Telling the Wood from the Trees: Ranking a Tree Species List to Aid Urban Afforestation in the Amazon
Abstract
1. Introduction
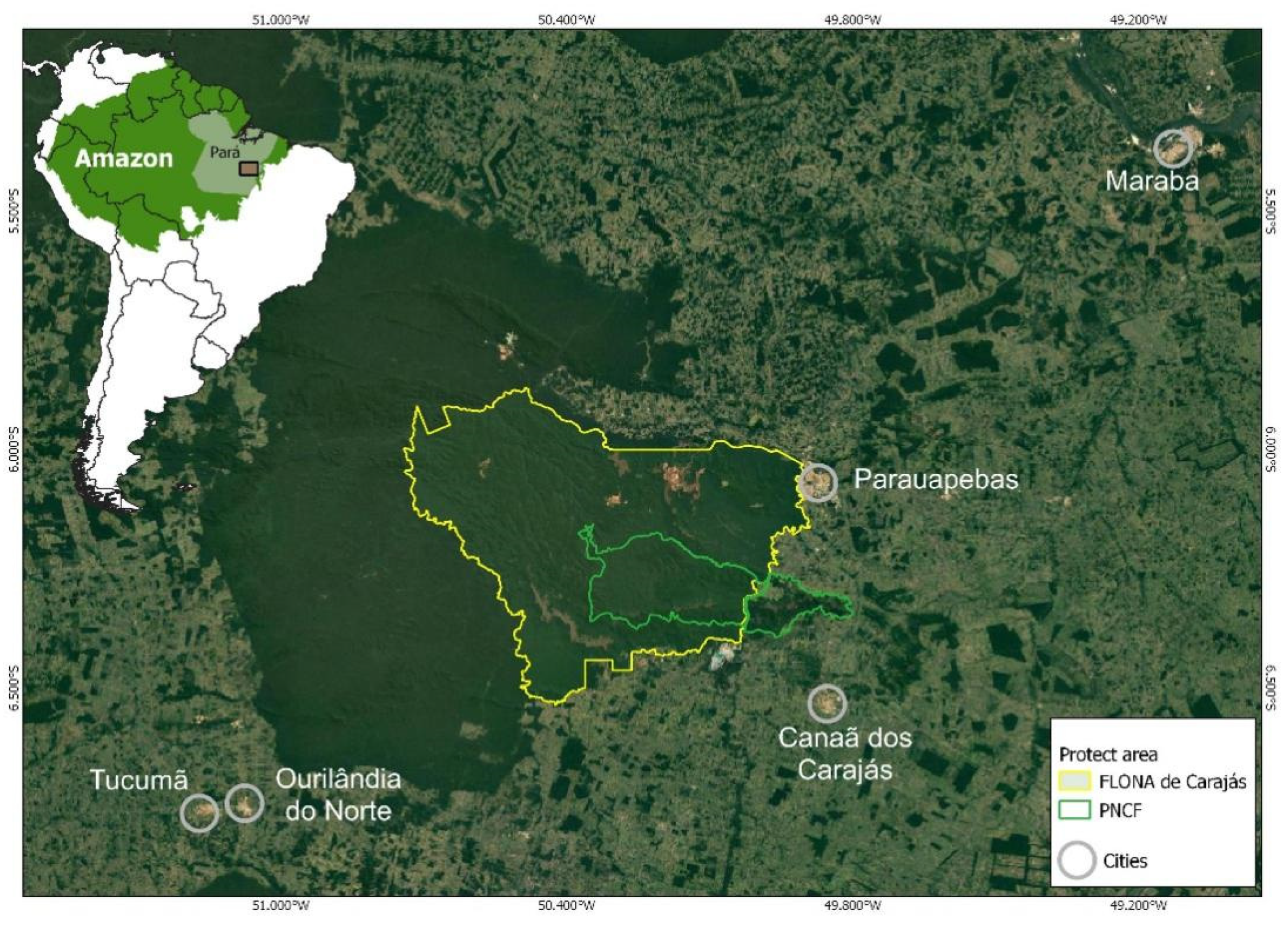
2. Material and Methods
3. Results
- Portraits of Selected Species
- Inga nobilis (Fabaceae)
- Inga umbellifera (Fabaceae)
- Swartzia arumateuana (Fabaceae)
- Swartzia brachyrachis (Fabaceae)
- Theobroma speciosum (Malvaceae)
- Bellucia grossularioides (Melastomataceae)
- Mouriri acutiflora (Melastomataceae)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hopkins, M.J.G. Are We Close to Knowing the Plant Diversity of the Amazon? An. Acad. Bras. Ciênc. 2019, 91, e20190396. [Google Scholar] [CrossRef] [PubMed]
- Brazil Flora Group [BFG]. Growing Knowledge: An Overview of Seed Plant Diversity in Brazil. Rodriguésia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Zappi, D.C.; Gastauer, M.; Ramos, S.; Nunes, S.; Caldeira, C.F.; Souza-Filho, P.W.; Guimarães, T.; Giannini, T.C.; Viana, P.L.; Lovo, J.; et al. Plantas Nativas Para Recuperação de Áreas de Mineração Em Carajás; Instituto Tecnológico Vale: Belém, Brazil, 2018; ISBN 978-85-943650-4-0. [Google Scholar]
- De Souza, D.O.; Do Nascimento, M.G.; dos Santos Alvalá, R.C. Influência do Crescimento Urbano sobre o Microclima de Manaus e Belém: Um Estudo Observacional (The influence of urban growth on the microclimate of Manaus and Belém: A observational study). Rev. Bras. Geogr. Física 2015, 8, 1109–1124. [Google Scholar] [CrossRef]
- Soares, A.C.S.; dos Santos, R.O.; Soares, R.N.; Cantuaria, P.C.; de Lima, R.B.; da Silva e Silva, B.M. Paradox of Afforestation in Cities in the Brazilian Amazon: An Understanding of the Composition and Floristic Similarity of These Urban Green Spaces. Urban For. Urban Green. 2021, 66, 127374. [Google Scholar] [CrossRef]
- Vieira, T.A.; Panagopoulos, T. Urban Forestry in Brazilian Amazonia. Sustainability 2020, 12, 3235. [Google Scholar] [CrossRef]
- Dos Santos, E.B.; Nogueira, F.M.; Talgatti, D.M. Plant Species Composition and the Perception of the Afforestation in Urban Public Green Spaces in a Municipality in Eastern Brazilian Amazon. Sustainability 2021, 13, 10332. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem Consequences of Biological Invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Gaertner, M.; Fisher, J.; Sharma, G.; Esler, K. Insights into Invasion and Restoration Ecology: Time to Collaborate towards a Holistic Approach to Tackle Biological Invasions. NeoBiota 2012, 12, 57–76. [Google Scholar] [CrossRef]
