Evaluation Study on Extraction of Anthocyanins from Red Cabbage Using High Pressure CO2 + H2O: A Fuzzy Logic Model and Metabolomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction Methods
2.3. Traditional Quality Evaluation Using Targeted Analysis
2.3.1. Total Monomeric Anthocyanins, Relative Yield of Anthocyanins, Total Phenolics Content and Polymeric Color
2.3.2. Color Property, pH and Half-Life
2.3.3. Statistical Analysis
2.4. Fuzzy Logic-Based Ranking Function
2.4.1. Fuzzification
2.4.2. Definition of Fuzzy Rules
2.4.3. Defuzzification of Output
2.5. Metabolomics Analysis
2.5.1. Sample Pretreatment and Data Acquisition
2.5.2. Individual Anthocyanins and Phenolics Identification
2.5.3. Data Processing and Potential Marker Putative Annotation
3. Results and Discussion
3.1. Traditional Quality Characters
3.2. Fuzzy Logic Ranking
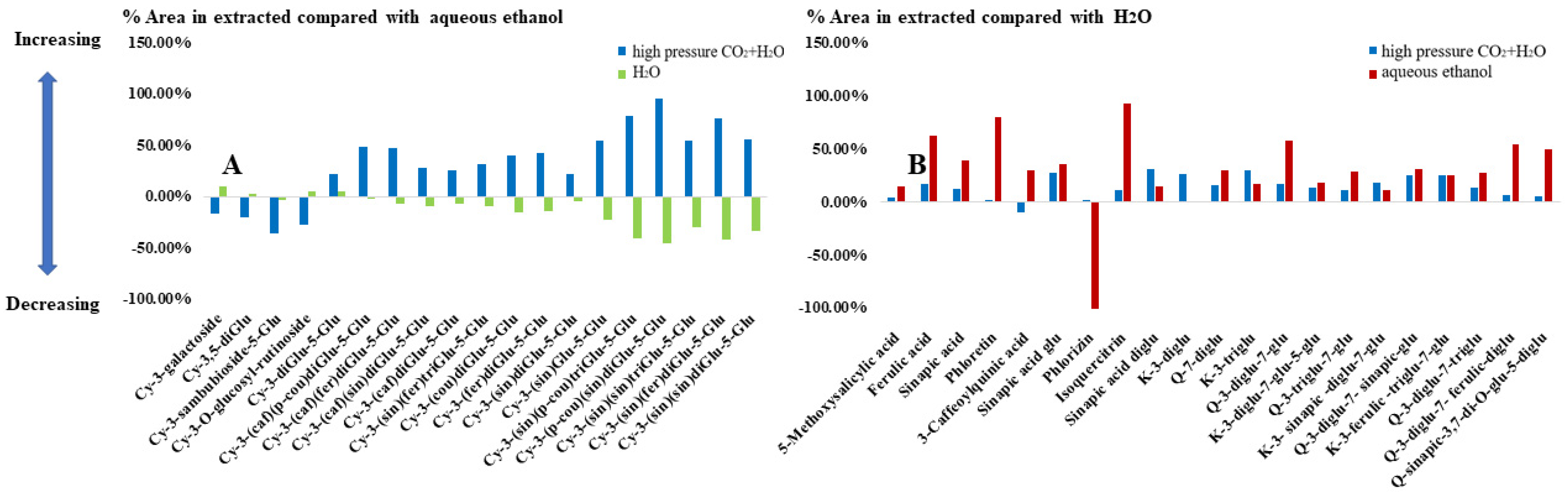
3.3. Targeted Metabolomics Analysis
3.4. Untargeted Metabolomics Analysis
3.4.1. PCA Analysis
3.4.2. Differential Analysis and Potential Marker Annotation
3.5. Extraction Mechanism of High-Pressure CO2 + H2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamatar, M.Y. Natural food colouring: A healthier alternative to artificial food colouring. In Proceedings of the Global Milling Conference: Saftey, Sustainability and Food Supply for the 21st Century, Chennai, India, February 2013. [Google Scholar]
- Christensen, C.M. Effects of Color on Aroma, Flavor and Texture Judgments of Foods. J. Food Sci. 1983, 48, 787–790. [Google Scholar] [CrossRef]
- Gao, H.-W.; Xu, Q.; Chen, L.; Wang, S.-L.; Wang, Y.; Wu, L.-L.; Yuan, Y. Potential Protein Toxicity of Synthetic Pigments: Binding of Poncean S to Human Serum Albumin. Biophys. J. 2008, 94, 906–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titova, N. 300 The Method of Antigen Specific Damage of Leucocytes by Food Additives in Patients with Bronchial Asthma. World Allergy Organ. J. 2012, 5, S97–S114. [Google Scholar] [CrossRef]
- Weber, R.W.; Hoffman, M.; Raine, A.D.; Nelson, H.S.; Rainejr, D. Incidence of bronchoconstriction due to aspirin, azo dyes, non-azo dyes, and preservatives in a population of perennial asthmatics. J. Allergy Clin. Immunol. 1979, 64, 32–37. [Google Scholar] [CrossRef]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The Realm of Microbial Pigments in the Food Color Market. Front. Sustain. Food Syst. 2021, 5, 584605. [Google Scholar] [CrossRef]
