Abstract
This study focused on what combination of anaerobic digestion (AD) temperature (ambient, mesophilic, and thermophilic) and olive mill waste (OMW) to dairy manure (DM) ratio mixture delivers the desired renewable energy and digestate qualities when using AD as olive mill waste treatment. OMW is widespread in the local environment in the North Sinai region, Egypt, which causes many environmental hazards if left without proper treatment. Three different mixtures consisting of OMW, dairy manure (DM), and inoculum (IN) were incubated under ambient, mesophilic, and thermophilic conditions for 45 days. The results showed that mixture B (2:1:2, OMW:DM:IN) at 55 °C produced more methane than at 35 °C and ambient temperature by 40% and 252%, respectively. Another aim of this study was to investigate the effects of the different concentrations of the digestate taken from each mixture on faba bean growth. The results showed that the maximum fresh weight values of the shoot system were observed at 10% and 15% for mixture B at ambient temperature. The best concentration value for the highest root elongation rate is a 5% addition of digestate mixture A at 55 °C, compared with other treatments.
1. Introduction
Greenhouse gases (GHGs) are added to the atmosphere by human activities. One of the most potent GHGs emitted to the atmosphere is methane, 90% of which comes from the decomposition of biomass.
Olive oil manufacturing in the Mediterranean countries produced around 1.4–1.8 million tons of olive oil, resulting in a significant quantity of solid waste every year (30 million m3 of solid waste) [1]. According to the Ministry of Agriculture and Land Reclamation, Egypt has around 497 km2 of planted olive trees, producing roughly 314,450 tons of olives. This operation generates large quantities of waste, both solid and liquid, that are made up of highly colored and refractory chemicals with high organic loading and complicated compositions. The North Sinai region produces 3000 tons/year of olives that are processed in 18 olive mills. During the milling season, OMW production exceeds 720 m3/day and an area of 74 km2. Olea cake (OC) is a solid byproduct from the olive oil extraction process that is made up of a variety of olive materials, including skins, woody endocarps, and seeds, and accounts for around 35% of the weight of the olives that were squeezed. It is a common agricultural waste product in the Mediterranean region. Due to technological and budgetary restrictions, no suitable solution for the safe disposal of OMW has been developed. The anaerobic digestion (AD) process is an effective approach to getting rid of an extensive variety of wastes [2].
Methane-enriched biogas is produced by AD, and it decreases organic pollutants while also making sustainable energy. Biogas and digestate are the primary end products of AD [3]. The AD of olive mill waste (OMW) faces many challenges due to the phenols and furans that are considered AD inhibitors. Some studies, however, suggest that their toxicity is linked to more complicated parameters, including the consortium of microbes, acclimation, individual concentration, and a combination of these inhibitory substances [4,5]. Low nitrogen content and the fast acidification (low pH) of OMW have been reported as problems for digestion [6,7]. Messineo et al. [8] found a low biodegradability of OMW due to high phenol content and an unbalanced nutrient ratio, which could be overcome by chemical addition, pretreatment, or co-digestion. The OMW AD process was unstable at high chemical oxygen demand (COD) concentrations due to polyphenol inhibitors, poor OMW alkalinity, and the absence of ammonia [9,10]. To overcome these issues, OMW AD requires dilution with water and the addition of a nitrogen source. Alkalinity is commonly corrected with NaHCO3, NaOH, or Ca(OH)2 [9,10]. However, the addition of chemicals is not environmentally friendly, and the dilution of OMW with water results in large volumes of unwanted effluents. In a recent study, swine manure has been used as a cost-effective way for treating OMW without the need to add nitrogen or chemicals to increase buffer capacity [1].
Angelidaki et al. [1] investigated the co-digestion of OMW with swine manure in an up-flow anaerobic sludge blanket (UASB) reactor under mesophilic conditions, and the results revealed that the co-digestion of OMW with swine manure gave the highest CH4 production rates. However, no other research had investigated the co-digestion of OMW with dairy manure to determine the optimal amount of dairy manure required for effective OMW digestion while investigating the effects of the digestate from each mixture in different concentrations on plant growth, as demonstrated in this study.
Adding a nitrogen-rich substrate like dairy manure to the mixture may help to lower the carbon-to-nitrogen (C:N) ratio and offer buffer capacity to keep the pH stable, both of which are important for increasing methane (CH4) generation [11]. Biogas generation from OMW improves both in quantity and quality under co-digestion processes [12,13,14].
The organic nitrogen is converted to ammonia during the AD process, and is considered a nitrogen source for the plants [15]. AD changes manure composition: the organic nitrogen content is reduced and ammonium nitrogen content is increased; the carbon content and dry organic matter are reduced; and the C:N ratio declines while the pH increases [16].
The agriculture-based AD reactor digestate is utilized as a fertilizer, with prior research investigating vegetable growth in hydroponic systems utilizing digestate as a growth medium [17,18,19]. Potassium (K), phosphorus (P), and nitrogen (N) are key plant nutrients [20,21]. The digestate contains sufficient concentrations of N, P, and K, and it could be used as a biofertilizer since they are easily available for plants [22]. Therefore, it could be used as a fertilizer to improve soil fertility and plant quality, and their immunity to biotic and abiotic agents [23]. Lošák et al. [24] in their studies conclude that the use of digestate improves the quality and yield of vegetables. The authors also reported that the use of digestate as a fertilizer increases the content of macro- and microelements in the soil and plants. In a study undertaken by Pivato et al. [25], they studied the potential emerging contaminants present in the digestate using ecotoxicological tests. They found that no significant negative effects have been observed on the plant growth, but rather that they improved plant growth and mineral content, which promotes the use of digestate as a biofertilizer in agriculture. The digestate is beneficial for the plants and, in addition, it also improves the structure of soil [26,27]. So, the possibility of using OMW as a fertilizer could be achieved when integrated and processed using AD. However, no other research had investigated the effects of employing digestate from a system that involves the co-digestion of OMW with dairy manure, as demonstrated in this study. Furthermore, co-digestion and thermophilic effects on energy production from OMW, as well as fertilizer quality, still need some investigation. The objectives of this study were (Figure 1): (1) assess the potential of using OMW in North Sinai and turning it into a renewable energy source; (2) to determine the quantity and quality of biogas produced from the co-digestion of different mixtures of OMW and dairy manure; (3) to investigate the impact of mesophilic (37 °C) and thermophilic (55 °C) conditions compared to uncontrolled temperatures on AD performance and biogas quantity and quality; and (4) to examine the impact of digestate on plant root elongation to investigate its importance after anaerobic digestion as a potential fertilizer.
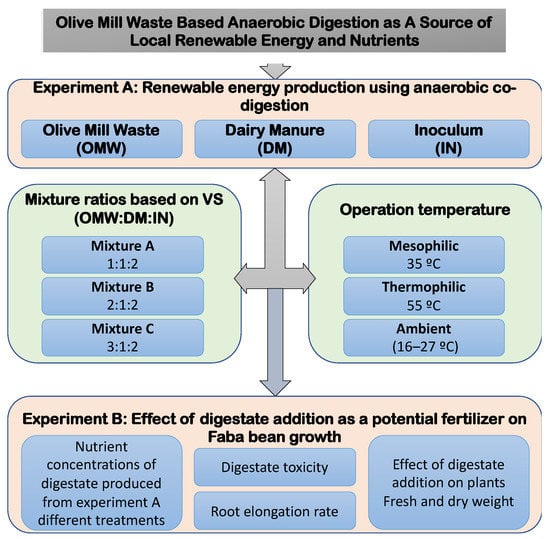
Figure 1.