- Da Silva, F.P.; Fadini, R.F. Observational and Experimental Evaluation of Hemiparasite Resistance in Trees in the Urban Afforestation of Santarém, Pará, Brazil. Acta Amaz. 2017, 47, 311–320. [Google Scholar] [CrossRef]
- Guimarães, M.M. A influência da arborização urbana e do ruído sobre a avifauna do Plano Piloto de Brasília. Master’s Thesis, Universidade de Brasília, Brasilia, Brazil, 2020. [Google Scholar]
- Moro, M.F.; Castro, A.S.F. A Check List of Plant Species in the Urban Forestry of Fortaleza, Brazil: Where Are the Native Species in the Country of Megadiversity? Urban Ecosyst. 2015, 18, 47–71. [Google Scholar] [CrossRef]
- Rufino, M.R.; Silvino, A.S.; Moro, M.F. Exóticas, exóticas, exóticas: Reflexões sobre a monótona arborização de uma cidade brasileira. Rodriguésia 2019, 70, e03562017. [Google Scholar] [CrossRef]
- Lima, J.R.; de Oliveira Filho, L.S. Publicações sobre arborização urbana na região Nordeste, Brasil. Rev. Soc. Bras. Arborização Urbana 2020, 15, 56–69. [Google Scholar] [CrossRef]
- Gomes, A.C.; de Figueredo, L.F.; Souza, J.T.A.; Souto, J.S.; de Lacerda, A.V. Flora of Public Squares in the City of Areia, Paraíba, Brazil. Floresta E Ambiente 2019, 26, e20170277. [Google Scholar] [CrossRef]
- Da Silva Ferro, C.C.; Oliveira, R.S.; Andrade, F.W.C.; da Rocha Souza, S.M.A. Inventário quali-quantitativo da arborização viária de um trecho da rodovia PA-275 no município de Parauapebas-PA. Rev. Soc. Bras. Arborização Urbana 2015, 10, 73–84. [Google Scholar] [CrossRef]
- Gama Neto, O. Arborização Urbana Em Belém: Diálogo Entre Tempos; Universidade Federal do Pará-Instituto de Tecnologia: Belém, Brazil, 2013. [Google Scholar]
- Moraes, R.P.; Pereira, J.A.A.; Silva, R.A.; de Souza Lopes, M.; de Xisto, F.A.; Silva, R.A.S. Challenges in Urban Afforestation of the Amazon: Exotic Species and Management / Desafios Da Arborização Urbana Na Amazônia: Espécies Exóticas e Gestão. Braz. J. Anim. Environ. Res. 2021, 4, 920–932. [Google Scholar] [CrossRef]
- Guilherme, F.A.G.; Silva, M.C.; Carneiro, D.N.M.; Nascimento, H.C.A.; Ressel, K.; Ferreira, W.C. Urban Arborization in Public Pathways of Four Cities in East Mato Grosso Do Sul (MS) Brazil. Ornam. Hortic. 2018, 24, 174–181. [Google Scholar] [CrossRef]
- Munduruku, D.K.; dos Santos Mesquita, N.; Guedes, T.M.; Munduruku, I.B.K.; Maestri, M.P.; de Sousa, S.F. Percepção ambiental e arborização urbana na praça do pescador e do parque da cidade, localizadas em Santarém, PA. Nat. Resour. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Pena, J.C.d.C.; Martello, F.; Ribeiro, M.C.; Armitage, R.A.; Young, R.J.; Rodrigues, M. Street Trees Reduce the Negative Effects of Urbanization on Birds. PLoS ONE 2017, 12, e0174484. [Google Scholar] [CrossRef]