- Chou, P.-H.; Matsui, S.; Misaki, K.; Matsuda, T. Isolation and Identification of Xenobiotic Aryl Hydrocarbon Receptor Ligands in Dyeing Wastewater. Environ. Sci. Technol. 2007, 41, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Nazarpoor, K.; Karimi, L. Eco-friendly dyeing of wool using natural dye from weld as co-partner with synthetic dye. J. Clean. Prod. 2011, 19, 1045–1051. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.; Tung, M.A.; Speers, R.A. Anthocyanins as food colorants—A review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Andersen, O.M.; Jordheim, M. Basic anthocyanin chemistry and dietary sources. Anthocyanins Health Dis. 2013, 13–90. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markakis, P. Stability of Anthocyanins in Foods—ScienceDirect. Anthocyanins Food Colors 1982, 163, 180. [Google Scholar]
- Tsuda, T. Anthocyanins as Functional Food Factors—Chemistry, Nutrition and Health Promotion—. Food Sci. Technol. Res. 2012, 18, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Turturică, M.; Oancea, A.M.; Râpeanu, G.; Bahrim, G. Anthocyanins: Naturally occuring fruit pigments with functional properties. Ann. Univ. Dunarea De Jos Galati. Fascicle VI Food Technol. 2015, 39, 9–24. [Google Scholar]
- Jafari, S.; Mahdavee, K.; Assadpour, E. Production of a natural color through microwave—Assisted extraction of saffron tepal’s anthocyanins. Food Sci. Nutr. 2019, 7, 1438–1445. [Google Scholar] [CrossRef]
- He, Y.; Wen, L.; Liu, J.; Li, Y.; Zheng, F.; Min, W.; Yue, H.; Pan, P. Optimisation of pulsed electric fields extraction of anthocyanin from Beibinghong Vitis Amurensis Rupr. Nat. Prod. Res. 2017, 32, 23–29. [Google Scholar] [CrossRef]
- Tiberio de Jesus, A.L.; Cristianini, M.; dos Santos, N.N.; Marostica Junior, M.R. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from acai (Euterpe oleracea Martius) pulp. Food Res. Int. 2020, 130, 108856. [Google Scholar] [CrossRef]
- Knorr, D. Impact of non-thermal processing on plant metabolites. J. Food Eng. 2003, 56, 131–134. [Google Scholar] [CrossRef]
- Briongos, H.; Illera, A.E.; Sanz, M.; Melgosa, R.; Beltrán, S.; Solaesa, A. Effect of high pressure carbon dioxide processing on pectin methylesterase activity and other orange juice properties. LWT 2016, 74, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of non thermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci. Technol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, J.; Zhang, Y.; Hu, X.; Liao, X.; Wang, Z. Extraction of anthocyanins from red cabbage using high pressure CO2. Bioresour. Technol. 2010, 101, 7151–7157. [Google Scholar]
