Phenol Adsorption Mechanism of Organically Modified Bentonite and Its Microstructural Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. OMB Preparation
2.3. OMB Characterization
2.4. Phenol Adsorption Test of OMB
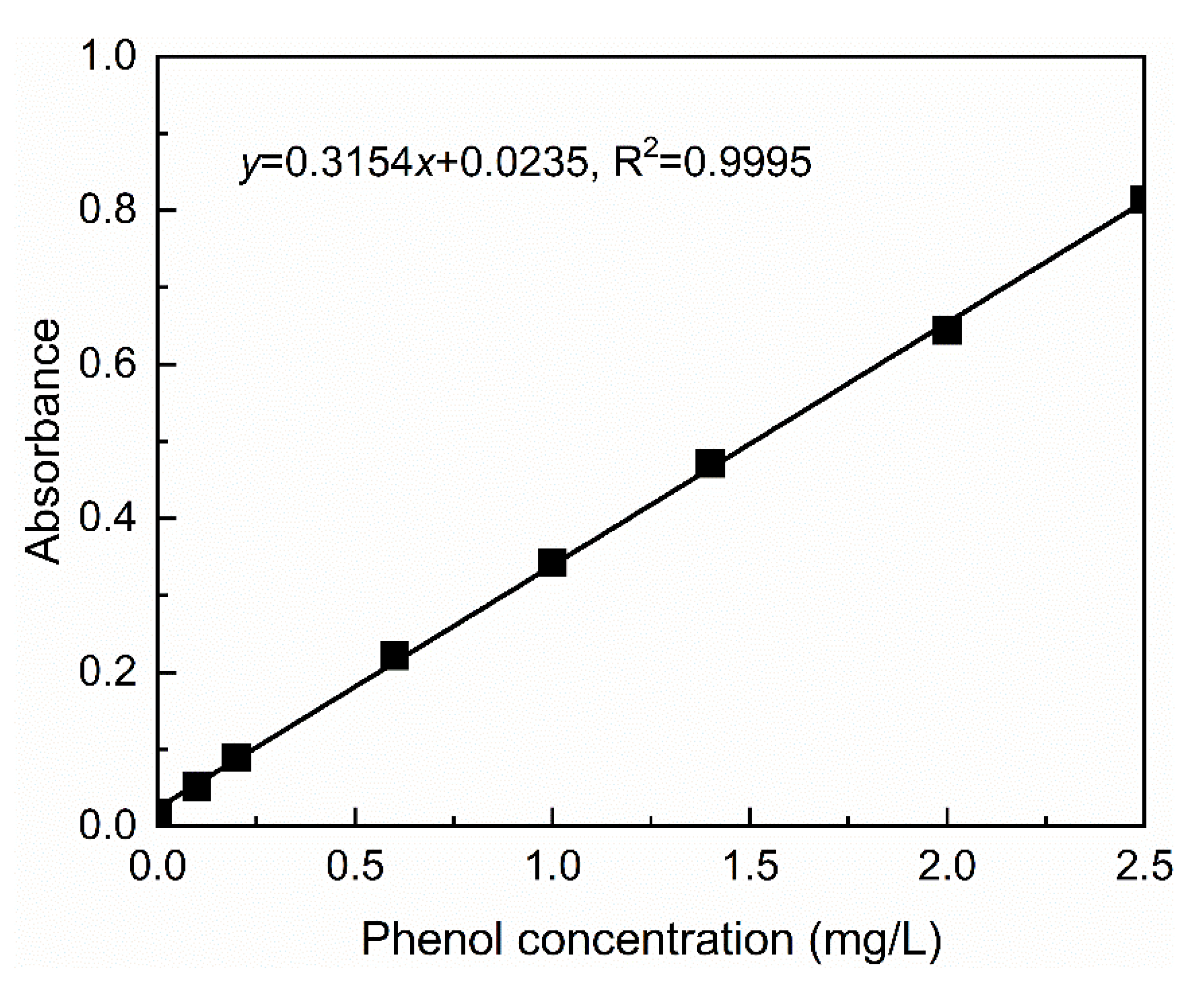
2.4.1. Determination of the Calibration Curve of Phenol Solution
2.4.2. Adsorption Test
2.4.3. Adsorption Kinetic Analysis of OMB
3. Results and Discussion
3.1. Effect of Phenol Solution Concentration
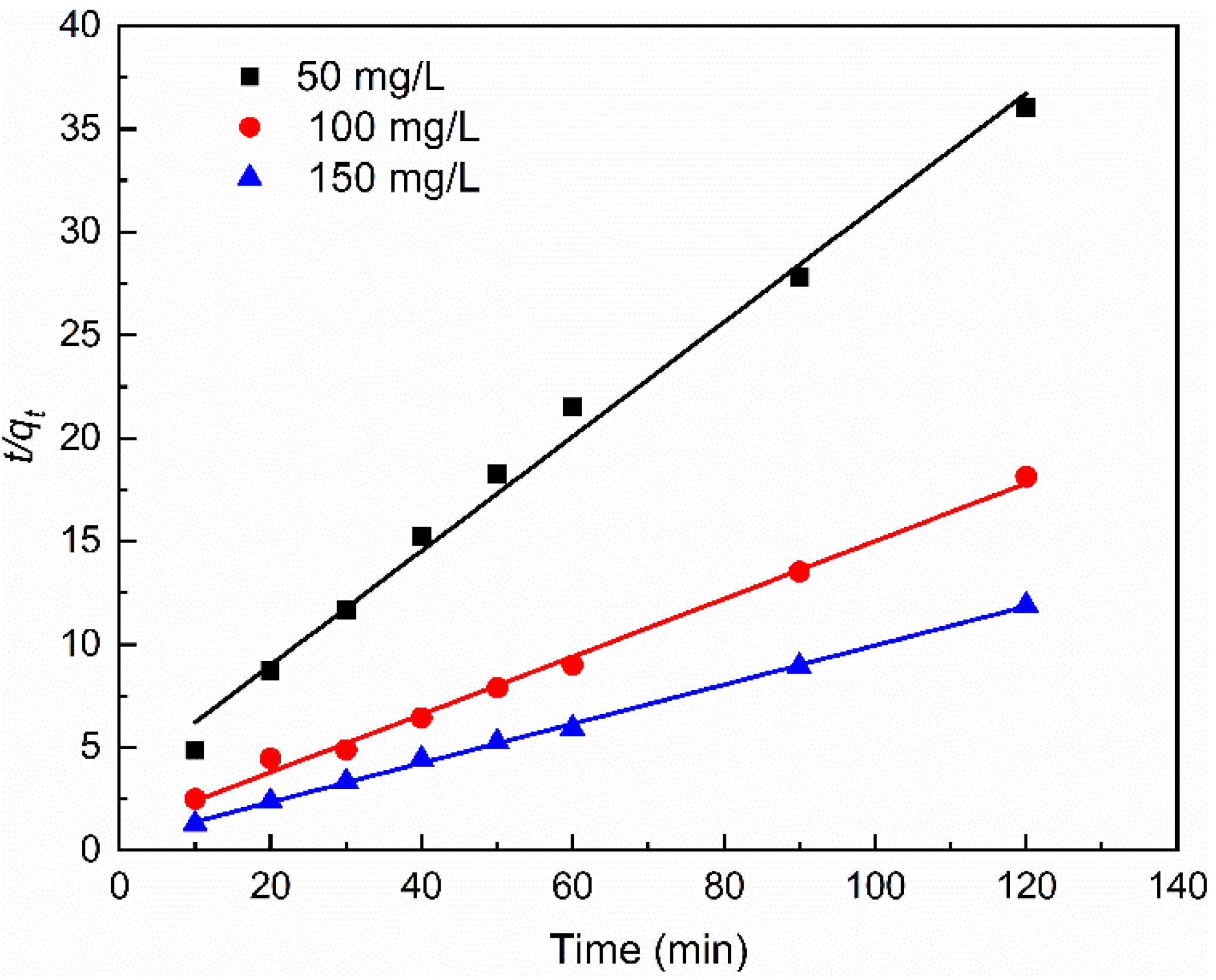
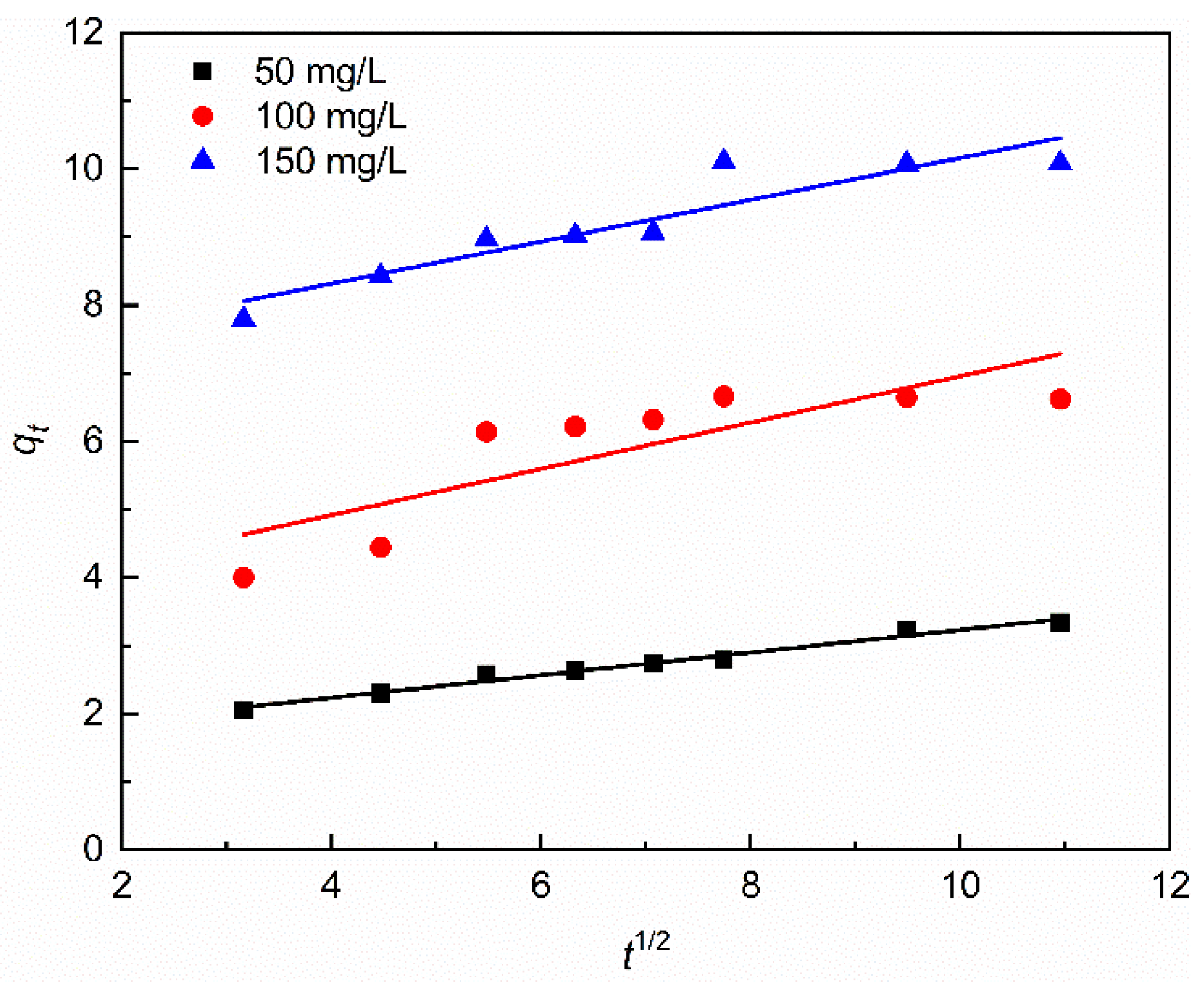
3.2. Adsorption Kinetic
3.3. Microscopic Characterization of OMB with Different Adsorption Times
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Q.; Huang, X.; Luo, Y.; Yuan, H.; Ren, T.; Li, X.; Xu, D.; Guo, X.; Wu, Y. Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr (VI). Chem. Eng. J. 2021, 407, 127050. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, Y.; Lei, Z.; Peng, J.; Xu, D.; Guo, X.; Wu, Y. Wood-derived nanocellulose hydrogel incorporating gold nanoclusters using in situ multistep reactions for efficient sorption and sensitive detection of mercury ion. Ind. Crop. Prod. 2021, 173, 114142. [Google Scholar] [CrossRef]
- Luo, Q.; Ren, T.; Lei, Z.; Huang, Y.; Huang, Y.; Xu, D.; Wan, C.; Guo, X.; Wu, Y. Non-toxic chitosan-based hydrogel with strong adsorption and sensitive detection abilities for tetracycline. Chem. Eng. J. 2022, 427, 131738. [Google Scholar] [CrossRef]