- Martini, A.; Biondi, D.; Batista, A.C. Urban Forest Components Influencing Microclimate and Cooling Potential. Rev. Árvore 2017, 41. [Google Scholar] [CrossRef]
- De Abreu-Harbich, L.V.; Labaki, L.C.; Matzarakis, A. Effect of Tree Planting Design and Tree Species on Human Thermal Comfort in the Tropics. Landsc. Urban Plan. 2015, 138, 99–109. [Google Scholar] [CrossRef]
- Wiesel, P.G.; Dresch, E.; de Santana, E.R.R.; Loboestan, E.A. Urban Afforestation and Its Ecosystem Balance Contribution: A Bibliometric Review. Manag. Environ. Qual. Int. J. 2021, 32, 453–469. [Google Scholar] [CrossRef]
- Beckmann-Wübbelt, A.; Fricke, A.; Sebesvari, Z.; Yakouchenkova, I.A.; Fröhlich, K.; Saha, S. High Public Appreciation for the Cultural Ecosystem Services of Urban and Peri-urban Forests during the COVID-19 Pandemic. Sustain. Cities Soc. 2021, 74, 103240. [Google Scholar] [CrossRef]
- Derks, J.; Giessen, L.; Winkel, G. COVID-19-Induced Visitor Boom Reveals the Importance of Forests as Critical Infrastructure. For. Policy Econ. 2020, 118, 102253. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Kark, S. Motivations for Conserving Urban Biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef]
- CBD Aichi Biodiversity Targets. Available online: https://www.cbd.int/sp/targets (accessed on 26 November 2021).
- Sirakaya, A.; Cliquet, A.; Harris, J. Ecosystem Services in Cities: Towards the International Legal Protection of Ecosystem Services in Urban Environments. Ecosyst. Serv. 2018, 29, 205–212. [Google Scholar] [CrossRef]
- Cirilo, B.B.; Almeida, O.T. Os limites à atuação do poder público municipal na gestão de recursos hídricos das bacias hídrográficas do rio Marapanim e do rio Itacaiúnas, estado do Pará. Geografares 2020, 31, 268–292. [Google Scholar]
- Zappi, D.C.; Hiura, A.L.; Barbosa-Silva, R.G. Lista de Espécies de Plantas Da Floresta e Dos Capões Sobre Canga; Instituto Tecnológico Vale: Belém, Brazil, 2019; p. 37. [Google Scholar]
- Flora do Brasil Flora Do Brasil Online 2020, Jardim Botânico Do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/reflora/listaBrasil/ConsultaPublicaUC/ResultadoDaConsultaNovaConsulta.do#CondicaoTaxonCP (accessed on 8 March 2020).
- INCT. Herbário Virtual Da Flora e Dos Fungos. Available online: http://inct.splink.org.br (accessed on 1 December 2021).