- Castro-Puyana, M.; Herrero, M. Metabolomics approaches based on mass spectrometry for food safety, quality and traceability. TrAC Trends Anal. Chem. 2013, 52, 74–87. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Huang, X.; Wang, X.; Yang, R.; Mao, J.; Wang, X.; Wang, X.; Zhang, Q.; Li, P. Identification of Nutritional Components in Black Sesame Determined by Widely Targeted Metabolomics and Traditional Chinese Medicines. Molecules 2018, 23, 1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinu, F.R. Grape and Wine Metabolomics to Develop New Insights Using Untargeted and Targeted Approaches. Fermentation 2018, 4, 92. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Hu, X.; Liao, X.; Chen, F.; Wu, J. Inactivation of Escherichia coli inoculated into cloudy apple juice exposed to dense phase carbon dioxide. Int. J. Food Microbiol. 2007, 118, 126–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, X.; Chen, F.; Wu, J.; Hu, X. Isolation, identification, and color characterization of cyanidin-3-glucoside and cyanidin-3-sophoroside from red raspberry. Eur. Food Res. Technol. 2007, 226, 395–403. [Google Scholar] [CrossRef]
- Zhang, J.; Iqbal, A.; Murtaza, A.; Zhou, X.; Xu, X.; Pan, S.; Hu, W. Effect of high pressure carbon dioxide on the browning inhibition of sugar-preserved orange peel. J. CO2 Util. 2021, 46, 101467. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. The effect of pigment matrix, temperature and amount of carrier on the yield and final color properties of spray dried purple corn (Zea mays L.) cob anthocyanin powders. Food Chem. 2017, 227, 376–382. [Google Scholar] [CrossRef]
- Zou, H.; Lin, T.; Bi, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of high hydrostatic pressure, high-pressure carbon dioxide and high-temperature short-time processing on quality of mulberry juice. Food Bioproc. Technol. 2016, 9, 217–231. [Google Scholar] [CrossRef]
- Rahul, K.; Payel, G.; Srinivasa Rao, P.; Rana, S.S.; Rahul, V.; Kambhampati, V. Sensory evaluation of microwave assisted ultra-sound treated soymilk beverage using fuzzy logic. J. Saudi Soc. Agric. Sci. 2021, 20, 257–264. [Google Scholar]
- Shahidi, B.; Sharifi, A.; Nasiraie, L.R.; Niakousari, M.; Ahmadi, M. Phenolic content and antioxidant activity of flixweed (Des-curainia sophia) seeds extracts: Ranking extraction systems bsed on fuzzy logic method. Sustain. Chem. Pharm. 2020, 16, 100245. [Google Scholar] [CrossRef]
- Balezentiene, L.; Streimikiene, D.; Balezentis, T. Fuzzy decision support methodology for sustainable energy crop selection. Renew. Sustain. Energy Rev. 2013, 17, 83–93. [Google Scholar] [CrossRef]