- Luo, Q.; Yuan, H.; Zhang, M.; Jiang, P.; Liu, M.; Xu, D.; Guo, X.; Wu, Y. A 3D porous fluorescent hydrogel based on amino-modified carbon dots with excellent sorption and sensing abilities for environmentally hazardous Cr (VI). J. Hazard. Mater. 2021, 401, 123432. [Google Scholar] [CrossRef]
- Yuan, H.; Ren, T.; Luo, Q.; Huang, Y.; Huang, Y.; Xu, D.; Guo, X.; Li, X.; Wu, Y. Fluorescent wood with non-cytotoxicity for effective adsorption and sensitive detection of heavy metals. J. Hazard. Mater. 2021, 416, 126166. [Google Scholar] [CrossRef]
- Annan, E.; Nyankson, E.; Agyei-Tuffour, B.; Armah, S.K.; Nkrumah-Buandoh, G.; Hodasi, J.A.M.; Oteng-Peprah, M. Synthesis and Characterization of Modified Kaolin-Bentonite Composites for Enhanced Fluoride Removal from Drinking Water. Adv. Mater. Sci. Eng. 2021, 2021, 6679422. [Google Scholar] [CrossRef]
- Diaz-Nava, M.; Olguin, M.; Solache-Rios, M. Adsorption of phenol onto surfactants modified bentonite. J. Incl. Phenom. Macro. 2012, 74, 67–75. [Google Scholar] [CrossRef]
- Uddin, F. Montmorillonite: An Introduction to Properties and Utilization; IntechOpen: London, UK, 2018. [Google Scholar]
- Min, F.; Wang, X.; Li, M.; Ni, Y.; Al-qadhi, E.; Zhang, J. Preparation of High-Porosity and High-Strength Ceramisites from Municipal Sludge Using Starch and CaCO3 as a Combined Pore-Forming Agent. J. Mater. Civil Eng. 2021, 33, 04020502. [Google Scholar] [CrossRef]
- Xu, H.; Shu, S.; Wang, S.; Zhou, A.; Jiang, P.; Zhu, W.; Fan, X.; Chen, L. Studies on the chemical compatibility of soil-bentonite cut-off walls for landfills. J. Environ. Manag. 2019, 237, 155–162. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Z.; Zhao, Y. Laboratory evaluation of hydraulic conductivity and chemical compatibility of bentonite slurry for grouting walls. Environ. Earth. Sci. 2021, 80, 569. [Google Scholar] [CrossRef]