- REFLORA, 2020 Reflora-Virtual Herbarium. Available online: http://reflora.jbrj.gov.br/reflora/herbarioVirtual (accessed on 1 November 2021).
- CEMIG. Manual de Arborização; CEMIG/Fundação Biodiversitas: Belo Horizonte, Brazil, 2011. [Google Scholar]
- Salomão, R.P.; Matos, A.H.; Rosa, N.A.; Bezerra, A.V. Seleção de Espécies Arbóreas Ornamentais para Arborização Urbana Adequada a Rede Elétrica, Estado do Pará. In Proceedings of the Anais do II Congresso de Inovação Tecnológica em Energia Elétrica, Salvador, Brazil, 13–14 November 2003; pp. 660–663. [Google Scholar]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Faegri, K.; Pijl, L.V.D. Principles of Pollination Ecology; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1-4832-9303-5. [Google Scholar]
- Pavoine, S.; Vallet, J.; Dufour, A.-B.; Gachet, S.; Daniel, H. On the Challenge of Treating Various Types of Variables: Application for Improving the Measurement of Functional Diversity. Oikos 2009, 118, 391–402. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; vs. 4.0.1; R Core Team R: Vienna, Austria, 2021. [Google Scholar]
- Thioulouse, J.; Dray, S.; Dufour, A.-B.; Siberchicot, A.; Jombart, T.; Pavoine, S. Multivariate Analysis of Ecological Data with Ade4; Springer: New York, NY, USA, 2018; ISBN 978-1-4939-8848-8. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Core Team R: Vienna, Austria, 2010. [Google Scholar]
- Sirayama, L.; Lima, O.F. Biodiversidade na Província Petrolífera de Urucu; PETROBRAS. CENPES: Rio de Janeiro, Brazil, 2008; ISBN 978-85-99891-04-9. [Google Scholar]
- Hands, M.R. The uses of Inga in the acid soils of the Rainforest zone: Alley-cropping sustainability and soil-regeneration. In The Genus Inga: Utilization; Pennington, T.D., Fernandes, E.C.M., Eds.; The Royal Botanic Gardens: Kew, UK, 1998; pp. 53–86. [Google Scholar]
- Tropical Botany. How to Prepare Inga Seeds for Sowing. Available online: https://tropicalbotany.wordpress.com/2013/12/29/inga-seeds-and-their-preparation-for-sowing/ (accessed on 1 December 2021).
- Souza, L.A.G.D.; Silva, M.F.D. Bioeconomical Potential of Leguminoseae from the Lower Negro River, Amazon, Brazil. Lyonia 2003, 5, 15–24. [Google Scholar]
- David, M.D.; Gonçalves, K.G.; Neto, G.G. A subfamília Mimosoideae (Fabaceae) para a flora de mato grosso, Brasil. Biodiversidade 2015, 4, 16. [Google Scholar]
- Lima, N.M.; Santos VN, C.; Porta, L. Chemodiversity, Bioactivity and Chemosystematics of the Genus Inga (FABACEAE): A Brief Review. Rev. Virtual Quím. 2018, 10, 459–473. [Google Scholar] [CrossRef]
- Coley, P.D.; Endara, M.-J.; Ghabash, G.; Kidner, C.A.; Nicholls, J.A.; Pennington, R.T.; Mills, A.G.; Soule, A.J.; Lemes, M.R.; Stone, G.N.; et al. Macroevolutionary Patterns in Overexpression of Tyrosine: An Anti-herbivore Defence in a Speciose Tropical Tree Genus, Inga (Fabaceae). J. Ecol. 2019, 107, 1620–1632. [Google Scholar] [CrossRef]
- Torke, B.M.; de Freitas Mansano, V. Increments to the Genus Swartzia (Leguminosae) from the Southern Amazonian Craton. Kew Bull. 2013, 68, 269–284. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil III, 2nd ed.; Instituto Plantarum de Estudos da Flora LTDA.: Nova Odessa-São Paulo, Brazil, 2016. [Google Scholar]
- Pinto, R.C. Lista de Espécies para Subsidiar A Restauração de Florestas Degradadas na Microrregião de Tomé-Açu do Estado do Pará; Graduação, Universidade Federal Rural da Amazônia: Belém, Brazil, 2018. [Google Scholar]
- Barbosa, L.; França, I.; Hernández Ruz, E.J.; Barbosa, L.; França, I.; Hernández Ruz, E.J. First Records of Theobroma Speciosum Fruits Dispersion. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2019, 43, 518–520. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil II, 5th ed.; Instituto Plantarum de Estudos da Flora LTDA.: Nova Odessa-São Paulo, Brazil, 2016. [Google Scholar]
- Gualberto, M.L.C.; Gama, V. Fitossociologia e potencial de espécies arbóreas em ecossistema sucessional na floresta nacional do tapajós, pará. Agroecossistemas 2014, 6, 42–57. [Google Scholar] [CrossRef][Green Version]
- Absy, M.L.; Bezerra, E.B.; Kerr, W.E. Plantas nectaríferas utilizadas por duas espécies de Melipona da Amazônia. Acta Amaz. 1980, 10, 271–282. [Google Scholar] [CrossRef]
- EMBRAPA. Goiaba de Anta-Portal Embrapa. Available online: https://www.embrapa.br/agrossilvipastoril/sitio-tecnologico/trilha-ecologica/especies/goiaba-de-anta (accessed on 3 January 2022).