- Gachovska, T.; Cassada, D.; Subbiah, J.; Hanna, M.; Thippareddi, H.; Snow, D. Enhanced Anthocyanin Extraction from Red Cabbage Using Pulsed Electric Field Processing. J. Food Sci. 2010, 75, E323–E329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charron, C.S.; Clevidence, B.A.; Britz, S.J.; Novotny, J.A. Effect of dose size on bioavailability of acylated and nonacylated an-thocyanins from red cabbage (Brassica oleracea L. var. capitata). J. Agric. Food Chem. 2007, 55, 5354–5362. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, Z.; Lin, L.-Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling Polyphenols in Five Brassica Species Microgreens by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization, Quantification, and Yearly Variation of the Naturally Occurring Polyphenols in a Common Red Variety of Curly Kale (Brassica oleracea L. convar. acephala var. sabellica cv. ‘Redbor’). J. Agric. Food Chem. 2010, 58, 11346–11354. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Cheng, H.; Wang, Q.; Wang, X.; Liao, X.; Xu, Z. Enhanced water extraction with high-pressure carbon dioxide on purple sweet potato pigments: Comparison to traditional aqueous and ethanolic extraction. J. CO2 Util. 2020, 40, 101188. [Google Scholar] [CrossRef]
- Nunes, A.N.; Carmo, C.S.D.; Duarte, C.M.M. Production of a natural red pigment derived from Opuntia spp. using a novel high pressure CO2 assisted-process. RSC Adv. 2015, 5, 83106–83114. [Google Scholar] [CrossRef]
- Klaykruayat, S.; Mahayothee, B.; Khuwijitjaru, P.; Nagle, M.; Müller, J. Influence of packaging materials, oxygen and storage temperature on quality of germinated parboiled rice. LWT 2019, 121, 108926. [Google Scholar] [CrossRef]
- Hart, A.; Kleinig, A. The role of oxygen in the ageing of bottled wine. Aust. NZ Wine Industry J. 2005, 20, 46–50. [Google Scholar]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Teixeira, N.; Cruz, L.; Brás, N.F.; Mateus, N.; Ramos, M.J.; De Freitas, V. Structural Features of Copigmentation of Oenin with Different Polyphenol Copigments. J. Agric. Food Chem. 2013, 61, 6942–6948. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Lima, J.C.; Freitas, A.; Shimizu, K.; Macanita, A.; Quina, F. Charge-Transfer Complexation as a General Phenomenon in the Copigmentation of Anthocyanins. J. Phys. Chem. A 2005, 109, 7329–7338. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Ollilainen, V.; Vahermo, M.; Yli-Kauhaluoma, J.; Heinonen, M. Identification of novel pyranoanthocyanins in berry juices. Eur. Food Res. Technol. 2005, 220, 239–244. [Google Scholar] [CrossRef]