- Liu, G.; Wu, L.; Ye, C.; Liu, Y.; Huang, Q.; Wen, M.; Liao, B.; Lu, T.; He, T. Study on controlling of cadmium pollution with fly ash-bentonite blocking wall. Chemosphere 2019, 228, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Xue, Q.; Liu, L.; Wang, S. Relationship between the shrinkage crack characteristics and the water content gradient of compacted clay liner in a landfill final cover. Soils Found. 2018, 58, 1435–1445. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Krol, M.M.; Rowe, R.K. Diffusion of TCE through soil-bentonite slurry walls. Soil Sediment. Contam. 2004, 13, 81–101. [Google Scholar] [CrossRef]
- Goodarzi, A.; Fateh, S.N.; Shekary, H. Impact of organic pollutants on the macro and microstructure responses of Na-bentonite. Appl. Clay Sci. 2016, 121, 17–28. [Google Scholar] [CrossRef]
- Liu, Y.; Bouazza, A.; Gates, W.; Rowe, R. Hydraulic performance of geosynthetic clay liners to sulfuric acid solutions. Geotext. Geomembr. 2015, 43, 14–23. [Google Scholar] [CrossRef]
- Ashmawy, A.K.; El-Hajji, D.; Sotelo, N.; Muhammad, N. Hydraulic performance of untreated and polymer-treated bentonite in inorganic landfill leachates. Clays Clay Miner. 2002, 50, 546–552. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, H.; Xiao, L. Formation mechanism of carbide slag composite sustained-alkalinity-release particles for the source control of acid mine drainage. Sci. Rep. 2021, 11, 23793. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.-s.; Xue, Q.; Chen, Z.; Du, Y.-j.; Wan, Y.; Liu, L.; Poon, C.S. Use of self-hardening slurry for trench cutoff wall: A review. Constr. Build. Mater. 2021, 286, 122959. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Guo, Y.X.; Liu, J.H.; Gates, W.P.; Zhou, C.H. Organo-modification of montmorillonite. Clays Clay Miner. 2020, 68, 601–622. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Cheng, J.; Pan, Y.; Yang, G.; Liu, Y.; Wang, L.; Leng, Y.; Tuo, X. Synthesis and characterization of CTAB-modified bentonite composites for the removal of Cs+. J. Radioanal. Nucl. Chem. 2021, 329, 451–461. [Google Scholar] [CrossRef]
- Guo, M.; Yang, G.; Zhang, S.; Zhang, Y.; Gao, C.; Zhang, C.; Zhang, P. Co-modification of Bentonite by CTAB and Silane and its Performance in Oil-Based Drilling Mud. Clays Clay Miner. 2020, 68, 646–655. [Google Scholar] [CrossRef]
- Al Rubai, H.F.; Hassan, A.K.; Sultan, M.S.; Abood, W.M. Kinetics of Adsorption of Reactive Red 120 Using Bentonite Modified by CTAB and Study the Effect of Salts. Nat. Environ. Pollut. Technol. 2021, 20, 281–289. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Modification of bentonite with cationic and nonionic surfactants: Structural and textural features. Materials 2019, 12, 3772. [Google Scholar] [CrossRef] [Green Version]
- Perelomov, L.; Mandzhieva, S.; Minkina, T.; Atroshchenko, Y.; Perelomova, I.; Bauer, T.; Pinsky, D.; Barakhov, A. The synthesis of organoclays based on clay minerals with different structural expansion capacities. Minerals 2021, 11, 707. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Zhao, M.; Wei, L.; Zheng, Y.; Liu, M.; Wang, J.; Qiu, Y. Structural effect of imidazolium-type ionic liquid adsorption to montmorillonite. Sci. Total. Environ. 2019, 666, 858–864. [Google Scholar] [CrossRef]
- Rivas-Rojas, P.C.; Ollier, R.P.; Alvarez, V.A.; Huck-Iriart, C. Enhancing the integration of bentonite clay with polycaprolactone by intercalation with a cationic surfactant: Effects on clay orientation and composite tensile properties. J. Mater. Sci. 2021, 56, 5595–5608. [Google Scholar] [CrossRef]
- Intachai, S.; Suppaso, C.; Khaorapapong, N. A Novel Process for Intercalating Alkylammonium Ions in a Thai Bentonite and its Effect on Adsorption Performance. Clays Clay Miner. 2021, 69, 477–488. [Google Scholar] [CrossRef]
- Tang, Y.; Slaný, M.; Yang, Y.; Li, S.; Qin, F.; Zhao, Y.; Zhang, Z.; Zhang, L. Highly active Mg–Al hydrotalcite for efficient O-methylation of phenol with DMC based on soft colloidal templates. J. Chem. Technol. Biot. 2022, 97, 79–86. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Lu, M.; Li, Z.; Wang, X.; Meng, y.; Yuxin, Z. Adsorption properties of organic bentonite for phenols in wastewater. J. Taiyuan Univ. Technol. 2018, 05, 681–685. [Google Scholar]
- Lin, T.; Wang, J.; Yin, X.; Wei, X. Modification of Bentonite and Its Application in Antimicrobial Material. Bentonite Modif. 2020, 5, 54–61. [Google Scholar]
- Zhang, L.; Tu, L.-y.; Liang, Y.; Chen, Q.; Li, Z.-s.; Li, C.-h.; Wang, Z.-h.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T. Research on the Characteristics of Organic Modified Bentonite for Phenol Removing. Henan Chem. Ind. 2016, 12, 21–23. [Google Scholar]