- Völtz, R.R.; Goldenberg, R. Mouriri in Flora Do Brasil 2020. Jardim Botânico Do Rio de Janeiro. Available online: http://Floradobrasil.Jbrj.Gov.Br/Reflora/Floradobrasil/FB19703 (accessed on 30 December 2021).
- Oliveira, F.D.S. Ecologia da polinização e análise da composição química do óleo floral de duas espécies de Mouriri (Melastomataceae) e a sua importância na atração dos visitantes florais. Doctoral Thesis, Universidade Federal do Maranhão, São Luis, Brazil, 2016. [Google Scholar]
- Vasconcelos, J.M.; Cardoso, T.V.; Sales, J.d.F.; Silva, F.G.; Vasconcelos Filho, S.C.; Santana, J.d.G. Métodos de superação de dormência em sementes de croada (Mouriri elliptica Mart). Ciênc. E Agrotecnol. 2010, 34, 1199–1204. [Google Scholar] [CrossRef]
- Albuquerque-Lima, S.; Diniz, U.M.; Machado, I.C.S. A Nectar Oasis for Urban Glossophaginae Bats: Temporal Resource Dynamics of the Chiropterophilous Crescentia Cujete (Bignoniaceae). Urban For. Urban Green 2022, 67, 127412. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Power, S.A.; Tjoelker, M.G.; Marchin, R.M.; Rymer, P.D. Contrasting Heat Tolerance of Urban Trees to Extreme Temperatures during Heatwaves. Urban For. Urban Green 2021, 66, 127387. [Google Scholar] [CrossRef]
- Ronquim, C.C.; Alvarez, I.A.; Cipriani, H.N.; Simonetti, J.C. Avaliação Dos Viveiros Produtores de Mudas Florestais Nativas de Rondônia; EMBRAPA Territorial: Campinas, Brazil, 2020. [Google Scholar]
- Pimentel da Silva, L.; de Souza, F.T. Urban Management: Learning from Green Infrastructure, Socioeconomics and Health Indicators in the Municipalities of the State of Paraná, Brazil, Towards Sustainable Cities and Communities. In Universities and Sustainable Communities: Meeting the Goals of the Agenda 2030; Leal Filho, W., Tortato, U., Frankenberger, F., Eds.; World Sustainability Series; Springer International Publishing: Cham, Switzerland, 2020; pp. 493–509. ISBN 978-3-030-30306-8. [Google Scholar]
- Tan, P.Y.; Wang, J.; Sia, A. Perspectives on Five Decades of the Urban Greening of Singapore. Cities 2013, 32, 24–32. [Google Scholar] [CrossRef]
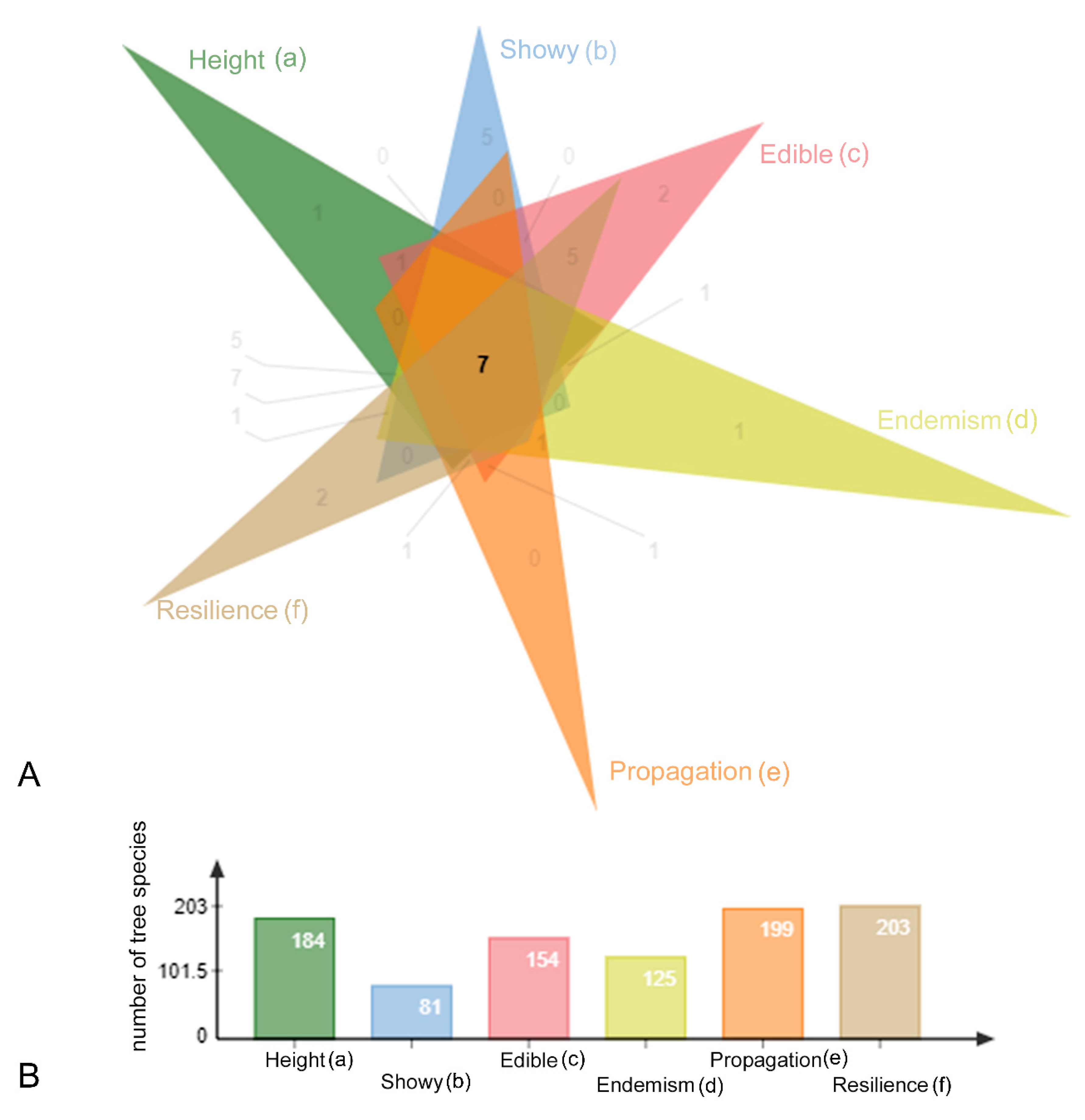



| Feature | Ideal/Adequate | |
|---|---|---|
| A | Appropriate height | Species that reach up to 10–15 m tall were preferred over taller species for street planting, however taller species were not eliminated from our list. |