- Eiro, M.J.; Heinonen, M. Anthocyanin Color Behavior and Stability during Storage: Effect of Intermolecular Copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef]
- Xie, F.-y.; Li, F.-f.; Zhang, S.; Bi, W.-w.; Zhang, X.-l.; Zhang, X.-n. Analysis of acylation modification of black rice anthocyanins using fourier transform infrared spectroscopy (FTIR). Spectrosc. Spect. Anal. 2018, 38, 2386–2389. [Google Scholar]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Sources and relative stabilities of acylated and nonacylated anthocyanins in bev-erage systems. J. Food Sci. Technol. 2021, 1–15. [Google Scholar] [CrossRef]
- Fenger, J.-A.; Moloney, M.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural anti-oxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019, 10, 6740–6751. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Könighofer, L.; Hogg, M.; Scharinger, A.; Buchweitz, M. Impact of B-Ring Substitution and Acylation with Hydroxy Cinnamic Acids on the Inhibition of Porcine α-Amylase by Anthocyanin-3-Glycosides. Foods 2020, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; LaRocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Zeng, F.; Zeng, H.; Ye, Y.; Zheng, S.; Zhuang, Y.; Liu, J.; Fei, P. Preparation of acylated blueberry anthocyanins through an en-zymatic method in an aqueous/organic phase: Effects on their colour stability and pH-response characteristics. Food Funct. 2021, 12, 6821–6829. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Azevedo, J.; Teixeira, N.; Araújo, P.; de Freitas, V.; Basílio, N.; Pina, F. On the Limits of Anthocyanins Co-Pigmentation Models and Respective Equations. J. Agric. Food Chem. 2021, 69, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Zhang, C.L.; Cheng, X.L.; Zhao, J.H.; Wang, L.C. Solubility of 3-Caffeoylquinic Acid in Different Solvents at 291–340 K. Russ. J. Phys. Chem. A 2017, 91, 2512–2517. [Google Scholar] [CrossRef]
- Santos, P.F.D.; André, L.; Ducousso, M.; Contamine, F.; Cézac, P. Experimental Measurements of CO2 solubility in aqueous MgCl2 solution at temperature between 323.15 and 423.15 K and pressure up to 20 MPa. J. Chem. Engi. Data 2021, 66, 4166–4173. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.; Xie, H.; Yang, Y.; Lin, C. Inactivation of Staphylococcus aureus and Escherichia coli by the synergistic action of high hydrostatic pressure and dissolved CO2. Int. J. Food Microbiol. 2010, 144, 118–125. [Google Scholar] [CrossRef]
- Lockwood, S.F.; O’Malley, S.; Mosher, G.L. Improved aqueous solubility of crystalline astaxanthin (3,3′-dihydroxy-beta, be-ta-carotene-4,4′-dione) by Captisol (sulfobutyl ether beta-cyclodextrin). J. Pharm. Sci. 2003, 92, 922–926. [Google Scholar] [CrossRef]