| Adsorbent Concentration, mg/L | 50 | 100 | 150 | |
|---|---|---|---|---|
| Pseudo-first-order kinetic equation | qe | 2.1802 | 2.9432 | 2.6267 |
| k1 | 0.0337 | 0.0533 | 0.0235 | |
| R12 | 0.8913 | 0.6985 | 0.7511 | |
| Pseudo-second-order kinetic equation | qe | 3.6054 | 7.1382 | 10.5252 |
| k2 | 0.0223 | 0.0196 | 0.0202 | |
| R22 | 0.9904 | 0.9946 | 0.9986 | |
| Intraparticle diffusion equation | C | 1.5720 | 3.5595 | 7.0880 |
| k3 | 0.1660 | 0.3398 | 0.3075 | |
| R32 | 0.9753 | 0.6378 | 0.8386 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Xu, E.; Qiu, Z.; Wu, T.; Wang, S.; Lu, Y.; Chen, G. Phenol Adsorption Mechanism of Organically Modified Bentonite and Its Microstructural Changes. Sustainability 2022, 14, 1318. https://doi.org/10.3390/su14031318
He H, Xu E, Qiu Z, Wu T, Wang S, Lu Y, Chen G. Phenol Adsorption Mechanism of Organically Modified Bentonite and Its Microstructural Changes. Sustainability. 2022; 14(3):1318. https://doi.org/10.3390/su14031318
Chicago/Turabian StyleHe, Haijie, Erpei Xu, Zhanhong Qiu, Tao Wu, Shifang Wang, Yuhua Lu, and Guannian Chen. 2022. "Phenol Adsorption Mechanism of Organically Modified Bentonite and Its Microstructural Changes" Sustainability 14, no. 3: 1318. https://doi.org/10.3390/su14031318
APA StyleHe, H., Xu, E., Qiu, Z., Wu, T., Wang, S., Lu, Y., & Chen, G. (2022). Phenol Adsorption Mechanism of Organically Modified Bentonite and Its Microstructural Changes. Sustainability, 14(3), 1318. https://doi.org/10.3390/su14031318