| B | Showy flowers | It has been established that the population is keen on trees that produce ornamental displays in different seasons. |
| C | Fruits attractive to wildlife and to humans | With the intention of including more ecological interactions in the urban spaces, species with fruits that are attractive to wildlife were given priority. Moreover, streets planted with fruiting trees are a huge bonus to develop links between people and plants from an early age. |
| D | Amazonian distribution | Trees that are endemic to the Amazon were given preference as the list was prepared for urban areas within this biome. |
| E | Known propagation methods | Scientific and horticultural knowledge were combined to score this feature. We have considered that, if the propagation of a genus is known, this knowledge is transferrable to the species. |
| F | Resilience (drought resistance and/or tolerance to full sun) | Even in the extremely humid Amazonian climate, urban weather conditions differ from the natural situation. Here the intention was to favour species adapted to growing at the edge of forests, in more seasonal forest types or in open vegetation in the Amazon to ensure that these can thrive in urban conditions. |
| Family | Species | Criteria | Pollination Syndrome | Phenology | |
|---|---|---|---|---|---|
| 1 | Annonaceae | Annona exsucca DC. | A, C–F | Beetle | Fl XI–V, Fr III–VI |
| 2 | Annonaceae | Xylopia aromatica (Lam.) Mart. | A–C, E, F | Beetle | Fl VIII–V, Fr III–VIII |
| 3 | Apocynaceae | Tabernaemontana flavicans Willd. ex Roem. & Schult. | A–C, E, F | Bee | Fl VIII–X, Fr IX–VII |
| 4 | Bixaceae | Cochlospermum orinocense (Kunth) Steud. | A–C, E, F | Bee | Fl IV–X, Fr VI–IV |
| 5 | Boraginaceae | Cordia goeldiana Huber | A, B, D–F | Bee, Hoverfly | Fl VI–X, Fr IX–XII |
| 6 | Burseraceae | Protium sagotianum Marchand | A, C–F | Bee | Fl VIII–I, Fr XI–VI |
| 7 | Chrysobalanaceae | Hirtella racemosa Lam. | A, B, D–F | Butterfly | Fl II–VII, Fr VIII–X |
| 8 | Clusiaceae | Clusia panapanari (Aubl.) Choisy | A–C, E, F | Bee | Fl II–XI, Fr IX–I |
| 9 | Clusiaceae | Symphonia globulifera L.f. | A–C, E, F | Bird, Hummingbird | Fl VII–II, VI–V, Fr VIII–II |
| 10 | Ebenaceae | Diospyros vestita Benoist | A, C–F | Insect | Fl VII–II, Fr VI–VII |
| 11 | Fabaceae | Abarema cochleata (Willd.) Barneby & J.W.Grimes | A, B, D–F | Bat, Moth | Fl VIII–I, Fr AY |
