Comparison of Bacterial Community Structure in PM2.5 within Broiler Houses under Different Rearing Systems in China
Abstract
:1. Introduction
2. Materials and Methods
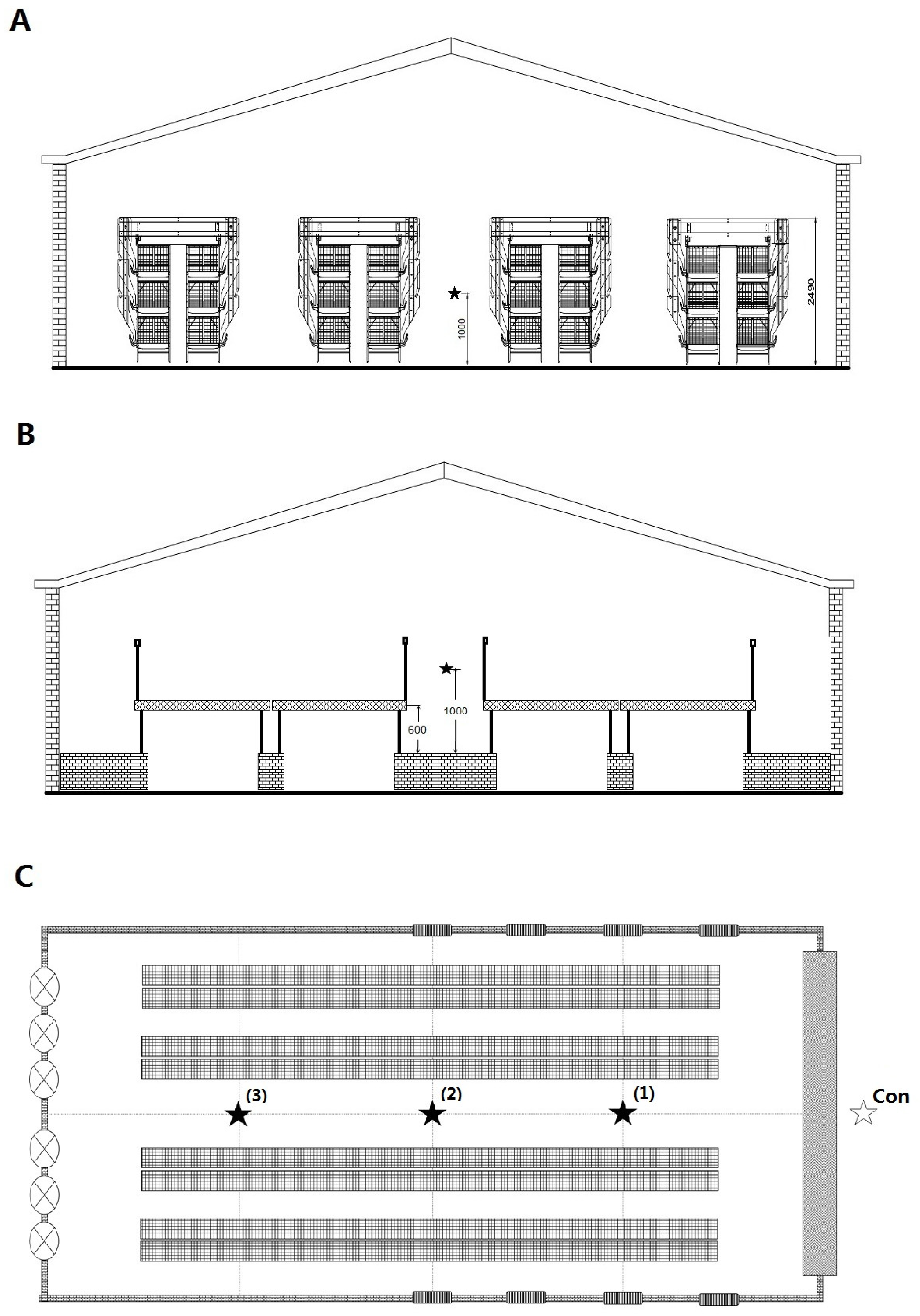
2.1. Characteristics of Broiler Houses
2.2. The Detection of Particulate Matter, Temperature, Relative Humidity, CO2 and NH3 in Broiler House
2.3. DNA Extraction and PCR Amplification
2.4. Illumina MiSeq Sequencing
2.5. Processing of Sequencing Data
2.6. Chemical Analysis
2.7. Statistical Analysis
3. Results
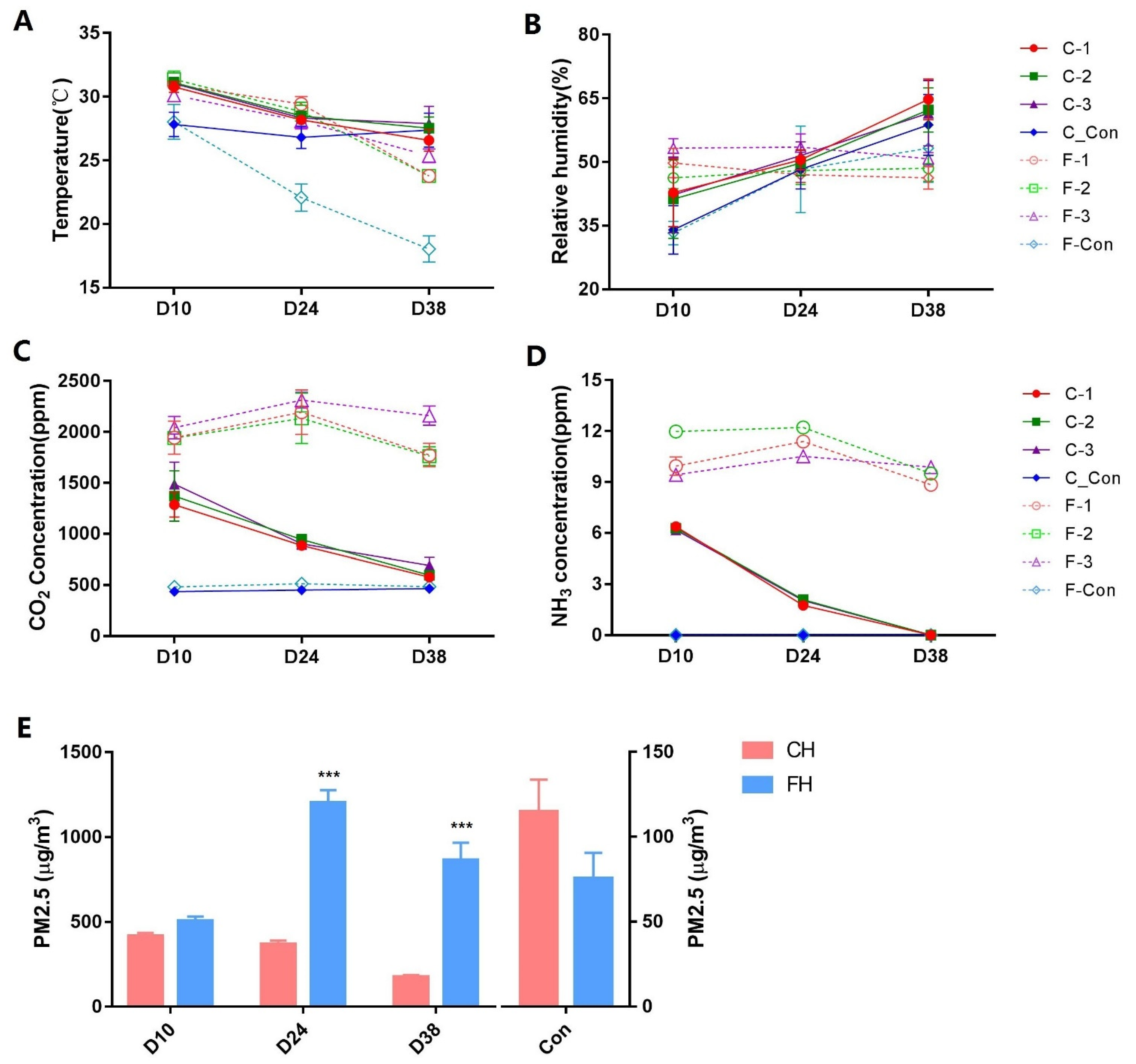
3.1. Variation in Environmental Control Variables and PM2.5
3.2. Water-Soluble Ion Concentrations in Indoor PM2.5
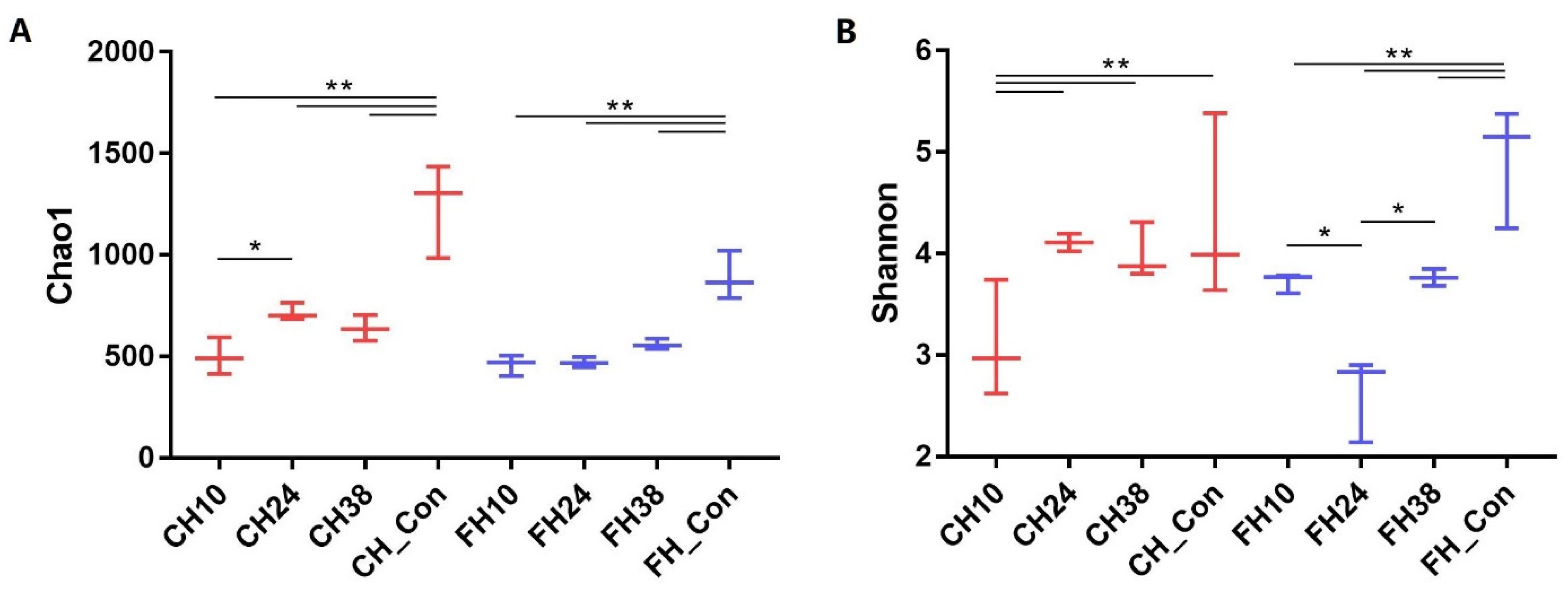
3.3. Airborne Bacterial Community Structure of PM2.5
3.4. Effects on Bacterial Community Structure in PM2.5
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zheng, X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Mosquera, J.; Koerkamp, P.; Ogink, N.; Aarnink, A.J.A. Emissions of particulate matter from animal houses in the Netherlands. Atmos. Environ. 2015, 111, 202–212. [Google Scholar] [CrossRef]
- Raisi, L.; Aleksandropoulou, V.; Lazaridis, M.; Katsivela, E. Size distribution of viable, cultivable, airborne microbes and their relationship to particulate matter concentrations and meteorological conditions in a mediterranean site. Aerobiologia 2013, 29, 233–248. [Google Scholar] [CrossRef]
- de Rooij, M.M.T.; Smit, L.A.M.; Erbrink, H.J.; Hagenaars, T.J.; Hoek, G.; Ogink, N.W.M.; Winkel, A.; Heederik, D.J.J.; Wouters, I.M. Endotoxin and particulate matter emitted by livestock farms and respiratory health effects in neighboring residents. Environ Int 2019, 132, 105009. [Google Scholar] [CrossRef]
- Dai, P.; Shen, D.; Tang, Q.; Huang, K.; Li, C. PM2.5 from a broiler breeding production system: The characteristics and microbial community analysis. Environ. Pollut. 2020, 256, 113368. [Google Scholar] [CrossRef]