Overall experiment goals and specific topics.
2. Materials and Methods
2.1. Experiment A: Renewable Energy Production Using Anaerobic Co-Digestion
2.1.1. Inoculum Preparation
The inoculum is considered an essential factor in increasing the biodegradation of any type of organic material during anaerobic digestion [28]. Many studies have collected inoculum from working plants [29]. In the current work, the inoculum was prepared 15 days before the basic experiment began. The sludge collected (dairy manure) was stored at 4 °C for 24 h before the inoculum preparation process. The inoculum mixture was prepared using 1 kg of dairy manure mixed with 3 L of fresh water. A 5 L glass digester was used to hold the inoculum mixture in anaerobic conditions. The inoculum was placed in thermophilic conditions (55 °C) without any pH adjustment for 15 days. It was fed and tightly closed with rubber caps and silicon glue, covered with an aluminum sheet to keep out light, and incubated in a water bath with an automatically controlled heater and thermostat system to operate the temperature at 55 °C and keep the digester’s temperature stable. A gasbag was used to collect the biogas produced by the inoculum reactor. The primary purpose of biogas collection was to assure the activity of the reactor [30].
2.1.2. Feedstock Preparation
This study collected fresh dairy manure from a small farm located in El-Arish, North Sinai, Egypt. Samples were collected and placed in an airtight sterile plastic bag to prevent aeration, then they were stored in a fridge at 4 °C under dark conditions, which helped slow down the bacteria metabolism and keep the properties of the manure stable until the start of the experiments [31].
Fresh OMW was collected from a two-phase oil mill plant located in El-Arish, North Sinai, Egypt. The OMW samples were collected from a plant with two olive oil processing lines; the first line processed 1.5 tons while the second processed 2.5–3 tons per season (September to December). The oil percentage was around 60–70% of the total weight while the OMW was about 30–40%. All the OMW is stored in the open air (outside the plant) with no cover. More than 25 kg of OMW was collected from different depths. The samples were homogenized and placed in a sterile plastic bag and tightly closed to prevent aeration, transported, and subsequently stored in the fridge at 4 °C under dark conditions for 2 days. The substrates were characterized in terms of total solids (TS) and volatile solids (VS) content, according to the standard methods introduced by American Public Health Association, APHA [32].
2.1.3. Total Solids/Volatile Solids Analysis
The TS content of a sample is the mass of solids remaining after a sample has been dried in a 105 °C oven for 24 h. The samples were carried out in triplicate, and the average values were considered for the evaluations. The VS is the remaining solids representing the fixed total, dissolved, or suspended solids while the ignition loses weight. The samples were carried out in triplicate and then placed in a furnace for two hours at 550 °C, and the average values were considered for the evaluations.
VS concentration indicates the substrate’s organic matter content and is used to estimate the effectively decomposable fraction [31]. TS and VS values are presented in Table 1. VS of the raw dairy manure, olive mill waste, and inoculum were measured to be used in the calculations of the different mixture ratios (Table 2).

Table 1.
Total solids (TS), volatile solids (VS) (dry weight (DW), and wet weight (WW) bases), with ± standard division for substrates, olive mill waste, dairy manure, and inoculum.

Table 2.
Experimental design detailing the operational temperature, the mixture ratios based on VS, and the quantity of olive mill waste (OMW), dairy manure (DM), and inoculum (IN) added to each triplicate anaerobic digestion reactor.
2.1.4. Reactor Design
The total number of reactors used in this experiment was 36. Each reactor’s empty size was a 1 L digester with an effective working volume of 750 mL, and 250 mL was left as headspace for gas holding. In batch-dark mode, the experiment was designed for 12 treatments in triplicates. The experiments were carried out under ambient temperatures (16.3–27.4 °C), mesophilic temperatures (35 ± 1.0 °C), and thermophilic temperatures (55 ± 1.0 °C). The substrates (OMW and DM) and inoculum were loaded at three different substrate–inoculum ratios based on VS (Table 2), as suggested by recent research [33,34,35].
2.1.5. Digestion Temperature
Two homemade 70 L water baths were created to heat the reactors. Each water bath was 60 cm in diameter, 25 cm in height. An automatically controlled water heater (220 V, 500 W; made in China) with a thermostat system (0–40 °C, 250 V/16 A; China) was used to maintain the temperature at mesophilic conditions (35 °C) while a 1000 W water heater (220–240 V, 1000 W; China) with a 40–80 °C thermostat system (40–80 °C, 250 V/15 A; manufactured by Thermix, Italy) was used to maintain the thermophilic conditions (55 °C). Water bath temperature was monitored by using a digital thermometer. The water bath was coated with insulation to reduce heat loss.
The daily ambient temperature was determined by using a digital thermometer (0–100 °C) every six hours. The experiments were performed without any pretreatments, i.e., changing the initial pH of substrates, the addition of trace elements, and any chemical or physical pretreatment at substrates.
Various mixtures from olive mill waste (OMW), dairy manure (DM), and inoculum (IN) were formed based on 1:1:2, 2:1:2, and 3:1:2 ratios, respectively. Inoculum only was also tested to determine the biogas production from inoculum compared to the mixtures (Table 2). The experiment was carried out in triplicate, and the average values were considered for the evaluations. The biogas volume was measured every day until the gas reached the minimum level (less than 1% of the cumulative gas). All reactors were tightly closed with rubber caps and silicone glue to prevent leakage of biogas.
2.1.6. Methane Concentration and Purification
Biogas was collected and measured daily with the water displacement method, as described in the previous research [36]. The CH4 concentration in the collected biogas was measured daily. The CH4 and CO2 concentrations were measured by absorbing CO2 in an alkaline liquid (2M KOH) [37]. The CO2 liquid scrubber’s pH was adjusted to 9.0 to make sure all CO2 in the biogas was completely absorbed. The amount of liquid replaced after the CO2 scrubber corresponds to the volume of CH4 produced. The difference between initial (before the CO2 scrubber) and final volume (after the CO2 scrubber) corresponds to the CO2 content in the biogas, since the H2S concentration was negligible compared with CO2 concentration [37].
2.1.7. Laboratory Measurements
The following parameters were analyzed for each sample by standard methods APHA [32]. Sample pH was measured using a pH meter. At the end of the digestion stage, total Kjeldahl nitrogen, total prosperous (TP), and potassium (K) were determined to prepare for the plant growth experiment. TKN and TP were analyzed on a Lachat autoanalyzer (QuikChem 8500, Hach Company, Loveland, CO, USA) using QuikChem methods 13-107-06-2-D for TKN and 13-115-01-1-B for TP. Total K concentration was determined using atomic absorption spectrophotometry.
2.2. Experiment B: Plant Experiment Using Digestate
2.2.1. Faba Bean Growth
Faba bean seeds (Vicia faba) were coated with rolls of paper towel and placed vertically under room temperature tap water for three days. Four seedlings were placed in Perspex strips on top of a 275 mL plastic beaker (Polyethylene terephthalate PET) filled to the brim with 1.0 mM CaCl2 and 5.0 mM H3BO3. The seedling growth experiments were placed at room temperature for 24 h in this basal solution. The strips were then transferred to a digestate containing solution for 7 days.