| 12 | Fabaceae | Bauhinia acreana Harms | A, B, D–F | Bat | Fl X–V, Fr XI–VI |
| 13 | Fabaceae | Bowdichia nitida Spruce ex Benth. | A, B, D–F | Bee | Fl IV–VII, Fr VI–IX |
| 14 | Fabaceae | Campsiandra laurifolia Benth. | A, B, D–F | Bat | Fl V–I, Fr VI–IV |
| 15 | Fabaceae | Cassia fastuosa Willd. ex Benth. | A, B, D–F | Bee | Fl VII–XI, Fr XI–VIII |
| 16 | Fabaceae | Cenostigma tocantinum Ducke | A, B, D–F | Bee | Fl IV–II, Fr V–XII |
| 17 | Fabaceae | Hymenaea parvifolia Huber | B–F | Bat | Fl XI–III, Fr AY |
| 18 | Fabaceae | Inga disticha Benth. | A–C, E, F | Bee, Butterfly | Fl VI–IX, Fr XI–VII |
| 19 | Fabaceae | Inga heterophylla Willd. | A–C, E, F | Bee | Fl XII–VI, IX, Fr V–XI, I |
| 20 | Fabaceae | Inga marginata Willd. | A–C, E, F | Bee | Fl VII–XII, Fr IX–IV |
| 21 | Fabaceae | Inga nobilis Willd. | A–F | Bat | Fl X–V, Fr II–VI |
| 22 | Fabaceae | Inga stipularis DC. | A–E | Moth, Bat | Fl II–VI, XI, Fr I–VII |
| 23 | Fabaceae | Inga umbellifera (Vahl) DC. | A–F | Moth | Fl V–IX, Fr VI–X |
| 24 | Fabaceae | Platymiscium filipes Benth. | A, B, D–F | Bee | Fl III–XI, Fr VII–III |
| 25 | Fabaceae | Senna multijuga (Rich.) H.S.Irwin & Barneby | A, B, D–F | Bee | Fl IV–XII, Fr VII–XII |
| 26 | Fabaceae | Swartzia arumateuana (R. S. Cowan) Torke & Mansano | A–F | Bee | Fl II–V, Fr IV–XII |
| 27 | Fabaceae | Swartzia brachyrachis Harms | A–F | Bee | Fl III–VI, Fr VI–II |
| 28 | Fabaceae | Tachigali paniculata Aubl. | A, B, D–F | Bee | Fr VI–XI, Fr VIII–II |
| 29 | Fabaceae | Zollernia paraensis Huber | A–C, E, F | Bee | Fl VIII–X, Fr XI–XII |
| 30 | Lecythidaceae | Eschweilera obversa (O.Berg) Miers | B–F | Bee | Fl V–VI, XI–I, Fr V–VI, XII |
| 31 | Malpighiaceae | Byrsonima spicata (Cav.) DC. | A–C, E, F | Bee | Fl VII–IV, Fr X–V, VII |
| 32 | Malpighiaceae | Byrsonima stipulacea A.Juss. | A–C, E, F | Bee | Fl X–I, Fr V–VII |
| 33 | Malpighiaceae | Lophanthera lactescens Ducke | A, B, D–F | Bee | Fl V, X, Fr V |
| 34 | Malvaceae | Matisia ochrocalyx K.Schum. | A, B, D–F | Bat | Fl I–VIII, Fr VIII–X, III |
| 35 | Malvaceae | Theobroma speciosum Willd. ex Spreng. | A–F | Fly | Fl VIII–XI, Fr X–II |
| 36 | Melastomataceae | Bellucia grossularioides (L.) Triana | A–F | Bee | Fl V–II, Fr VIII–IV |
| 37 | Melastomataceae | Bellucia mespiloides (Miq.) Macbr. | B–F | Bee | Fl IX–II, VII, Fr X–II |
| 38 | Melastomataceae | Miconia ampla Triana | A, C–F | Bee | Fl I–IV, Fr III–VII |
