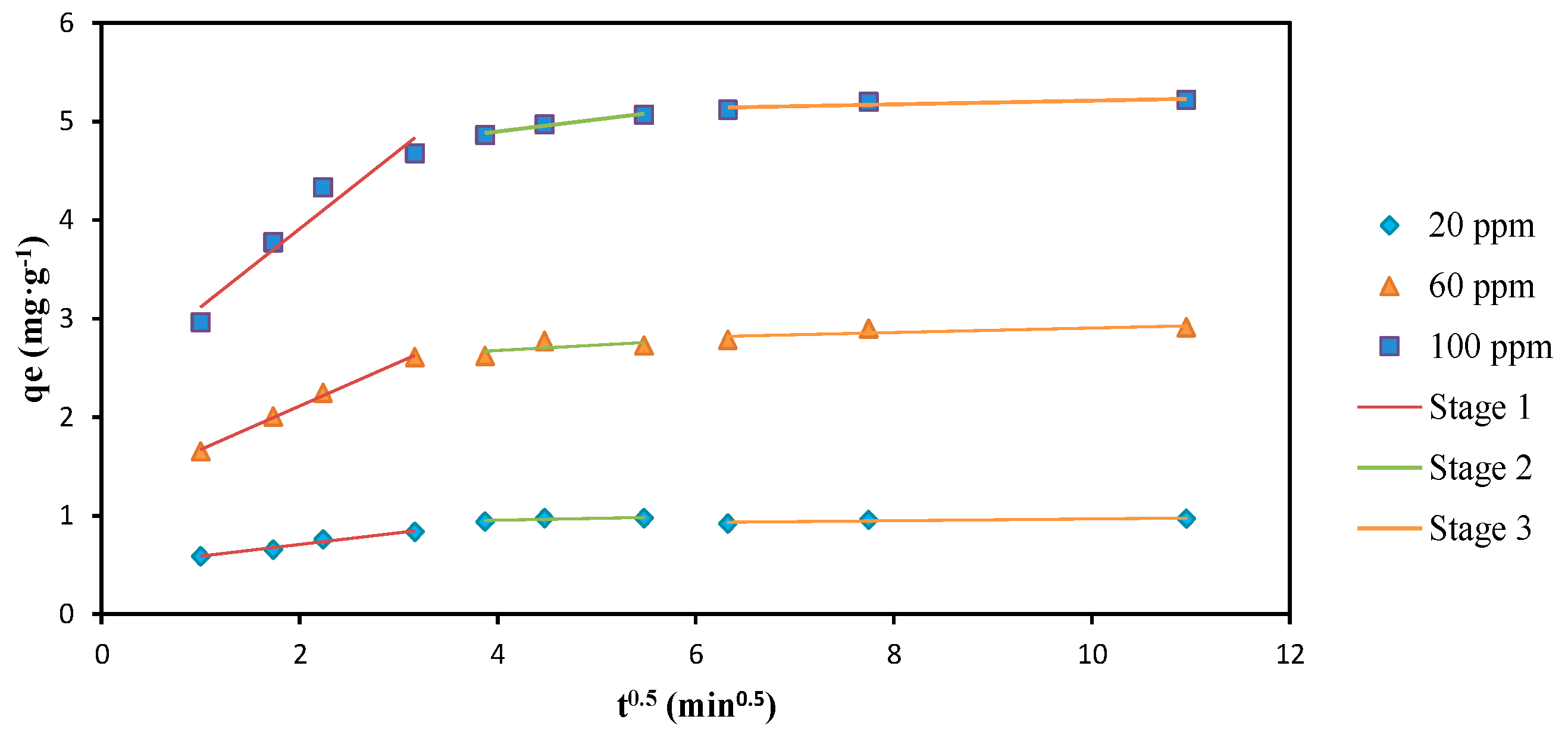
3.5. Adsorption Kinetics
3.6. Adsorption Isotherms
3.7. Adsorption Thermodynamics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- EPA. Learn about Lead. Available online: https://www.epa.gov/lead/learn-about-lead (accessed on 3 November 2021).
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- WHO. Lead Poisoning and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 3 November 2021).