2.2.2. Experimental Design and Digestate Spike
The experimental work was designed as described by Kopittke et al. [38], in a laboratory maintained at ca. 24 °C at Arish University, Egypt. Faba bean seeds (Vicia faba) were coated with rolls of paper towel and placed vertically under room temperature tap water for three days. Four seedlings were placed in 2 mm diameter holes in 10 mm-wide Perspex strips placed on the top of a plastic beaker (polyethylene terephthalate (PET)) filled with 275 mL solution of 1.0 mM CaCl2 and 5.0 mM H3BO3. The seedlings were grown for approximately 24 h in this basal solution before transferring the strips to different concentrations of digestate (0.0, 2.50, 5.0, 10.0 and 15.0%) for the exposure period (typically 24 h for toxicity measurements and 7 days for characterizing plant growth). Each concentration of tested digestate was conducted in triplicates, resulting in a total of 432 plants in the three digested slurries at the three digestion temperatures (mesophilic, thermophilic, and ambient) and 12 plants in the control (plant grown in basal solution only) (Figure 2).
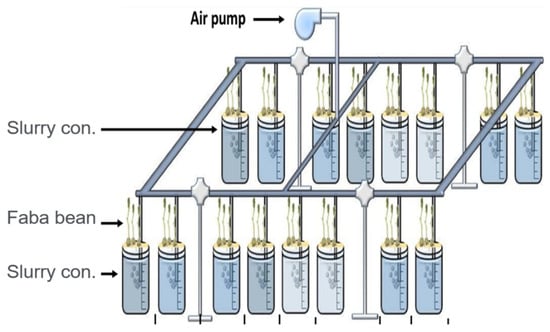
Figure 2.
Schematic representation shows the overall experimental layout with a four-hole strip used to hold the plant in the solution phase.
Immediately after transferring the strips, and at set times up until 24 h thereafter, each Perspex strip was placed horizontally 300 mm beneath a digital camera (Sony DLSAR A2, Tokyo, Japan) mounted on a tripod. A digital image was captured, and the strips were replaced on the beaker. This took ca. 30 s ensuring minimal disruption to root growth. The length of each root was determined using ImageJ processing—an analysis software available free of charge at https://imagej.net/Welcome [39]. After recording the plant root length at 0 and 24 h, root elongation rate (RER, mmh−1) was calculated as follows:
where R0 and Rt are the length (mm) of each root at zero and T time (T = 24 h).
RER = (Rt − R0 )/T
The average fresh and dry weight of the plants after 7 days of exposure time to digestate was recorded. The fresh weight was measured directly using digital balance, while dry weight was measured after oven drying at 60 °C for 24 h.
2.2.3. Toxicity Model EC50
The toxicity model was calculated by the log-logistic model using MS Excel 2016. The RSD (residual standard deviation) [40] and correlation coefficient have been used to assess the model performance for root elongation rate (RER) affected by different digestate concentrations after 24 h of the exposure time to the digestate. Toxicity dose was calculated as EC50 value described by Ritz et al. [41]. By far, the log-logistic models are the most used dose–response models [42] that can calculate EC50 as follows:
where e and m are the equation coefficient values, m is denoted as EC50, and is added digestate concentrations to the bean plant (mg L−1) from all digesters.
2.2.4. Length, and Fresh and Dry Weight of Root and Shoot System
The average length of the faba bean root and shoot system and their fresh and dry weight after germination was recorded. ImageJ software was used to monitor the length parameter using the Sony digital camera (DLSAR A2). The fresh weight was measured directly using digital balance, while dry weight was measured after oven drying at 60 °C for 24 h.
2.3. Statistical Analysis
Each treatment was conducted in three replicates, and the results were presented as mean ± standard deviation (SD). The statistical analysis of experimental data utilized the Parried t-test and Pearson correlation. Each of the experimental values was compared to the corresponding control. Moreover, there was a comparison of 95% confidence intervals using ANOVA analysis using Minitab ® statistical software Version 17.1.0.
3. Results and Discussion
3.1. Experiment A: Renewable Energy Production Using Anaerobic Co-Digestion
3.1.1. Biogas Production
The biogas production values were normalized by gVS of the substrate being added to each treatment to easily compare the results presented in this study to other studies (Figure 3). In anaerobic digestion, the choice of substrate plays an important role, either in terms of maximizing the biogas yield and/or in the economy of the process [43,44]. The cumulative biogas was produced from mixture B (504 ± 0.4 mL/gVS for 55 °C; 394 ± 3.8 mL/gVS for 35 °C), which was higher than that of A and C by 58% and 30%, respectively, for 55 °C, while it was 53% and 177%, respectively, for 35 °C.
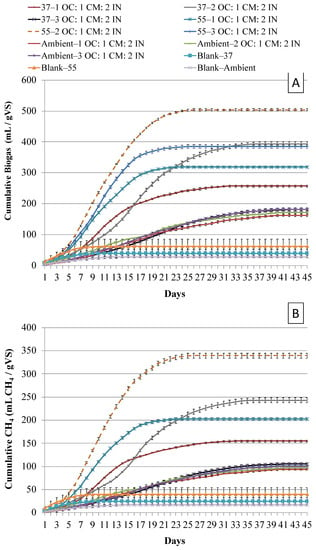
Figure 3.
(A) Cumulative biogas and (B) methane production normalized by gVS added in each treatment at different operational temperatures. Error bars represent the standard deviation of triplicates.
The biogas production for mixtures A, B, and C at 55 °C was higher than that of 35 °C by 24%, 28%, and 113%, respectively. The treatments A, B, and C produced more biogas under 55 °C and 35 °C compared to ambient temperature. The lowest cumulative biogas obtained from mixture A at ambient temperature (16–27 °C) was 162.3 mL/gVS, while mixture A produced 257 ± 1.7 mL/gVS at 35 °C, and 318 ± 1.1 mL/ gVS at 55 °C (Figure 3). Biogas production from mixtures A, B, and C at thermophilic conditions were 96%, 195%, and 112.7% higher than biogas production at ambient temperature, respectively. At the same time, treatments A and B produced 85% and 130% more biogas under 35 °C compared to ambient temperature, respectively.
This finding showed the efficiency of using thermophilic over the mesophilic and ambient temperatures. The increase in the digestion temperature can increase the biodegradation rate of organic material [8]. Kim et al. [45] showed that thermophilic digestion at 55 °C was more efficient in increasing AD biogas production from food waste compared to mesophilic. Moreover, recent research found that the removal rate of soluble chemical oxygen demand (sCOD) under thermophilic conditions (83%) was higher than the sCOD removal (76%) under Mesophilic conditions [8].
Moreover, Pandey et al. [46] observed the influence of temperature in the anaerobic digestion of DM, which was performed at low (25 °C), mesophilic (37 °C), and thermophilic temperatures (52.5 °C). The authors found that the biogas production at thermophilic (52.5 °C) and mesophilic temperature digestions (37 °C) were 49 and 17 times higher, respectively, and higher than that of digestion at a low temperature (25 °C). Prasad [47] found more biogas production was promoted while increasing the digestion temperature.
Rubio et al. [48] found a mixture of OMW:DM (85:15, weight-based ratio) causes the inhibition of methanogenesis and low biodegradability while using a two-phase digestion [48]. The methanogenesis inhibition is due to the high concentration of propionic acid existing in OMW. The authors suggested using DM with different mixing ratios to prevent inhibition problems in the AD process.