- Mantell, C.; Rodríguez, M.; de la Ossa, E.M. A Screening Analysis of the High-Pressure Extraction of Anthocyanins from Red Grape Pomace with Carbon Dioxide and Cosolvent. Eng. Life Sci. 2003, 3, 38–42. [Google Scholar] [CrossRef]
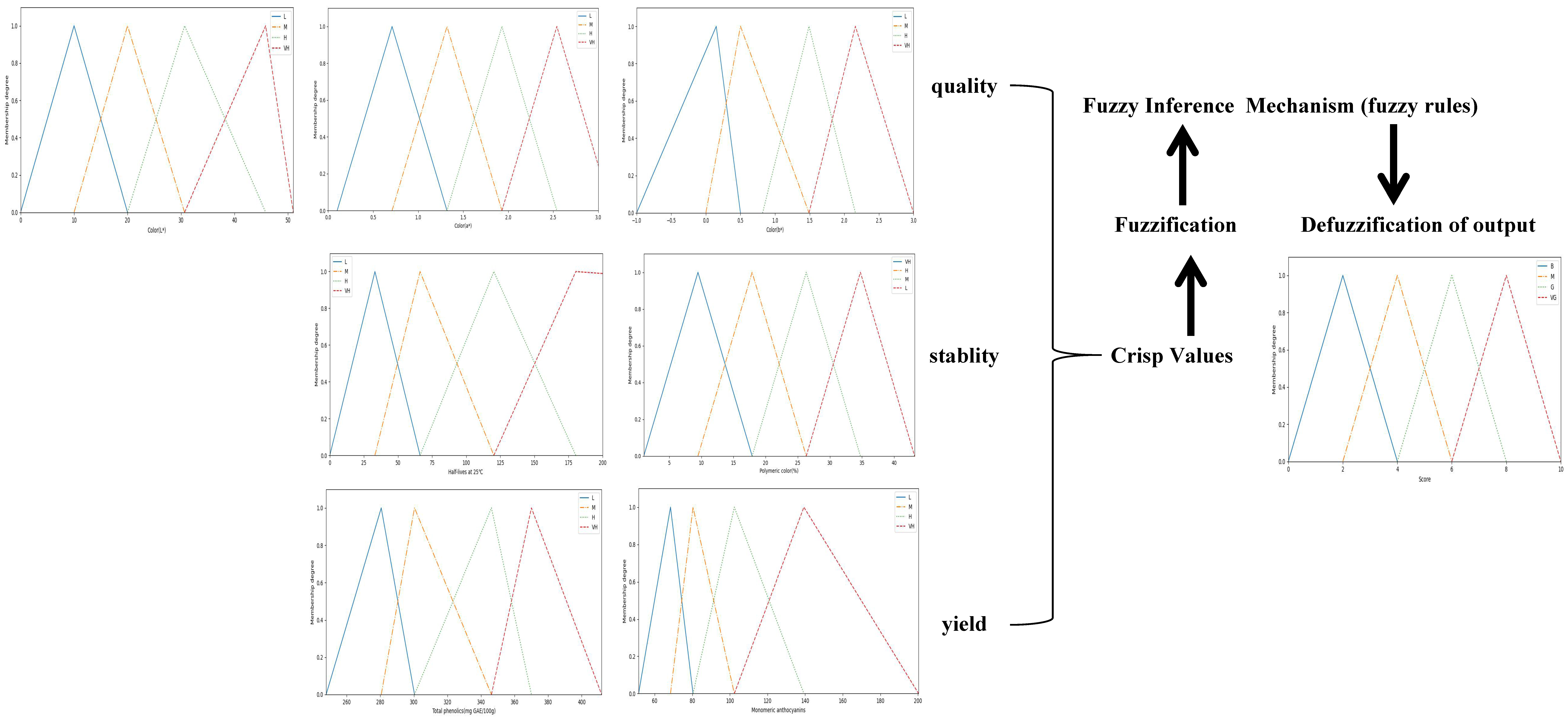



| Examples of Fuzzy Rules |
|---|
| If (Color(L*) is L) and (Color(C*) is L) and (c) (Color(h*) is L) and (Total Phenolic is L) and (Monomeric Anthocyanins is L) and (Polymeric Color is H)and (Half-lives at 25 °C is L) then (Score is VB) |
| If (Color(L*) is H) and (Color(C*) is H) and (c) (Color(h*) is H) and (Total Phenolic is H) and (Monomeric Anthocyanins is H) and (Polymeric Color is H)and (Half-lives at 25 °C is L) then (Score is B) |
| If (Color(L*) is H) and (Color(C*) is H) and (c) (Color(h*) is H) and (Total Phenolic is M) and (Monomeric Anthocyanins is M) and (Polymeric Color is L)and (Half-lives at 25 °C is H) then (Score is M) |
| If (Color(L*) is H) and (Color(C*) is H) and (c) (Color(h*) is H) and (Total Phenolic is H) and (Monomeric Anthocyanins is H) and (Polymeric Color is L)and (Half-lives at 25 °C is L) then (Score is G) |
| If (Color(L*) is H) and (Color(C*) is VH) and (c) (Color(h*) is H) and (Total Phenolic is H) and (Monomeric Anthocyanins is H) and (Polymeric Color is L)and (Half-lives at 25 °C is VH) then (Score is VG) |
| H2O | Aqueous Ethanol | High-Pressure CO2 + H2O | ||
|---|---|---|---|---|
| Total phenolics (mg GAE/100 g) | 185.83 ± 0.77 c | 314.26 ± 1.53 a | 355.32 ± 3.47 b | |
| Total monomeric anthocyanins (mg C3G/100 g) | 73.88 ± 0.54 a | 92.10 ± 1.08 a | 157.71 ± 0.98 b | |
| The ratio of total phenolics to total monomeric anthocyanins | 2.52 | 3.41 | 2.25 | |
| L* | 37.53 ± 0.55 b | 42.08 ± 0.72 a | 33.51 ± 0.26 c | |
| Color | a* | 1.43 ± 0.92 c | 1.01 ± 1.14 b | 1.78 ± 0.57 a |
| b* | 1.48 ± 1.16 a | −0.41 ± 1.54 b | 1.27 ± 0.77 a | |
| Matrix pH | 2.37 ± 0.02 a | 3.22 ± 0.08 a | 2.36 ± 0.04 b | |
| Polymeric color (%) | 15.42 ± 2.72% b | 32.93 ± 3.30% a | 10.27 ± 0.04% b | |
| Half-lives at 25 °C, dark (days) | 59 (R2 = 0.88) | 46 (R2 = 0.93) | 139 (R2 = 0.89) | |
| Tentative Annotation | RT (min) | m/z | |||
|---|---|---|---|---|---|
| [M] + | [M] + Fragments | ||||
| Anthocyanin | Cyanidin-3-galactoside | 7.97 | 449.1071 | 344 | 287 |
| Cyanidin-3,5-diglucoside | 8.23 | 611.1617 | 449 | 287 | |
| Cyanidin-3-sambubioside-5-glucoside | 8.43 | 743.2011 | 611 | 419 | |
| Cyanidin 3-O-glucosyl-rutinoside | 8.65 | 757.2101 | 611 | 443 | |