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 5th ed.; McGraw-Hill: Singapore, 1993. [Google Scholar]
- Shamim, S. Biosorption of Heavy Metals. Biosorption 2018, 2, 21–49. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Lopez-Cantillo, K.; Vidales-Hernandez, K.; Villabona-Ortíz, A.; Acevedo-Correa, D. Kinetics and bioadsortion equilibrium of lead and cadmium in batch systems with cocoa shell (Theobroma Cacao L.). Contemp. Eng. Sci. 2018, 11, 1111–1120. [Google Scholar] [CrossRef][Green Version]
- Shekinah, P.; Kadirvelu, K.; Kanmani, P.; Senthilkumar, P.; Subburam, V. Adsorption of lead(II) from aqueous solution by activated carbon prepared from Eichhornia. J. Chem. Technol. Biotechnol. 2002, 77, 458–464. [Google Scholar] [CrossRef]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef]
- Heraldy, E.; Lestari, W.W.; Permatasari, D.; Arimurti, D.D. Biosorbent from tomato waste and apple juice residue for lead removal. J. Environ. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar] [CrossRef]
- Noboa Zaldumbide, M.A. Nanocelulosa de Tagua Como Agente Para Captación y Recuperación de Metales Pesados y Preciosos en Cuerpos de Agua; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2017. [Google Scholar]
- Baird, R.B.; Eaton Andrew, D.; Rice Eugene, W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Campos, N.F.; Barbosa, C.M.B.M.; Rodríguez-Díaz, J.M.; Duarte, M.M.M.B. Removal of naphthenic acids using activated charcoal: Kinetic and equilibrium studies. Adsorpt. Sci. Technol. 2018, 36, 1405–1421. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.M.; García, J.O.P.; Sánchez, L.R.B.; da Silva, M.G.C.; da Silva, V.L.; Arteaga-Pérez, L.E. Comprehensive Characterization of Sugarcane Bagasse Ash for Its Use as an Adsorbent. BioEnergy Res. 2015, 8, 1885–1895. [Google Scholar] [CrossRef]
- Jaerger, S.; dos Santos, A.; Fernandes, A.N.; Almeida, C.A.P. Removal of p-Nitrophenol from Aqueous Solution Using Brazilian Peat: Kinetic and Thermodynamic Studies. Water Air Soil Pollut. 2015, 226, 236. [Google Scholar] [CrossRef]
- Andrade, C.A.; Zambrano-Intriago, L.A.; Oliveira, N.S.; Vieira, J.S.; Quiroz-Fernández, L.S.; Rodríguez-Díaz, J.M. Adsorption Behavior and Mechanism of Oxytetracycline on Rice Husk Ash: Kinetics, Equilibrium, and Thermodynamics of the Process. Water Air Soil Pollut. 2020, 231, 103. [Google Scholar] [CrossRef]
- Zambrano-Intriago, L.A.; Gorozabel-Mendoza, M.L.; Córdova Mosquera, A.; Delgado-Demera, M.H.; Duarte, M.M.M.B.; Rodríguez-Díaz, J.M. Kinetics, equilibrium, and thermodynamics of the blue 19 dye adsorption process using residual biomass attained from rice cultivation. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Le, H.T.N.; Do, T.S.; Pham, V.T.; Lam Tran, D.; Ho, V.T.T.; Tran, T.V.; Nguyen, D.C.; Nguyen, T.D.; Bach, L.G.; et al. Metal-Organic Framework MIL-53(Fe) as an Adsorbent for Ibuprofen Drug Removal from Aqueous Solutions: Response Surface Modeling and Optimization. J. Chem. 2019, 2019, 5602957. [Google Scholar] [CrossRef]
- Tovar, C.; Herrera, A.; Ruiz, E. Kinetic and isotherms of biosorption of Hg(II) using citric acid treated residual materials. Ing. Y Compet. 2016, 18, 117–127. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Dai, J.; Chen, X.; He, J.; Chang, Z.; Yan, Y.; Li, C. Hierarchical porous carbon materials derived from a waste paper towel with ultrafast and ultrahigh performance for adsorption of tetracycline. RSC Adv. 2016, 6, 72985–72998. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lakshmi, U.R.; Srivastava, V.C.; Mall, I.D.; Lataye, D.H. Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for Indigo Carmine dye. J. Environ. Manag. 2009, 90, 710–720. [Google Scholar] [CrossRef]