In the current study, the results show the co-digestion of DM with OMW (mixture B) under thermophilic conditions represents an effective solution to achieve a successful AD of OMW. The improvement in the AD process was due to the co-digestion of DM improving the digestion process by providing the necessary buffer needed for digestion [11].
3.1.2. Biogas Composition
In this study, biogas composition (CH4 and CO2) was analyzed daily for 45 days of the digestion period. The methane content ranged from 55.5% to 68.2%. The CO2 content ranged from 31.8% to 44.5%. The maximum level of CH4 (68.2%) corresponded with mixture C at a thermophilic temperature (55 °C), while the minimum level of CH4 (55.5%) corresponded with mixture C at an ambient temperature (Figure 3).
Mixture B produced the highest cumulative CH4 (340 ± 3.0 mL CH4/gVS) compared to other treatments. While mixture B produced 243 ± 3.2 mL CH4/gVS at mesophilic conditions. Mixture C produced 202 ± 1.4 mL CH4/gVS at thermophilic conditions while being reduced to 105 ± 1.8 mL CH4/gVS at mesophilic conditions. The results showed an increase in cumulative CH4 from mixture B in comparison to mixture C by 131% and 29% at mesophilic and thermophilic conditions, respectively. From our study, the produced biogas quality and quantity in thermophilic conditions was more efficient compared to mesophilic and ambient temperatures.
The maximum CO2 concentration was 44.5% for mixture C at an ambient temperature, while the minimum level of CO2 was 31.8% for mixture C at a thermophilic temperature.
Our study is in agreement with a study by İnce et al. [49], which showed that digestion at a thermophilic temperature (55 °C) increases the amount of biogas without affecting biogas composition.
3.1.3. Effect of Mesophilic and Thermophilic Temperatures on Biogas Production Based on Digestion Period
While the overall biogas production efficiency from thermophilic temperatures was higher than the other treatments, the treatments produced 100% of the methane and biogas production in the first 25–26 days of the 41-day digestion period, while the maximum digestion period was observed with the ambient temperature treatments. In a mesophilic temperature, biogas production takes place at 37 days from the beginning of biogas production to the end of biogas production. The results show a clear relationship between temperature and the digestion period. The thermophilic digestion temperature leads to an increase in the biodegradability of organic material, which also leads to an increase in the cumulative biogas production, while reducing the digestion period. Lv et al. [50] noticed an improvement in biogas production, biodegradation of organic matter, and reduced hydraulic retention time while observing the co-digestion of corn stalk and DM under mesophilic conditions. Jacob et al. [51] found an increase in methane volume from DM digested at 40 °C compared to that digested at 30 °C and room temperature. Zhu et al. [52] found that low digestion temperature leads to an increased digestion period and decreased biogas production as a deleterious effect on methanogenesis bacteria. The decrease in the biogas production in all ambient temperature treatments is due to the fluctuation of temperature, which affects methanogens. Methanogenic microbes are affected by changes in temperature, which must be maintained to keep methanogens active [53,54,55,56].
Based on the results, the recommended mixture was mixture B (2:1:2, OMW:DM:IN) at 55 °C, which produced more methane than 35 °C and ambient by 40% and 252%, respectively. The findings from this research presents an efficient method to reduce the greenhouse gas emissions from OMW by capturing methane. Methane emissions from OMW, especially in the Mediterranean countries, which collectively produce 30 million m3 of solid waste, are considered a global warming challenge [1]. Using the co-digestion of DM with OMW supports sustainable development and produces a clean source of energy, which helps to reduce the greenhouse gasses if AD systems are used to treat the OMV. Another aim of this study was not only to produce clean energy from OMW (Experiment A) but also to investigate the effects of different concentrations of digestate from each mixture on plant growth (Experiment B). Compared to synthetic fertilizers, using digestate as fertilizer could help reduce the direct and indirect greenhouse gas emissions (such as production, transportation, and use) from the agriculture sector.
3.2. Experiment B: Effect of Digestate Addition as a Potential Fertilizer on Faba Bean Growth
3.2.1. Nutrient Concentrations of Digestate Produced from Experiment A (Different Treatments) and Used in Experiment B as Fertilizer
The digestate from different mixtures under different temperatures (37 °C, 55 °C, and ambient) were analyzed for total N, P, and K as a proxy of nutrient concentrations of the proposed fertilizers. The digestate shows various nutrient concentrations of N, P, and K. Figure 4 shows the concentrations of N, P, and K of mixture A, mixture B, mixture C, and blank (inoculum alone) produced under 37 °C, 55 °C, and ambient temperatures.
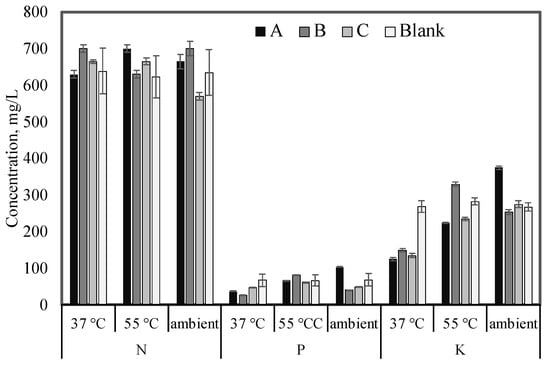
Figure 4.
Nutrient concentrations (NPK, mg/L) of produced digestate under different temperature conditions (mesophilic, thermophilic, and ambient). Error bars represent the standard deviation of triplicates.
The results showed that the maximum average value of N, P, and K, regardless of incubation temperature, were mixtures B (677 mg N/L), A (69.7 mg P/L), and blank (273 mg K/L), respectively. However, the ambient temperature showed promising nutrient content for mixture B and A for N and K, respectively. It is noteworthy that cumulative biogas production from mixture A is lower than that produced from mixture B at ambient temperature. Mixture A, at ambient temperature, showed a 10%, 21%, and 12% increase in N, P, and K, respectively, more than mixture B at 55 °C (Figure 4).
Digestate has a significant amount of NPK nutrient content and could be used as a potential source for soil fertility, individually or with mineral fertilizer applications [57]. Feng et al. [58] reported the main physical and chemical properties of digestate, which contains 95.5% water, a total nitrogen of 4.2 g/kg, a total phosphorus of 0.27 g/kg, a total potassium of 1.15 g/kg, and a large amount of humic acid ammonium. Arthurson [59] confirms that the digested residues contain 25% more accessible ammonium (NH4+-N) than untreated manure.
3.2.2. Effect of Digestate Addition on Fresh and Dry Weight of Plants
The results of the fresh and dry weight of plant exposure to different concentrations of tested slurries were presented in Figure 5. The results showed that the maximum fresh weight values of the plants were observed at 10% and 15% for mixture B at ambient temperature (Figure 5). This could be due to the enrichment of the N concentration of the added digestate, as seen in Figure 4. The general results show an increase of fresh plant weight with an increase of the digestate concentrations. The most effective addition was the 10% concentration, reflecting the highest fresh weight throughout the whole dataset, although there is no significant difference between all tested slurries according to the one-way AONVA test. However, all additional levels of digestate showed a significant difference in fresh plant weight compared to the control (p < 0.01). Moreover, a significant relationship has been found between digestate concentrations and fresh plant weight. Mixture A and B at 37 °C temperature-produced digestate showed the highest significant value at probability <0.01 where r > 0.97, followed by mixture B (r = 0.92, p = 0.03) and A (r = 0.88, p = 0.04), produced by digestate at ambient and 55 °C temperatures, respectively.