- Fujiyoshi, S.; Tanaka, D.; Maruyama, F. Transmission of Airborne Bacteria across Built Environments and Its Measurement Standards: A Review. Front. Microbiol. 2017, 8, 2336. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.; Wu, S.; Dai, P.Y.; Li, Y.S.; Li, C.M. Distribution of particulate matter and ammonia and physicochemical properties of fine particulate matter in a layer house. Poult. Sci. 2018, 97, 4137–4149. [Google Scholar] [CrossRef]
- Yan, L.; Lv, Z.; An, S.; Xing, K.; Zhou, G. Effects of rearing system and narasin on growth performance, gastro-intestinal development and gut microbiota of broilers. Poult. Sci. 2020, 100, 100840. [Google Scholar] [CrossRef]
- Kadykalo, S.; Roberts, T.; Thompson, M.; Wilson, J.; Lang, M.; Espeisse, O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 2017, 51, 304–310. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Geng, T.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Glckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dan, G.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.Y.; Li, X.; Yang, X.; Shinkai, T.; Zhang, Y.; Wang, X.; Mackie, R.I. Monitoring airborne biotic contaminants in the indoor environment of pig and poultry confinement buildings. Environ. Microbiol. 2012, 14, 1420–1431. [Google Scholar] [CrossRef]
- Just, N.; Lecours, P.B.; Marcoux-Voiselle, M.; Kirychuk, S.; Veillette, M.; Singh, B.; Duchaine, C. Bacterial diversity characterization of bioaerosols from cage-housed and floor-housed poultry operations. Environ. Res. 2013, 59, 46–50. [Google Scholar] [CrossRef]
- Cambra-López, M.; Aarnink, A.J.A.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Xu, E.; Jiang, L.; Tang, J.; Li, M.; Zhao, X.; Chen, G.; Zhu, H.; Yu, X.; et al. Bacterial communities in PM2.5 and PM10 in broiler houses at different broiler growth stages in spring. Pol. J. Vet. Sci. 2019, 22, 495–504. [Google Scholar]