| 39 | Melastomataceae | Miconia egensis Cogn. | A, C–F | Bee | Fl III, IX–XI, Fr III, X–XI |
| 40 | Melastomataceae | Mouriri acutiflora Naudin | A–F | Bee | Fl VIII–XII, Fr X–II |
| 41 | Melastomataceae | Mouriri brachyanthera Ducke | A, C–F | Bee | Fl I, IV–V, XII, Fr I, V–VII |
| 42 | Melastomataceae | Mouriri cearensis Huber | A–C, E, F | Bee | Fl X–XII, Fr IV–V, X, XII |
| 43 | Melastomataceae | Mouriri vernicosa Naudin | A–E | Bee | Fl V–VIII, X–XII, Fr VII–XI |
| 44 | Myrtaceae | Eugenia anastomosans DC. | A, C–F | Bee, Fly | Fl X–XII, Fr VII |
| 45 | Myrtaceae | Eugenia ramiflora Desv. ex Ham. | A, C–F | Bee | Fl X–XII, Fr I |
| 46 | Myrtaceae | Myrcia pyrifolia (Desv. ex Ham.) Nied. | A, C–F | Bee, Fly | Fl X–I, Fr III–VI, VIII, XII |
| 47 | Myrtaceae | Psidium acutangulum DC. | A, C–F | Bee | Fl VI–X, F. IV–VI, IX |
| 48 | Verbenaceae | Citharexylum macrochlamys Pittier | A, C–F | Moth | Fl I–IV, VIII, Fr I–IV, VIII |
| 49 | Vochysiaceae | Erisma uncinatum Warm. | B–F | Bee | Fl VIII–XI, Fr VII–I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappi, D.C.; Lovo, J.; Hiura, A.; Andrino, C.O.; Barbosa-Silva, R.G.; Martello, F.; Gadelha-Silva, L.; Viana, P.L.; Giannini, T.C. Telling the Wood from the Trees: Ranking a Tree Species List to Aid Urban Afforestation in the Amazon. Sustainability 2022, 14, 1321. https://doi.org/10.3390/su14031321
Zappi DC, Lovo J, Hiura A, Andrino CO, Barbosa-Silva RG, Martello F, Gadelha-Silva L, Viana PL, Giannini TC. Telling the Wood from the Trees: Ranking a Tree Species List to Aid Urban Afforestation in the Amazon. Sustainability. 2022; 14(3):1321. https://doi.org/10.3390/su14031321
Chicago/Turabian StyleZappi, Daniela C., Juliana Lovo, Alice Hiura, Caroline O. Andrino, Rafael G. Barbosa-Silva, Felipe Martello, Livia Gadelha-Silva, Pedro L. Viana, and Tereza C. Giannini. 2022. "Telling the Wood from the Trees: Ranking a Tree Species List to Aid Urban Afforestation in the Amazon" Sustainability 14, no. 3: 1321. https://doi.org/10.3390/su14031321
APA StyleZappi, D. C., Lovo, J., Hiura, A., Andrino, C. O., Barbosa-Silva, R. G., Martello, F., Gadelha-Silva, L., Viana, P. L., & Giannini, T. C. (2022). Telling the Wood from the Trees: Ranking a Tree Species List to Aid Urban Afforestation in the Amazon. Sustainability, 14(3), 1321. https://doi.org/10.3390/su14031321