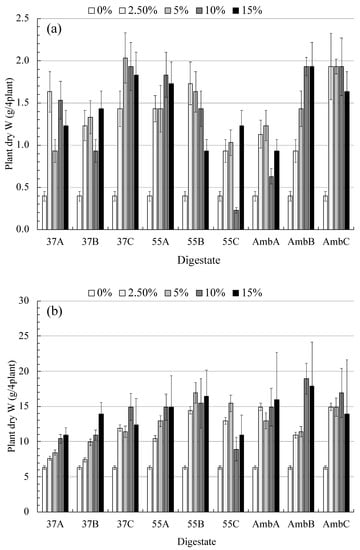
Figure 5.
Dry (a) and fresh (b) weight (g) of the faba bean plant as affected by different concentrations (0, 2.5, 5, 10, and 15%) of digestate. Error bars represent the standard deviation of triplicates.
On the other hand, the dry weight result of the faba bean plant was presented in Figure 5, as affected by different concentrations of digestate. The maximum dry weight values of the plant were observed at mixture C for the addition of 5% at 37 °C. The result in dry weight from mixture C at a mesophilic temperature is 8% more than the dry weight from mixture B at an ambient temperature. A one way ANOVA test displayed insignificant differences between different treatments. However, all addition levels of digestate show significant differences in dry plant weight compared with the control (p < 0.01). It is interesting to report that a significant correlation has only been found between mixture B at ambient temperature with dry plant weight (r = 0.92, p = 0.03).
Similarly, Makdi et al. [60] confirmed that due to the high available nutrient content, digestate application resulted in significantly higher aboveground biomass yields in the case of winter and spring wheat than that of the farmyard manure and undigested slurry treatment. Field experiments with the application of equivalent amounts of total N indicate that the uptake of N from liquid digested animal slurry (digestate) equaled that of undigested slurry after surface application, despite the higher NH4+-N content of the digestate [60,61].
Vegetation pot and field trials with vegetables show that the digestate application resulted in comparable or better yields in comparison to mineral fertilizers for kohlrabi [62], tomatoes and green peppers [63], and summer watermelon [64]. Garfi et al. [65] in a field trial experiment, documented that the digestate is an appropriate substitute of manure pre-compost for potato fertilization.
3.2.3. Effect of Digestate Addition on Root Elongation Rate
In an attempt to show the best concentration values for root elongation rate, Figure 6 shows a 5% addition of digestate—mixture A at 55 °C has the highest root elongation rate compared to other treatments. Generally speaking, the root elongation rates were increased until the concentration of 5%, then declined afterward with an exception in the case of mixture A and B at ambient temperatures. In comparison to mixture B at thermophilic AD, which gives 37% biogas production more than mixture A at thermophilic AD, the latest one is exceeded in RER.
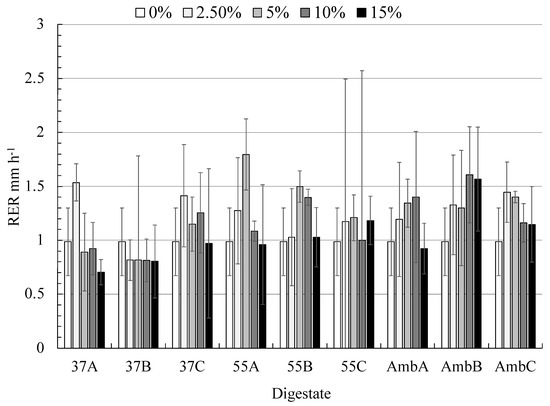
Figure 6.
The root elongation rate (mm h−1) of the faba bean plant was affected by different concentrations of digestates. Error bars represent the standard error of triplicates.
These results clearly show a potential toxicity effect of digestate in the case of mixtures A and B at 37 °C. Therefore, the calculation of EC50 generated from the log-logistic model has been applied in the next section to determine the toxicity effects of digestate to the faba bean plant.
3.2.4. Digestate Toxicity to Faba Bean, EC50
Some toxic organic and inorganic pollutants could influence the effective utilization of digestate to be used as plant fertilizers, such as polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated biphenyls (PCB), polyaromatic hydrocarbons (PAH), perfluorinated alkyl compounds (PFCs), linear alkylbenzene sulfonates (LASs), nonylphenols/nonylphenol ethoxylates (NP/NPEOs), and polybrominated diphenyl ethers (PBDEs) [66]. Recent research has focused on treatment methods for digestate, which could be a potential requirement for the risk evaluation of digestate, especially toxicity. Therefore, the first stage in determining the toxicity level of the obtained digestate from the current work is to undertake a simple toxicity experiment that would predict its environmental influence and therefore the prerequisite for processing treatments. Therefore, a hydroponic experiment was used regarding these concerns to ensure all materials were in direct contact with the soft root tissue of the faba bean plant, as described by Ostwald [67].
The results showed that the digestate additions reduced root growth substantially compared to that of the control. However, the effective toxic dose (so-called EC50) for different addition amounts of digestate was different. Figure 7 shows RER as a function of different concentrations of different digestates (log scaled). The results obtained from the log-logistic model showed an EC50 of 18.1% for mixture A at 37 °C. On the other hand, the same value obtained in the cases of mixtures B and C were 16.7 and 15.8%, respectively. There is no significant difference between the toxicity level of mixture B and C, but there is with A. In addition, the same values for mixture A, B, and C at 55 °C were 19.04, 16.04, and 15.8%, respectively.
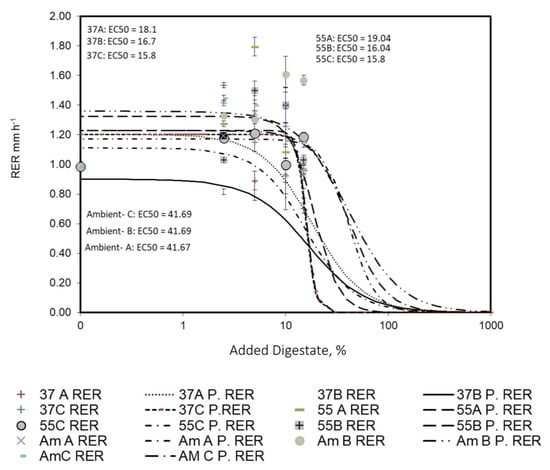
Figure 7.
Root elongation rate (RER, mm h−1) of faba bean roots as a function of added different concentrations of digestate. The solid fitting lines represent the fitting of a log-logistic model (predicted RER; P. RER); the error bar represents the SD of the three replicates, and EC50 was calculated according to the log-logistic model.