- Li, Z.; Zheng, W.; Wei, Y.; Li, B.; Wang, Y.; Zheng, H. Prevention of particulate matter and airborne culturable bacteria transmission between double-tunnel ventilation layer hen houses. Poult. Sci. 2019, 98, 2392–2398. [Google Scholar] [CrossRef]
- Yang, W.; Guo, M.; Liu, G.; Yu, G.; Wang, P.; Wang, H.; Chai, T. Detection and analysis of fine particulate matter and microbial aerosol in chicken houses in Shandong Province, China. Poult. Sci. 2017, 97, 995–1005. [Google Scholar] [CrossRef]
- Blais-Lecours, P.; Perrott, P.; Duchaine, C. Non-culturable bioaerosols in indoor settings: Impact on health and molecular approaches for detection. Atmos. Environ. 2015, 110, 45–53. [Google Scholar] [CrossRef]
- Duquenne, P. On the Identification of Culturable Microorganisms for the Assessment of Biodiversity in Bioaerosols. Ann. Work Expo. Health 2018, 62, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dungan, R.S.; Leytem, A.B. Qualitative and quantitative methodologies for determination of airborne microorganisms at concentrated animal-feeding operations. World J. Microbiol. Biotechnol. 2009, 25, 1505–1518. [Google Scholar] [CrossRef]
- Xu, Z.; Kai, W.; Wu, Y.; Shen, F.; Chen, Q.; Li, M.; Yao, M.; Vishal, S. Enhancing Bioaerosol Sampling by Andersen Impactors Using Mineral-Oil-Spread Agar Plate. PLoS ONE 2013, 8, e56896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.; Grinshpun, S.A.; Martuzevicius, D.; Adhikari, A.; Crawford, C.M.; Luo, J.; Reponen, T. Relationship between indoor and outdoor bioaerosols collected with a button inhalable aerosol sampler in urban homes. Indoor Air 2006, 16, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 2013, 47, 12097. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gil, T.; Acuña, J.J.; Fujiyoshi, S.; Tanaka, D.; Noda, J.; Maruyama, F.; Jorquera, M.A. Airborne bacterial communities of outdoor environments and their associated influencing factors. Environ. Int. 2020, 145, 106156. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Jia, R.; Qiu, T.; Han, M.; Wang, X. Size-related bacterial diversity and tetracycline resistance gene abundance in the air of concentrated poultry feeding operations. Environ. Pollut. 2017, 220 Pt B, 1342–1348. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.; Tang, J.; Li, M.; Zhao, X.; Zhu, H.; Yu, X.; Li, Y.; Feng, T.; Zhang, X. Analyses of Aerosol Concentrations and Bacterial Community Structures for Closed Cage Broiler Houses at Different Broiler Growth Stages in Winter. J. Food Prot. 2018, 81, 1557–1564. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, X.; Xiao, H.; Xiao, H. Structural Characteristics and Functional Implications of PM2.5 Bacterial Communities During Fall in Beijing and Shanghai, China. Front. Microbiol. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Park, E.H.; Heo, J.; Kim, H.; Yi, S.M. The major chemical constituents of PM2.5 and airborne bacterial community phyla in Beijing, Seoul, and Nagasaki. Chemosphere 2020, 254, 126870. [Google Scholar] [CrossRef]
- Tan, J.; Duan, J.; Zhen, N.; He, K.; Hao, J. Chemical characteristics and source of size-fractionated atmospheric particle in haze episode in Beijing. Atmos. Res. 2015, 167, 24–33. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Zhang, D.; Li, B.; Wang, E.; Yuan, H. Concentration and Community of Airborne Bacteria in Response to Cyclical Haze Events During the Fall and Midwinter in Beijing, China. Front. Microbiol. 2018, 9, 1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.F.; Fan, X.Y.; Pan, K.L.; Li, H.Y.; Sun, L.X. Diversity, abundance and activity of ammonia-oxidizing microorganisms in fine particulate matter. Sci. Rep. 2016, 6, 38785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Zou, Z.; Zheng, Y.; Li, J.; Shen, F.; Wu, C.Y.; Wu, Y.; Hu, M.; Yao, M. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Sci. Total Environ. 2016, 550, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; McLetchie, S.; Knight, R.; Fierer, N. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME J. 2011, 5, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Xu, C.; Xu, X.; Zhu, C.; Li, J.; Lv, G. Characteristics of atmospheric bacterial and fungal communities in PM2.5 following biomass burning disturbance in a rural area of North China Plain. Sci. Total Environ. 2019, 651 Pt 2, 2727–2739. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, R.; Rao, J.; Xiao, Y.; Zhang, Z.; Yang, B.; Cao, D.; Zhong, H.; Ning, P.; Shang, Y.; et al. Transcriptionally Active Lung Microbiome and Its Association with Bacterial Biomass and Host Inflammatory Status. mSystems 2018, 3, e00199-18. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Qi, J.; Shao, C.; Zhong, X.; Gao, D.; Cao, W.; Gao, J.; Bai, R.; Long, G.; Chu, C. Concentration and size distribution of total airborne microbes in hazy and foggy weather. Sci. Total Environ. 2016, 541, 1011–1018. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, H.; Yao, X.; Zhou, M.; Wang, J.; He, Z.; Zhang, H.; Lou, L.; Mao, W.; et al. Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environ. Pollut. 2018, 233, 483–493. [Google Scholar] [CrossRef]
- Innocente, E.; Squizzato, S.; Visin, F.; Facca, C.; Rampazzo, G.; Bertolini, V.; Gandolfi, I.; Franzetti, A.; Ambrosini, R.; Bestetti, G. Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Sci. Total Environ. 2017, 593–594, 677–687. [Google Scholar] [CrossRef]