| Cyanidin 3-diglucoside-5-glucoside | 8.85 | 773.2106 | 611 | 449 | |
| Cyanidin 3-(sinapoyl)glucoside-5-glucoside | 11.3 | 817.2172 | 655 | 449 | |
| Cyanidin 3-(p-coumaroyl)diglucoside-5-glucoside | 10.87 | 919.249 | 757 | 449 | |
| Cyanidin 3-(caffeoyl)diglucoside-5-glucosides | 10.37 | 935.2488 | 773 | 287 | |
| Cyanidin 3-(feruloyl)diglucoside-5-glucoside | 10.99 | 949.2602 | 787 | 449 | |
| Cyanidin 3-(sinapoyl)diglucoside-5-glucoside | 11.02 | 979.2708 | 817 | 449 | |
| Cyanidin 3-(caffeoyl)(p-coumaroyl)diglucoside-5-glucoside | 9.29 | 1081.2835 | 919 | 752 | |
| Cyanidin 3-(caffeoyl)(feruloyl)diglucoside-5-glucoside | 9.39 | 1111.2956 | 949 | 787 | |
| Cyanidin 3-(p-coumaroyl)(sinapoyl)diglucoside-5-glucoside | 11.57 | 1125.3063 | 979 | 449 | |
| Cyanidin 3-(caffeoyl)(sinapoyl)diglucoside-5-glucoside | 9.47 | 1141.2498 | 979 | 817 | |
| Cyanidin 3-(sinapoyl)(feruloyl)diglucoside-5-glucoside | 11.69 | 1155.3173 | 993 | 449 | |
| Cyanidin 3-(sinapoyl)(sinapoyl)diglucoside-5-glucoside | 11.73 | 1185.3325 | 1023 | 817 | |
| Cyanidin 3-(sinapoyl)(p-coumaroyl) triglucoside-5-glucoside | 11.48 | 1287.3596 | 1125 | 979 | |
| Cyanidin 3-(sinapoyl)(feruloyl)triglucoside-5-glucoside | 10.47 | 1317.3758 | 1155 | 979 | |
| Cyanidin 3-(sinapoyl)(sinapoyl)triglucoside-5-glucoside | 11.64 | 1347.3844 | 1155 | 963 | |
| Phenolic | 3-Caffeoylquinic acid | 5.6 | 355.1011 | 217 | 193 |
| Kaempferol-3-triglucoside | 7.8 | 771.1981 | 447 | 284 | |
| 5-Methoxysalicylic acid | 8.1 | 169.0488 | 109 | 64 | |
| Isoquercitrin | 8.7 | 465.1032 | 91 | 64 | |
| Kaempferol-3-ferulic -triglucoside-7-glucoside | 9.3 | 1109.2278 | 947 | 771 | |
| Quercetin-3-diglucoside-7-triglucoside | 9.4 | 1111.313 | 787 | 625 | |
| Sinapic acid glucoside | 9.6 | 385.1074 | 385 | 223 | |
| Quercetin-3-diglucoside-7-glucoside | 9.7 | 787.2181 | 625 | 463 | |
| Kaempferol-3-diglucoside-7-glucoside-5-glucoside | 10.3 | 933.2577 | 771 | 447 | |
| Sinapic acid diglucoside | 10.6 | 547.1658 | 385 | 223 | |
| Quercetin-7-diglucoside | 10.7 | 625.1434 | 625 | 301 | |
| Phloretin | 10.8 | 275.0922 | 259 | 109 | |
| Quercetin-3-triglucoside-7-glucoside | 10.9 | 949.2839 | 787 | 462 | |
| Quercetin-3-diglucoside-7-sinapic-glucoside | 10.9 | 1095.3069 | 771 | 609 | |
| Kaempferol-3- sinapic-diglucoside-7-glucoside | 11 | 977.2552 | 815 | 609 | |
| Kaempferol-3-diglucoside | 11.2 | 609.1489 | 609 | 284 | |
| Quercetin-3-diglucoside-7-ferulic-diglucoside | 11.6 | 1125.3225 | 949 | 625 | |
| Phlorizin | 11.6 | 437.144 | 275 | 259 | |