- Riyap, H.I.; Bewa, C.N.; Banenzoué, C.; Tchakouté, H.K.; Rüscher, C.H.; Kamseu, E.; Bignozzi, M.C.; Leonelli, C. Microstructure and mechanical, physical and structural properties of sustainable lightweight metakaolin-based geopolymer cements and mortars employing rice husk. J. Asian Ceram. Soc. 2019, 7, 199–212. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.-B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, R.P.S.; Chandrasekaran, V. Adsorption of lead (II) ions by activated carbons prepared from marine green algae: Equilibrium and kinetics studies. Int. J. Ind. Chem. 2014, 5, 10. [Google Scholar] [CrossRef]
- Salihi, I.U.; Kutty, S.R.M.; Isa, M.H. Adsorption of Lead ions onto Activated Carbon derived from Sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2017, 201, 012034. [Google Scholar] [CrossRef]
- Staroń, P.; Płecka, A.; Chwastowski, J. Lead Sorption by Chrysanthemum indicum: Equilibrium, Kinetic, and Desorption Studies. Water Air Soil Pollut. 2021, 232, 22. [Google Scholar] [CrossRef]
- Qaiser, S.; Saleemi, A.R.; Umar, M. Biosorption of lead from aqueous solution by Ficus religiosa leaves: Batch and column study. J. Hazard. Mater. 2009, 166, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Kul, A.R.; Caliskan, N. Equilibrium and Kinetic Studies of the Adsorption of Zn(II) Ions onto Natural and Activated Kaolinites. Adsorpt. Sci. Technol. 2009, 27, 85–105. [Google Scholar] [CrossRef]
- Chwastowski, J.; Staroń, P. Influence of Saccharomyces cerevisiae yeast cells immobilized on Cocos nucifera fibers for the adsorption of Pb(II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127735. [Google Scholar] [CrossRef]
- Sekar, M.; Sakthi, V.; Rengaraj, S. Kinetics and equilibrium adsorption study of lead(II) onto activated carbon prepared from coconut shell. J. Colloid Interface Sci. 2004, 279, 307–313. [Google Scholar] [CrossRef]
- Ghahremani, D.; Mobasherpour, I.; Mirhosseini, S.A. Sorption thermodynamic and kinetic studies of Lead removal from aqueous solutions by nano Tricalcium phosphate. Bull. Soc. R. Sci. Liege 2017, 86, 96–112. [Google Scholar] [CrossRef]




| Parameters | Co (mg·L−1) | ||
|---|---|---|---|
| 20 | 60 | 100 | |
| qe,exp | 0.9594 ± 0.0672 | 2.9022 ± 0.1265 | 5.2000 ± 0.1875 |
| Pseudo-first order | |||
| qe,cal (mg·g−1) | 0.9211 | 2.7081 | 4.9353 |
| k1 (min−1) | 0.6333 | 0.6298 | 0.6743 |
| R2 | 0.6075 | 0.7088 | 0.7915 |
| X2 | 0.1136 | 0.2420 | 0.2775 |
| Pseudo-second order | |||
| qe,cal (mg·g−1) | 0.9687 | 2.8524 | 5.1885 |
| k2 (g·mg−1·min−1) | 1.1176 | 0.3687 | 0.2171 |
| R2 | 0.8620 | 0.9320 | 0.9736 |
| X2 | 0.0369 | 0.0547 | 0.0362 |
| Elovich | |||
| β (g·mg−1) | 11.0310 | 3.6203 | 2.0958 |
| α (mg·g−1·min−1) | 83.1308 | 179.1824 | 530.8156 |
| R2 | 0.8423 | 0.9164 | 0.8904 |
| X2 | 0.0333 | 0.0533 | 0.1223 |
| Bangham | |||
| KB (mg·g−1·s–α) | 0.6491 | 1.8813 | 3.5107 |
| αB | 0.1021 | 0.1066 | 0.0997 |
| R2 | 0.7982 | 0.8724 | 0.8402 |
| X2 | 0.0438 | 0.0848 | 0.1831 |
| Intraparticle diffusion | |||
| Kdif1 (mg·g−1·min–0.5) | 0.1198 | 0.4419 | 0.7950 |
| C1 | 0.4683 | 1.2320 | 2.3217 |
| R21 | 0.9681 | 0.9967 | 0.9351 |
| Kdif2 (mg·g−1·min−0.5) | 0.0180 | 0.0528 | 0.1231 |
| C2 | 0.8832 | 2.4670 | 4.4025 |
| R22 | 0.6388 | 0.3060 | 0.9630 |
| Kdif3 (mg·g−1·min–0.5) | 0.0095 | 0.0231 | 0.0189 |
| C3 | 0.8728 | 2.6754 | 5.0231 |