- Xu, C.; Wei, M.; Chen, J.; Wang, X.; Zhu, C.; Li, J.; Zheng, L.; Sui, G.; Li, W.; Wang, W.; et al. Bacterial characterization in ambient submicron particles during severe haze episodes at Ji’nan, China. Sci. Total Environ. 2017, 580, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Zhang, L.; Jiang, X.; Gao, P. Comparison of chemical composition and airborne bacterial community structure in PM2.5 during haze and non-haze days in the winter in Guilin, China. Sci. Total Environ. 2019, 655, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.Y.; Yang, X.R.; Zhu, Y.G.; Hong, Y.W.; Su, J.Q. Spatial and seasonal variation of the airborne microbiome in a rapidly developing city of China. Sci. Total Environ. 2019, 665, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Zhang, Y.; Lee, J.; Su, J.; Gates, R.S. Characterization of trace elements and ions in PM10 and PM2.5 emitted from animal confinement buildings. Atmos. Environ. 2011, 45, 7096–7104. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Wang, W.; Ye, X.; Isa, A.M.; Khan, M.T.; Sa, R.; Liu, L.; Ma, T.; Zhang, H. Comparison of Bacterial Community Structure in PM2.5 within Broiler Houses under Different Rearing Systems in China. Sustainability 2022, 14, 1357. https://doi.org/10.3390/su14031357
Khan I, Wang W, Ye X, Isa AM, Khan MT, Sa R, Liu L, Ma T, Zhang H. Comparison of Bacterial Community Structure in PM2.5 within Broiler Houses under Different Rearing Systems in China. Sustainability. 2022; 14(3):1357. https://doi.org/10.3390/su14031357
Chicago/Turabian StyleKhan, Imran, Wenxing Wang, Xiaobin Ye, Adamu Mani Isa, Muhammad Tariq Khan, Renna Sa, Lei Liu, Teng Ma, and Hongfu Zhang. 2022. "Comparison of Bacterial Community Structure in PM2.5 within Broiler Houses under Different Rearing Systems in China" Sustainability 14, no. 3: 1357. https://doi.org/10.3390/su14031357
APA StyleKhan, I., Wang, W., Ye, X., Isa, A. M., Khan, M. T., Sa, R., Liu, L., Ma, T., & Zhang, H. (2022). Comparison of Bacterial Community Structure in PM2.5 within Broiler Houses under Different Rearing Systems in China. Sustainability, 14(3), 1357. https://doi.org/10.3390/su14031357