Finally, the same values for mixtures A, B, and C at ambient temperature were almost 41.7 for all treatments. These findings confirm that the highest toxicity levels were observed in mixture C at both 37 and 55 °C, while the lowest toxicity levels were observed at all mixture types at ambient temperature levels. It is well known that the lowest EC50 has the highest toxicity levels and vice versa. The possible cause of digestate toxicity to plants could be the N content, in the form of ammonia. Enriched landfill leachate with NH4-N was possibly the most important issue of toxicity for some plant species [68]. Although N concentration showed the highest levels in all samples, perhaps the most readily available forms of N could exist in mixture C digested at 37 and 55 °C. The inhibition of the root elongation rate has been previously confirmed as a result of using enriched animal manure digestate with ammonia [69]. Surprisingly, Gell et al. [70] did not find the reason for the lack of toxicity in a pig manure digestate for plants in their study. The lack of digestate toxicity to the plants was also documented by many researchers, but the conditions were hardly comparable with the present work. Some studies were performed under very-controlled conditions [71], different exposure times [72], or different kinds and doses of digested slurries [73]. The absence of an international standard method in biogas plants, and the major variety of organic raw material, requires a risk assessment based on each circumstance [74] and makes the comparison between studies more difficult. However, based on the results, the recommended mixture was mixture B at ambient temperature, which increased the maximum fresh weight values of the shoot system.
The potential of biogas digestates as fertilizers has gained great attention due to their beneficial plant nutrient profiles and content of organic materials and, hence, their potential to reduce the use of mineral fertilizers [75]. Kumar et al. confirmed in 2015 that the concentration of toxic heavy metals in digestate is very low compared to synthetic fertilizers. The use of digestate as a fertilizer reduces the use of synthetic fertilizers, thereby reducing costs, and digestate is also environmentally friendly [76].
The current work showed that using the digestate obtained from the different proposed mixtures at different temperatures could be safely used at a concentration lower than EC50 levels. In a recent study, the use of appropriate concentrations of digestate as a fertilizer is encouraged, since it causes plant growth and yield improvements under doses of 20% [77]. However, due to insufficient confidence in its quality and safety, as well as unfamiliarity with the produced digestate, the use of these residues is still limited. To close the knowledge gaps, biogas digestates should be evaluated for their short- and long-term effects before their large-scale application to arable land. Short-term soil effects could be very different from effects in the long run. The short-term effects reflect the situation at the time of fertilization, when a high amount of fertilizer is added in relation to the short-term needs of the soil and plant system. This imbalanced input is common practice in agriculture, but induces stress to the microbial ecosystem and may affect critical soil functions, such as those within the carbon and nitrogen cycle. Therefore, more work is needed to evaluate the actual effect of added digestate to the soil microorganism before large-scale application in the agricultural sector.
4. Conclusions
The anaerobic digestion of OMW was shown to be viable, with CH4 production enhanced by co-digestion with DM and by increasing the temperature of digestion. The results show thermophilic anaerobic digestion was more efficient than ambient and mesophilic anaerobic digestion. Co-digestion, especially mixture B (2:1:2, OMW:DM:IN) in thermophilic conditions, successfully reduced the digestion period while increasing the quantity of methane production. The study also determined the effect of digestate from each mixture on faba bean growth. The results showed that the fresh weight values of the shoot system were increased by using digestate, with the best fresh weight values being achieved from the use of mixture B at ambient temperature conditions compared with other treatments. Thus, the co-digestion of OMW and DM (mixture B: 2:1:2, OMW:DM:IN) can produce clean energy and reduce greenhouse gasses, while the digestate could be used for land application as a fertilizer and source for NPK.
Author Contributions
Conceptualization: M.A., A.H., M.R., M.E.-k. and E.R.M.; methodology: M.A., A.H. and E.R.M.; validation: M.A., A.H. and E.R.M.; formal analysis: M.A., A.H. and E.R.M.; investigation: M.A.; resources: A.H. and E.R.M.; data curation: M.A.; writing—original draft preparation: M.A., A.H. and E.R.M.; writing—review and editing: M.A., A.H., M.R., M.E.-k. and E.R.M.; visualization: M.A., M.R., M.E.-k. and E.R.M.; supervision: A.H. and E.R.M.; project administration: A.H. and E.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aangelidaki, I.; Ahrin, B.K.; Deng, H.; Schmidt, J.E. Anaerobic digestion of olive oil mill effluents together with swine manure in UASB reactors. Water Sci. Technol. 2002, 45, 213–218. [Google Scholar] [CrossRef]
- Maragkaki, A.E.; Vasileiadis, I.; Fountoulakis, M.; Kyriakou, A.; Lasaridi, K.; Manios, T. Improving biogas production from anaerobic co-digestion of sewage sludge with a thermal dried mixture of food waste, cheese whey and olive mill wastewater. Waste Manag. 2018, 71, 644–651. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Caroca, E.; Serrano, A.; Borja, R.; Jiménez, A.; Carvajal, A.; Braga, A.F.M.; Rodriguez-Gutierrez, G.; Fermoso, F.G. Influence of phenols and furans released during thermal pretreatment of olive mill solid waste on its anaerobic digestion. Waste Manag. 2021, 120, 202–208. [Google Scholar] [CrossRef]
- Trujillo-Reyes, Á.; Cubero-Cardoso, J.; Rodríguez-Gutiérrez, G.; García-Martín, J.F.; Rodríguez-Galán, M.; Borja, R.; Serrano, A.; Fermoso, F.G. Extraction of phenolic compounds and production of biomethane from strawberry and raspberry extrudates. Biochem. Eng. J. 2019, 147, 11–19. [Google Scholar] [CrossRef]
- Inan, H.; Dimoglo, A.; Şimşek, H.; Karpuzcu, M. Olive oil mill wastewater treatment by means of electro-coagulation. Sep. Purif. Technol. 2004, 36, 23–31. [Google Scholar] [CrossRef]
- Al bkoor Alrawashdeh, K. Improving anaerobic co-digestion of sewage sludge with thermal dried olive mill wastewater. Waste Biomass Valorization 2019, 10, 2213–2219. [Google Scholar] [CrossRef]
- Messineo, A.; Maniscalco, M.P.; Volpe, R. Biomethane recovery from olive mill residues through anaerobic digestion: A review of the state of the art technology. Sci. Total Environ. 2020, 703, 135508. [Google Scholar] [CrossRef]
- El Gnaoui, Y.; Sounni, F.; Bakraoui, M.; Karouach, F.; Benlemlih, M.; Barz, M.; El Bari, H. Anaerobic co-digestion assessment of olive mill wastewater and food waste: Effect of mixture ratio on methane production and process stability. J. Environ. Chem. Eng. 2020, 8, 103874. [Google Scholar] [CrossRef]
- Bouknana, D.; Hammouti, B.; Salghi, R.; Jodeh, S.; Zarrouk, A.; Warad, I.; Aouniti, A.; Sbaa, M. Physicochemical characterization of olive oil mill wastewaters in the eastern region of Morocco. J. Mater. Environ. Sci. 2014, 5, 1039–1058. [Google Scholar]
- Achi, C.G.; Hassanein, A.; Lansing, S. Enhanced biogas production of cassava wastewater using zeolite and biochar additives and manure co-digestion. Energies 2020, 13, 491. [Google Scholar] [CrossRef] [Green Version]
- Camarillo, R.; Rincón, J. Effect of inhibitory compounds on the two-phase anaerobic digestion performance of diluted wastewaters from the alimentary industry. Chem. Eng. J. 2012, 193, 68–76. [Google Scholar] [CrossRef]
- Cavinato, C.; Bolzonella, D.; Pavan, P.; Fatone, F.; Cecchi, F. Mesophilic and thermophilic anaerobic co-digestion of waste activated sludge and source sorted biowaste in pilot-and full-scale reactors. Renew. Energy 2013, 55, 260–265. [Google Scholar] [CrossRef]
- Cabbai, V.; Ballico, M.; Aneggi, E.; Goi, D. BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manag. 2013, 33, 1626–1632. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Mon, A.T.; Oxberger, B. Biogas Production From Farmyard Manure. Landtechnik 2000, 143–148. Available online: http://ramiran.uvlf.sk/doc00/Documents/Session%20V/PA24.pdf (accessed on 24 December 2021).