| Quercetin sinapic-3,7-di-O-glucoside-5-diglucoside | 11.7 | 1155.3181 | 993 | 787 | |
| Sinapic acid | 12.4 | 223.058 | 108 | 178 | |
| Ferulic acid | 12.5 | 193.0505 | 161 | 134 | |
| Category | Name | Mass | m/z | RT (min) | Formula | Error (ppm) | p-Value (t) | FC (Pressure CO2 + H2O/H2O) |
|---|---|---|---|---|---|---|---|---|
| Alkaloid | Betaine | 117.15 | 118.0857 | 1.99 | C5H11NO2 | 0.3558 | 0.0047 | 2.5988 |
| Phenylalanine betaine | 207.27 | 208.1328 | 8.71 | C12 H17NO2 | −0.1996 | 0.1965 | 2.0554 | |
| Lenticin | 246.30 | 247.1438 | 9.87 | C14H18N2O2 | −0.2809 | 0.1025 | 2.7830 | |
| Indole | 117.15 | 118.0632 | 9.16 | C8H7N | 0.8293 | 1.23 × 10−12 | 4.0135 | |
| 2-Methylindole | 131.17 | 132.0797 | 9.26 | C9H9N | −0.7040 | 1.16 × 10−7 | 12.3634 | |
| 3-Formylindole | 145.16 | 146.0597 | 9.15 | C9H7NO | 0.7957 | 2.33 × 10−13 | 3.6899 | |
| Nucleic Acid | Guanine | 151.13 | 152.0550 | 6.64 | C5H5N5O | −0.5112 | 0.0006 | 3.2886 |
| Amino Acid | Phenylalanine | 165.19 | 166.0856 | 8.04 | C9H11NO2 | 0.7581 | 6.37 × 10−13 | 3.3084 |
| Tyrosine | 181.20 | 182.0812 | 6.71 | C9H11NO3 | 0.7611 | 8.29 × 10−8 | 3.1048 | |
| Tryptophan | 204.23 | 205.0965 | 9.15 | C11H12N2O2 | 0.7147 | 3.21 × 10−9 | 3.0079 | |
| Proline | 115.13 | 116.0693 | 7.05 | C5H9NO2 | 0.7304 | 6.57 × 10−10 | 3.8139 | |
| Isoleucine | 131.17 | 132.1021 | 6.94 | C6H13NO2 | 0.7625 | 8.63 × 10−13 | 3.0268 | |
| Triethyl phosphate | 182.15 | 183.079 | 14.04 | (C2H5O)3PO | −0.3679 | 0.0262 | 2.3128 | |
| Fatty Acid | Triethylcitrate | 276.28 | 277.1264 | 14.86 | C12H20O7 | −0.301 | 0.0406 | 2.3636 |
| Tri-isobutylphosphate | 266.31 | 267.1718 | 16.5 | C12H27O4P | 0.7346 | 7.17 × 10−6 | 3.6693 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yang, S.; Xu, L.; Wang, X.; Mi, L.; Wang, K.; Liao, X.; Xu, Z. Evaluation Study on Extraction of Anthocyanins from Red Cabbage Using High Pressure CO2 + H2O: A Fuzzy Logic Model and Metabolomic Analysis. Sustainability 2022, 14, 1369. https://doi.org/10.3390/su14031369
Wang B, Yang S, Xu L, Wang X, Mi L, Wang K, Liao X, Xu Z. Evaluation Study on Extraction of Anthocyanins from Red Cabbage Using High Pressure CO2 + H2O: A Fuzzy Logic Model and Metabolomic Analysis. Sustainability. 2022; 14(3):1369. https://doi.org/10.3390/su14031369
Chicago/Turabian StyleWang, Bingfeng, Shini Yang, Lei Xu, Xue Wang, Lu Mi, Kewen Wang, Xiaojun Liao, and Zhenzhen Xu. 2022. "Evaluation Study on Extraction of Anthocyanins from Red Cabbage Using High Pressure CO2 + H2O: A Fuzzy Logic Model and Metabolomic Analysis" Sustainability 14, no. 3: 1369. https://doi.org/10.3390/su14031369






