Effect of Banana-Waste Biochar and Compost Mixtures on Growth Responses and Physiological Traits of Seashore Paspalum Subjected to Six Different Water Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. X-ray Diffraction and FTIR
2.2. Experimental Design
2.3. Gas-Exchange, Chlorophyll-Content, and Chlorophyll-Fluorescence Measurements
2.4. Relative Water Content (RWC)
2.5. Leaf Stomatal Traits
2.6. Statistics
3. Results
3.1. Characterization of Banana-Waste Biochar
X-ray Diffraction and FTIR
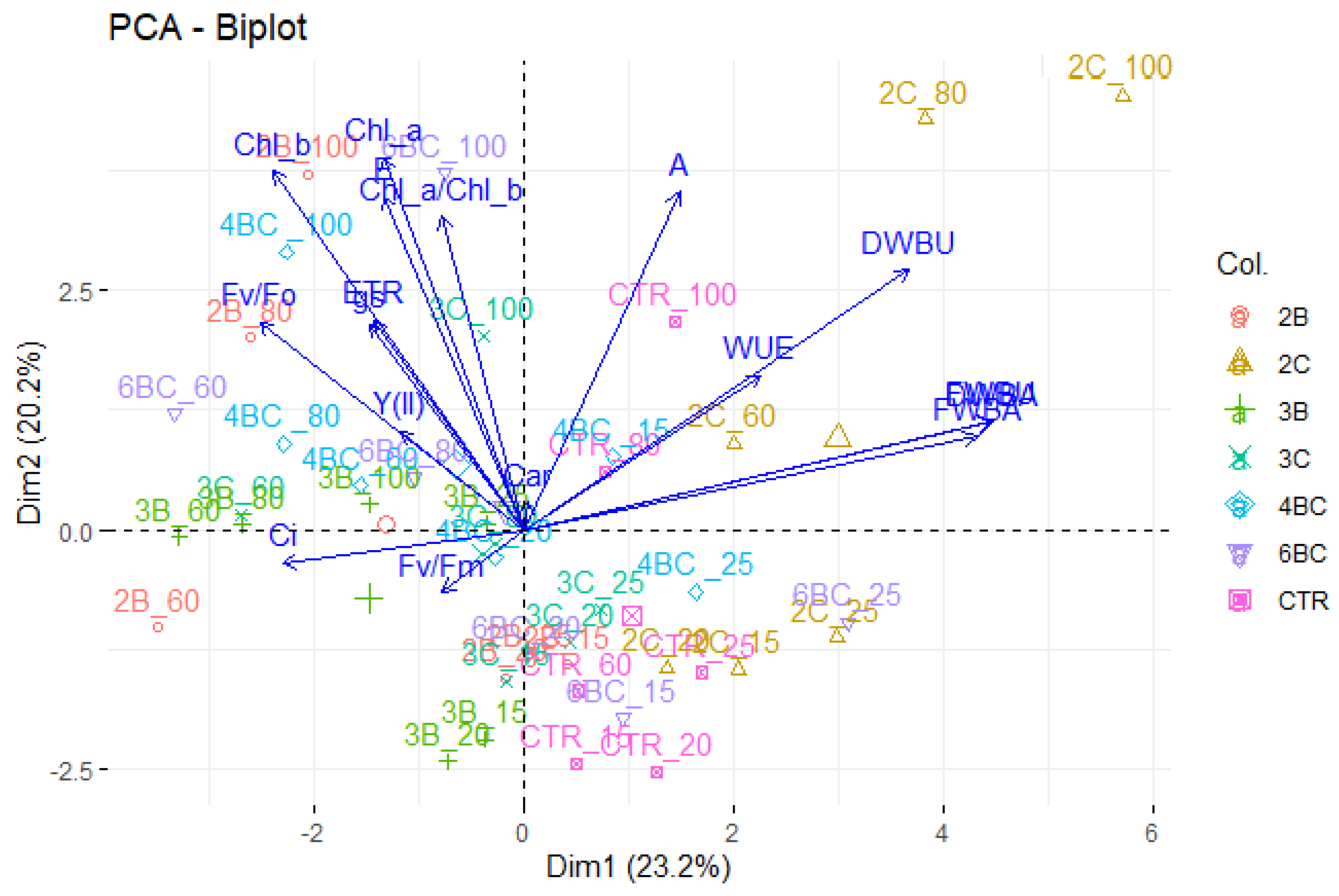
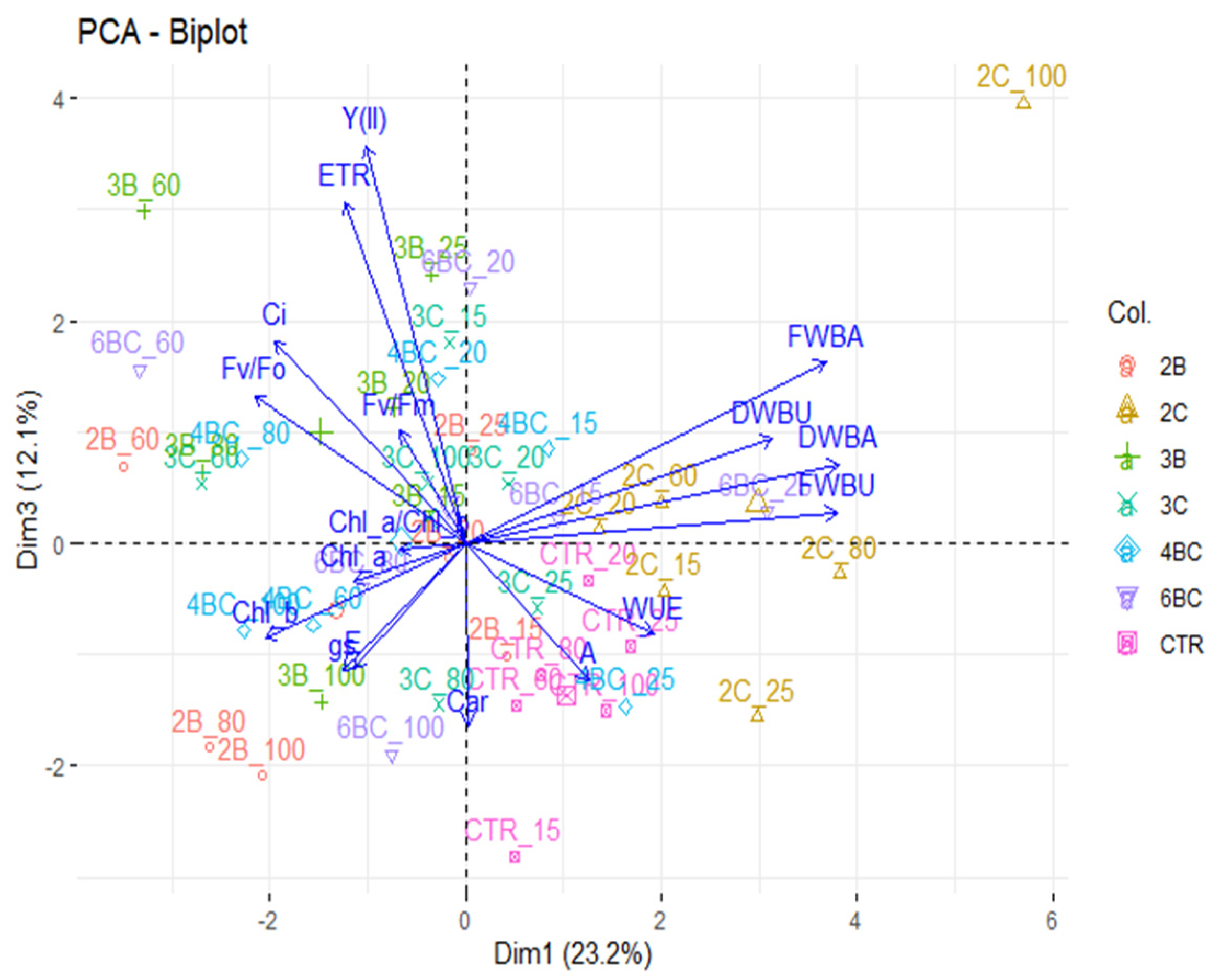