- Siddique, M.N.I.; Khalid, Z.B.; Ibrahim, M.Z.B. Effect of additional nutrients on Bio-methane production from anaerobic digestion of farming waste: Feasibility & Fertilizer recovery. J. Environ. Chem. Eng. 2019, 8, 103569. [Google Scholar] [CrossRef]
- Hassanein, A.; Naresh Kumar, A.; Lansing, S. Impact of electro-conductive nanoparticles additives on anaerobic digestion performance—A review. Bioresour. Technol. 2021, 342, 126023. [Google Scholar] [CrossRef]
- Ayala-Parra, P.; Liu, Y.; Field, J.A.; Sierra-Alvarez, R. Nutrient recovery and biogas generation from the anaerobic digestion of waste biomass from algal biofuel production. Renew. Energy 2017, 108, 410–416. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; Xu, H.; Wang, C.; Zhang, Y.; Chen, J.; Jia, B. Mesophilic anaerobic co-digestion of acorn slag waste with dairy manure in a batch digester: Focusing on mixing ratios and bio-based carbon accelerants. Bioresour. Technol. 2019, 286, 121394. [Google Scholar] [CrossRef]
- Zhang, C.; Yun, S.; Li, X.; Wang, Z.; Xu, H.; Du, T. Low-cost composited accelerants for anaerobic digestion of dairy manure: Focusing on methane yield, digestate utilization and energy evaluation. Bioresour. Technol. 2018, 263, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Bai, X.; Li, H.; Lu, Z.; Zhou, Y.; Wang, Y.; Cao, J.; Huang, Z. Nutrients removal and biomass production from anaerobic digested effluent by microalgae: A review. Int. J. Agric. Biol. Eng. 2019, 12, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture–the effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef]
- Lošák, T.; Válka, T.; Elbl, J.; Kintl, A.; Keutgen, A.; Keutgen, N.; Demková, L.; Árvay, J.; Varga, L.; Hnátková, H.; et al. Fertilization with magnesium-and sulfur-supplemented digestate increases the yield and quality of kohlrabi. Sustainability 2020, 12, 5733. [Google Scholar] [CrossRef]
- Pivato, A.; Vanin, S.; Raga, R.; Lavagnolo, M.C.; Barausse, A.; Rieple, A.; Laurent, A.; Cossu, R. Use of digestate from a decentralized on-farm biogas plant as fertilizer in soils: An ecotoxicological study for future indicators in risk and life cycle assessment. Waste Manag. 2016, 49, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Yun, S.; Zhang, C.; Xu, H.; Wang, Z. Steel slag as accelerant in anaerobic digestion for nonhazardous treatment and digestate fertilizer utilization. Bioresour. Technol. 2019, 282, 331–338. [Google Scholar] [CrossRef]
- Yun, S.; Fang, W.; Du, T.; Hu, X.; Huang, X.; Li, X.; Zhang, C.; Lund, P.D. Use of bio-based carbon materials for improving biogas yield and digestate stability. Energy 2018, 164, 898–909. [Google Scholar] [CrossRef]
- Mezzanotte, V.; Bertani, R.; Innocenti, F.D.; Tosin, M. Influence of inocula on the results of biodegradation tests. Polym. Degrad. Stab. 2005, 87, 51–56. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019, 275, 200–206. [Google Scholar] [CrossRef]
- Vikrant, D.; Shekhar, P. Generation of Biogas from Kitchen Waste -Experimental Analysis. Int. J. Eng. Sci. Invent. ISSN 2013, 2, 15–19. [Google Scholar]
- Carotenuto, C.; Guarino, G.; Morrone, B.; Minale, M. Temperature and ph effect on methane production from buffalo manure anaerobic digestion. Int. J. Heat Technol. 2016, 34, S425–S429. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American, P., Ed.; APHA: Washington, DC, USA, 1999; ISBN 0875532357. [Google Scholar]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Moody, L.; Burns, R.; Wu-Haan, W.; Spajic, R. Use of biochemical methane potential (BMP) assays for predicting and enhancing anaerobic digester performance. In Proceedings of the 44th Croatian and the 4th International Symposium on Agriculture, Opatija, Croatia, 16–20 February 2009. [Google Scholar]
- Aylin Alagöz, B.; Yenigün, O.; Erdinçler, A. Enhancement of anaerobic digestion efficiency of wastewater sludge and olive waste: Synergistic effect of co-digestion and ultrasonic/microwave sludge pre-treatment. Waste Manag. 2015, 46, 182–188. [Google Scholar] [CrossRef]
- Salam, B.; Biswas, S.; Rabbi, M.S. Biogas from mesophilic anaerobic digestion of cow dung using silica gel as catalyst. Procedia Eng. 2015, 105, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Pham, C.H.; Triolo, J.M.; Cu, T.T.T.; Pedersen, L.; Sommer, S.G. Validation and recommendation of methods to measure biogas production potential of animal manure. Asian-Australas. J. Anim. Sci. 2013, 26, 864–873. [Google Scholar] [CrossRef] [Green Version]
- Kopittke, P.M.; Kinraide, T.B.; Wang, P.; Blamey, F.P.C.; Reichman, S.M.; Menzies, N.W. Alleviation of Cu and Pb rhizotoxicities in cowpea (Vigna unguiculata) as related to ion activities at root-cell plasma membrane surface. Environ. Sci. Technol. 2011, 45, 4966–4973. [Google Scholar] [CrossRef]
- Ferreira, T.; Rasband, W. ImageJ User Guide-IJ 1.46r. 2012; pp. 1–198. Available online: https://imagej.nih.gov/ij/docs/guide/user-guide.pdf (accessed on 24 December 2021).
- Marzouk, E.R.; Chenery, S.R.; Young, S.D. Predicting the solubility and lability of Zn, Cd, and Pb in soils from a minespoil-contaminated catchment by stable isotopic exchange. Geochim. Cosmochim. Acta 2013, 123, 1–16. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Vliet, L.; Ritz, C. Statistics for Analyzing Ecotoxicity Test Data. In Encyclopedia of Aquatic Ecotoxicology; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.L.; Esposito, G.; Pirozzi, F. Dark fermentation of complex waste biomass for biohydrogen production by pretreated thermophilic anaerobic digestate. J. Environ. Manage. 2015, 152, 43–48. [Google Scholar] [CrossRef]
- Raja, I.A.; Wazir, S. Biogas Production: The Fundamental Processes. Univers. J. Eng. Sci. 2017, 5, 29–37. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Pandey, P.K.; Soupir, M.L. Impacts of Temperatures on Biogas Production in Dairy Manure Anaerobic Digestion. Int. J. Eng. Technol. 2012, 4, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.D. Empirical Study on Factors Affecting Biogas Production. ISRN Renew. Energy 2012, 2012, 136959. [Google Scholar] [CrossRef] [Green Version]
- Rubio, J.A.; Romero, L.I.; Wilkie, A.C.; García-Morales, J.L. Mesophilic Anaerobic Co-digestion of Olive-Mill Waste With Cattle Manure: Effects of Mixture Ratio. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- İnce, E.; İnce, M.; Önkal Engin, G. Comparison of thermophilic and mesophilic anaerobic treatments for potato processing wastewater using a contact reactor. Glob. Nest J. 2017, 19, 318–326. [Google Scholar] [CrossRef]
- Lv, Z.; Feng, L.; Shao, L.; Kou, W.; Liu, P.; Gao, P.; Dong, X.; Yu, M.; Wang, J.; Zhang, D. The effect of digested manure on biogas productivity and microstructure evolution of corn stalks in anaerobic cofermentation. Biomed Res. Int. 2018, 2018, 5214369. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.H.; Al-Fawwaz, A.T.; Al-Shira’h, H.H. Evaluation and optimization of methane production from different manure types. Jordan J. Biol. Sci. 2018, 11. Available online: https://jjbs.hu.edu.jo/files/v11n3/Paper%20Number%2013.pdf (accessed on 24 December 2021).