| R23 | 0.8020 | 0.6263 | 0.7359 |
| X2 | 0.0021 | 0.0051 | 0.0279 |
| Isotherms | Parameters | Temperature (K) | |||
|---|---|---|---|---|---|
| 288 | 298 | 308 | 318 | ||
| qe,exp | 6.9468 ± 0.1838 | 7.0366 ± 0.3067 | 7.1277 ± 0.4989 | 6.7492 ± 0.3571 | |
| Langmuir | qmax(mg·g−1) | 21.5886 | 22.0348 | 18.2598 | 10.8976 |
| KL(L·mg−1) | 0.0372 | 0.0417 | 0.0577 | 0.0912 | |
| RL | 0.57–0.16 | 0.55–0.15 | 0.46–0.11 | 0.35–0.07 | |
| R2 | 0.9629 | 0.9638 | 0.9843 | 0.9899 | |
| X2 | 0.3020 | 0.2988 | 0.1599 | 0.0767 | |
| Freundlich | KF [(mg·g−1)·(L·mg−1)1/n] | 0.8989 | 0.9997 | 1.1574 | 1.2587 |
| 1/n | 0.8087 | 0.8114 | 0.7651 | 0.5958 | |
| R2 | 0.9497 | 0.9509 | 0.9851 | 0.9779 | |
| X2 | 0.3949 | 0.3898 | 0.1960 | 0.2018 | |
| Toth | KT (mg·g−1) | 0.9024 | 18.7226 | 1.5253 | 1.8444 |
| αT (L·mg−1) | −0.0511 | 22.8995 | 1.8839 | 1.8410 | |
| 1/z | 0.1933 | 0.9628 | 0.3316 | 0.5241 | |
| R2 | 0.9496 | 0.9637 | 0.9860 | 0.9855 | |
| X2 | 0.4010 | 0.2260 | 0.1669 | 0.1161 | |
| Redlich–Peterson | KRP (L·g−1) | 3.8057 | 1.0153 | 2.2980 | 0.9100 |
| αRP (L·mg−1)β | 3.2150 | 0.0986 | 1.0487 | 0.0525 | |
| g | 0.2391 | 0.7638 | 0.3625 | 1.1452 | |
| R2 | 0.9505 | 0.9603 | 0.9855 | 0.9900 | |
| X2 | 0.3976 | 0.3352 | 0.1832 | 0.0799 | |
| Sips | qms (mg·g−1) | 8.9454 | 9.1036 | 13.0179 | 10.3128 |
| Ks (L·mg−1) | 0.0316 | 0.0400 | 0.0734 | 0.0923 | |
| ns | 1.8296 | 1.8175 | 11.515 | 1.0454 | |
| R2 | 0.9819 | 0.9823 | 0.9818 | 0.9900 | |
| X2 | 0.4221 | 0.4134 | 0.1604 | 0.0802 | |
| Temperature (K) | ΔG° (KJ·mol−1) | ΔH° (KJ·mol−1) | ΔS° (J·K−1) |
|---|---|---|---|
| Langmuir | |||
| 288 | −18.0468 | 22.7687 | 141.7204 |
| 298 | −19.4640 | ||
| 308 | −20.8812 | ||
| 318 | −22.2984 | ||
| Sips | |||
| 288 | −17.6957 | 26.2589 | 152.6201 |
| 298 | −19.2219 | ||
| 308 | −20.7481 | ||
| 318 | −22.2743 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Prado, G.A.; Benavides-García, A.B.; Zambrano-Intriago, L.A.; Maddela, N.R.; Quiroz-Fernández, L.S.; Baquerizo-Crespo, R.J.; Rodríguez-Díaz, J.M. Novel Application of Tagua Shell (Phytelephas aequatorialis) as Adsorbent Material for the Removal of Pb(II) Ions: Kinetics, Equilibrium, and Thermodynamics of the Process. Sustainability 2022, 14, 1309. https://doi.org/10.3390/su14031309
Chávez-Prado GA, Benavides-García AB, Zambrano-Intriago LA, Maddela NR, Quiroz-Fernández LS, Baquerizo-Crespo RJ, Rodríguez-Díaz JM. Novel Application of Tagua Shell (Phytelephas aequatorialis) as Adsorbent Material for the Removal of Pb(II) Ions: Kinetics, Equilibrium, and Thermodynamics of the Process. Sustainability. 2022; 14(3):1309. https://doi.org/10.3390/su14031309
Chicago/Turabian StyleChávez-Prado, Gino Alexander, Adams Brayan Benavides-García, Luis Angel Zambrano-Intriago, Naga Raju Maddela, Luis Santiago Quiroz-Fernández, Ricardo José Baquerizo-Crespo, and Joan Manuel Rodríguez-Díaz. 2022. "Novel Application of Tagua Shell (Phytelephas aequatorialis) as Adsorbent Material for the Removal of Pb(II) Ions: Kinetics, Equilibrium, and Thermodynamics of the Process" Sustainability 14, no. 3: 1309. https://doi.org/10.3390/su14031309
APA StyleChávez-Prado, G. A., Benavides-García, A. B., Zambrano-Intriago, L. A., Maddela, N. R., Quiroz-Fernández, L. S., Baquerizo-Crespo, R. J., & Rodríguez-Díaz, J. M. (2022). Novel Application of Tagua Shell (Phytelephas aequatorialis) as Adsorbent Material for the Removal of Pb(II) Ions: Kinetics, Equilibrium, and Thermodynamics of the Process. Sustainability, 14(3), 1309. https://doi.org/10.3390/su14031309