- Zhu, G.; Jha, A.K. Psychrophilic dry anaerobic digestion of cow dung for methane production: Effect of inoculum. Sci. Asia 2013, 39, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Ling, Q.; Hassanein, A.A.M.; Ayhan, A. New Factor (Quality of Temperature) Affecting Directly the Biogas Production and Solved by Solar Heating Models. Nat. Environ. Pollut. Tech. 2016, 15, 761–766. [Google Scholar]
- Turco, M.L.M.A.A. Treatment of Biogas for Feeding High Temperature Fuel Cells; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Isaksson, S. Biogas Production at High Ammonia Levels: The Importance of Temperature and Trace Element Supplementation on Microbial Communities 2018. Available online: https://www.diva-portal.org/smash/get/diva2:1203787/FULLTEXT01.pdf (accessed on 24 December 2021).
- Donoso-Bravo, A.; Retamal, C.; Carballa, M.; Ruiz-Filippi, G.; Chamy, R. Influence of temperature on the hydrolysis, acidogenesis and methanogenesis in mesophilic anaerobic digestion: Parameter identification and modeling application. Water Sci. Technol. 2009, 60, 9–17. [Google Scholar] [CrossRef]
- Ahamd, M.; Zeshan, M.S.H.; Nasim, M.; Zahir, Z.A.; Nadeem, S.M.; Nazli, F.; Jamil, M. Improving the productivity of cucumber through combined application of organic fertilizers and pseudomonas fluorescens. Pak. J. Agric. Sci. 2015, 52. Available online: https://pakjas.com.pk/papers/2511.pdf (accessed on 24 December 2021).
- Feng, H.; Qu, G.F.; Ning, P.; Xiong, X.F.; Jia, L.J.; Shi, Y.K.; Zhang, J. The resource utilization of anaerobic fermentation residue. Procedia Environ. Sci. 2011, 11, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Arthurson, V. Closing the global energy and nutrient cycles through application of biogas residue to agricultural land - potential benefits and drawbacks. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Makdi, M.; Tomcsik, A.; Orosz, V. Digestate: A New Nutrient Source - Review. In Biogas; Kumar, S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 295–310. ISBN 978-953-51-0204-5. [Google Scholar]
- Möller, K.; Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosystems 2008, 82, 209–232. [Google Scholar] [CrossRef]
- Lošák, T.; Zatloukalová, A.; Szostková, M.; Hlušek, J.; Fryč, J.; Vítěz, T. Comparison of the effectiveness of digestate and mineral fertilisers on yields and quality of kohlrabi (Brassica oleracea, L.). Acta Univ. Agric. Silvic. Mendel. Brun. 2011, 59, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Kouřimská, L.; Poustková, I.; Babička, L. The use of digestate as a replacement of mineral fertilizers for vegetables growing. Sci. Agric. Bohem. 2012, 43, 121–126. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Garfí, M.; Gelman, P.; Comas, J.; Carrasco, W.; Ferrer, I. Agricultural reuse of the digestate from low-cost tubular digesters in rural Andean communities. Waste Manag. 2011, 31, 2584–2589. [Google Scholar] [CrossRef]
- Suominen, K.; Verta, M.; Marttinen, S. Hazardous organic compounds in biogas plant end products-Soil burden and risk to food safety. Sci. Total Environ. 2014, 491–492, 192–199. [Google Scholar] [CrossRef]
- Ostwald, W. On the assumed isomerism of red and yellow mercury oxide and the surface-tension of solid bodies. Z. Phys. Chem. Stochiom. Verwandtschaftslehre 1900, 34, 495–503. [Google Scholar] [CrossRef]
- Tigini, V.; Prigione, V.; Varese, G.C. Mycological and ecotoxicological characterisation of landfill leachate before and after traditional treatments. Sci. Total Environ. 2014, 487, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.H.; Cheung, Y.H.; Cheung, C.L. The effects of ammonia and ethylene oxide in animal manure and sewage sludge on the seed germination and root elongation of Brassica parachinensis. Environ. Pollut. Ser. A Ecol. Biol. 1983, 30, 109–123. [Google Scholar] [CrossRef]
- Gell, K.; van Groenigen, J.W.; Cayuela, M.L. Residues of bioenergy production chains as soil amendments: Immediate and temporal phytotoxicity. J. Hazard. Mater. 2011, 186, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Różyło, K.; Oleszczuk, P.; Jośko, I.; Kraska, P.; Kwiecińska-Poppe, E.; Andruszczak, S. An ecotoxicological evaluation of soil fertilized with biogas residues or mining waste. Environ. Sci. Pollut. Res. 2015, 22, 7833–7842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Santos, T.; Palma, P.; Sengo, J.; Morais, M.C.; Cunha-Queda, C. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: Benefits versus limiting factors. Waste Manag. 2015, 40, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Cucina, M.; Pezzolla, D.; Tacconi, C.; Gigliotti, G. Anaerobic co-digestion of a lignocellulosic residue with different organic wastes: Relationship between biomethane yield, soluble organic matter and process stability. Biomass Bioenergy 2021, 153, 106209. [Google Scholar] [CrossRef]
- Nag, R.; Auer, A.; Markey, B.K.; Whyte, P.; Nolan, S.; O’Flaherty, V.; Russell, L.; Bolton, D.; Fenton, O.; Richards, K.; et al. Anaerobic digestion of agricultural manure and biomass – Critical indicators of risk and knowledge gaps. Sci. Total Environ. 2019, 690, 460–479. [Google Scholar] [CrossRef]
- Herrmann, A.; Sieling, K.; Wienforth, B.; Taube, F.; Kage, H. Short-term effects of biogas residue application on yield performance and N balance parameters of maize in different cropping systems. J. Agric. Sci. 2013, 151, 449–462. [Google Scholar] [CrossRef]
- Kumar, S.; Malav, L.C.; Malav, M.K.; Khan, S.A. Biogas Slurry: Source of Nutrients for Eco-friendly Agriculture. Int. J. Ext. Res. 2015, 2, 42–46. [Google Scholar]
- Baştabak, B.; Koçar, G. A review of the biogas digestate in agricultural framework. J. Mater. Cycles Waste Manag. 2020, 22, 1318–1327. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).