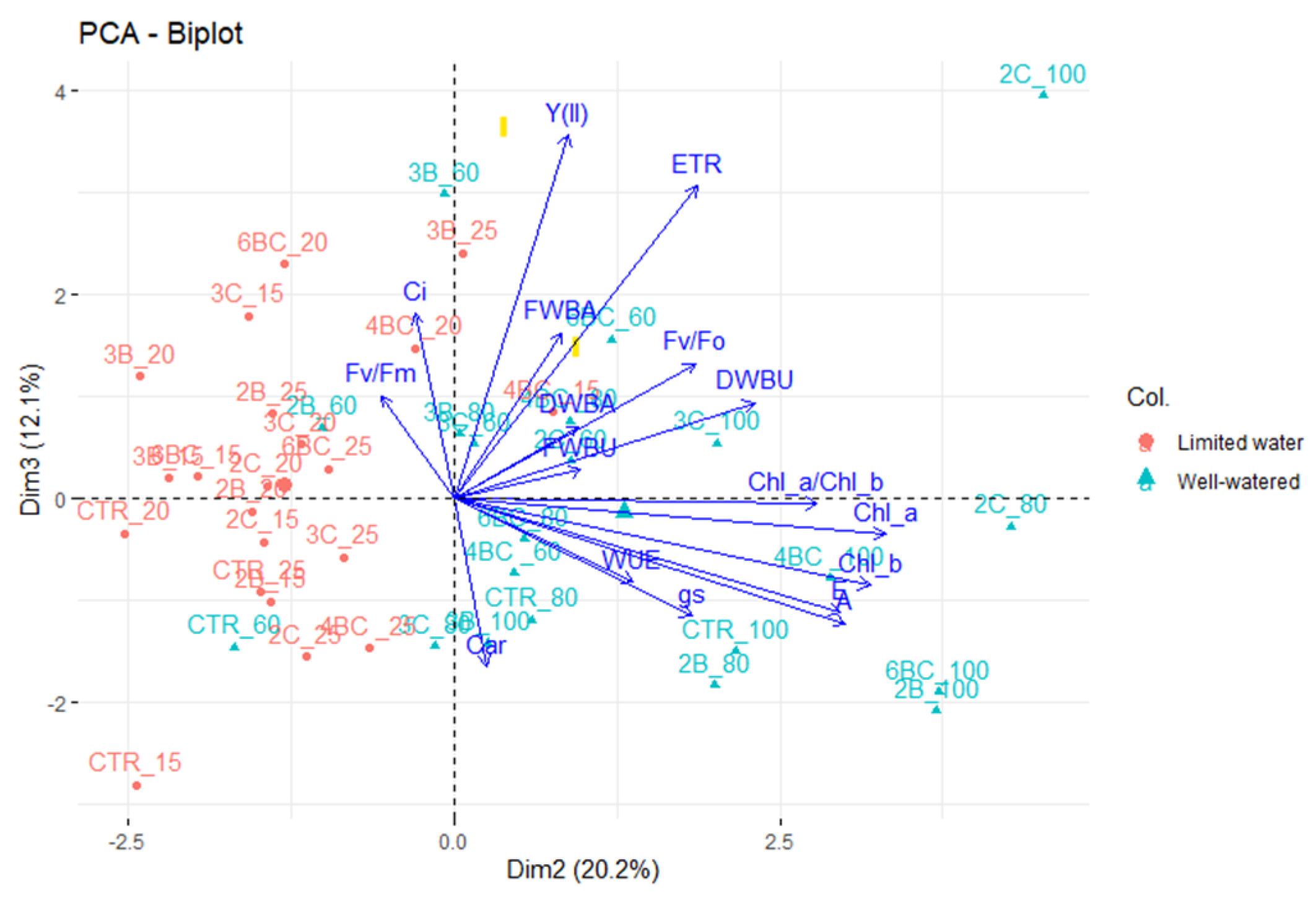
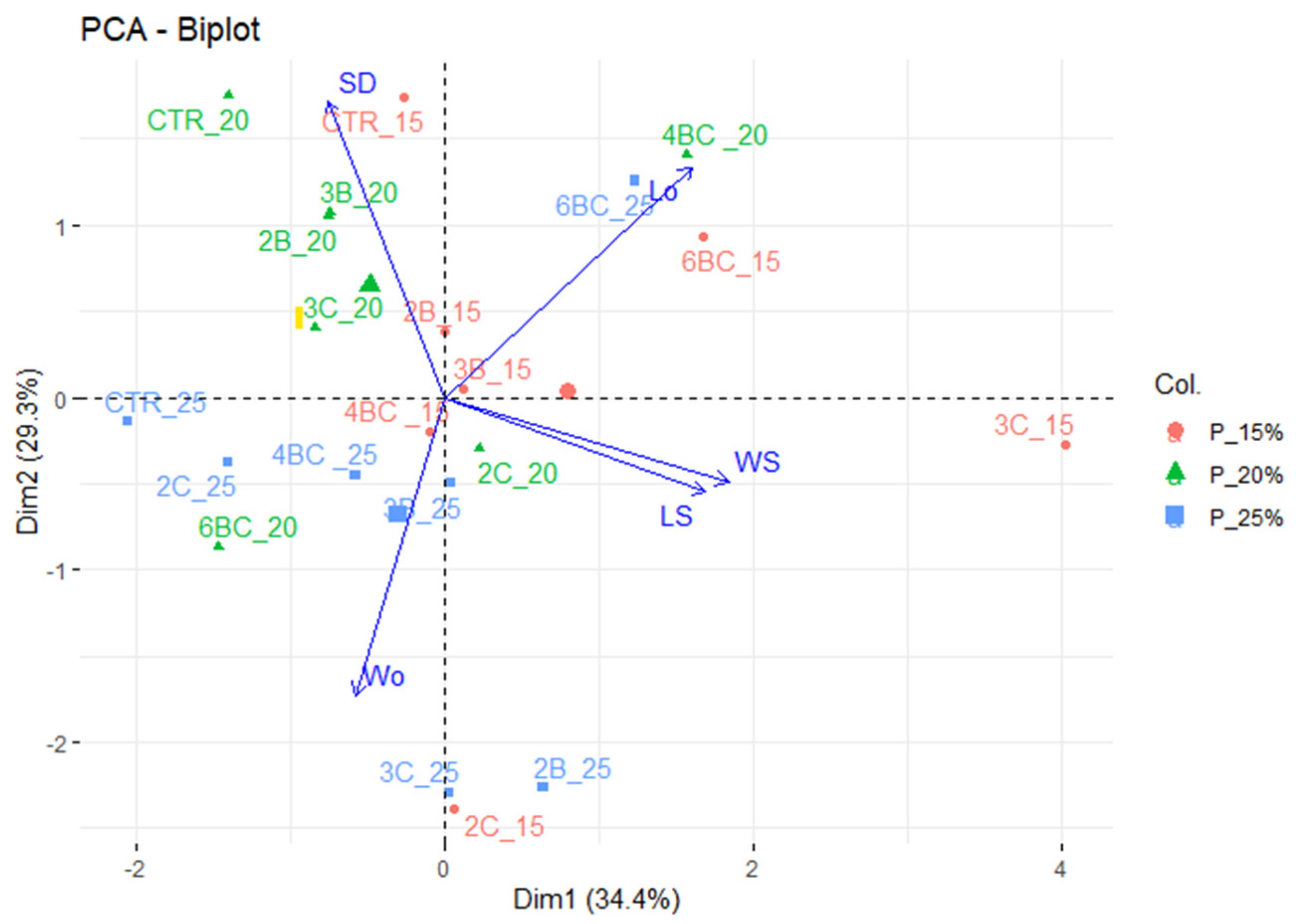
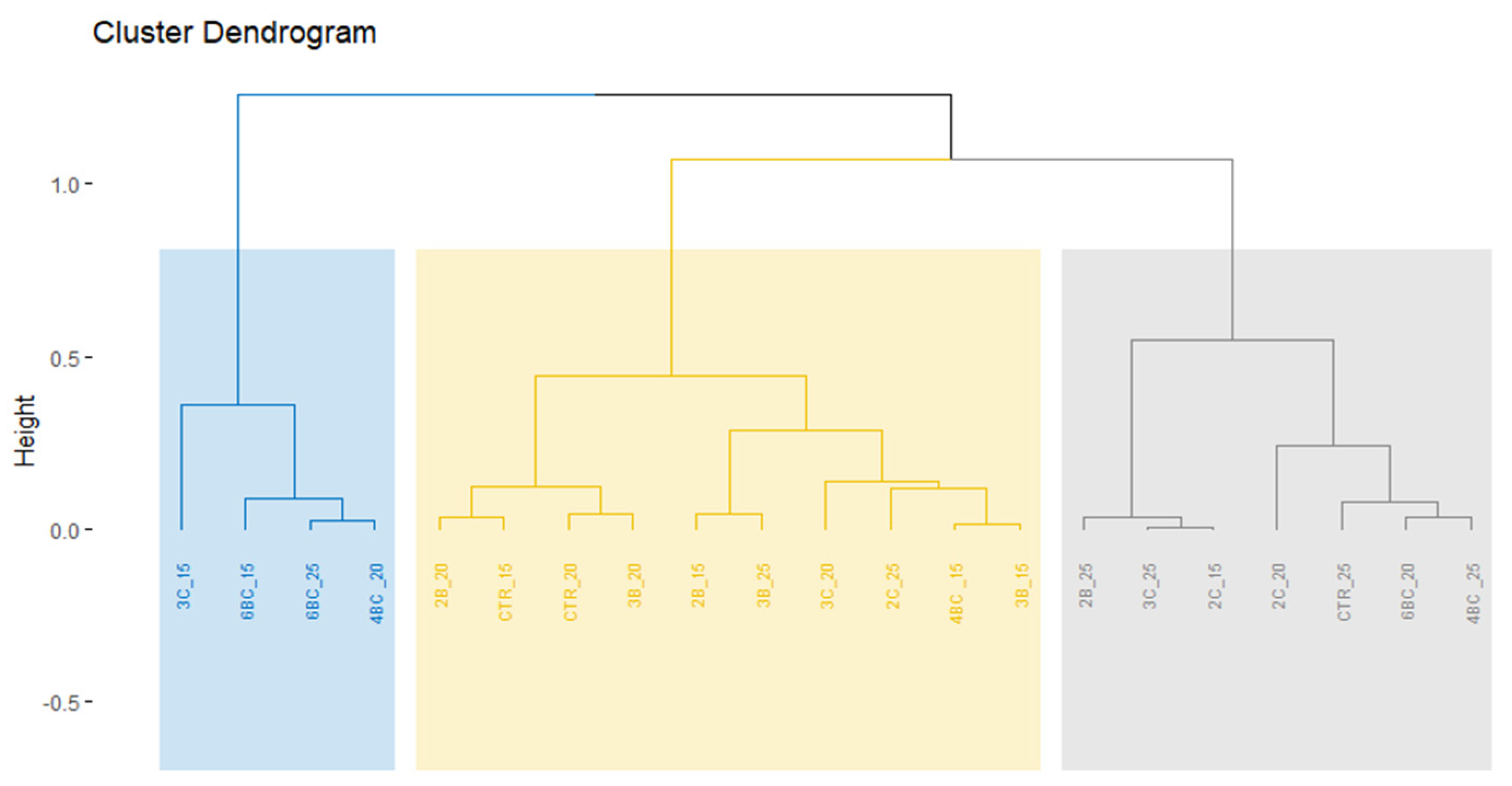
3.2. Physiological Traits of P. vaginatum
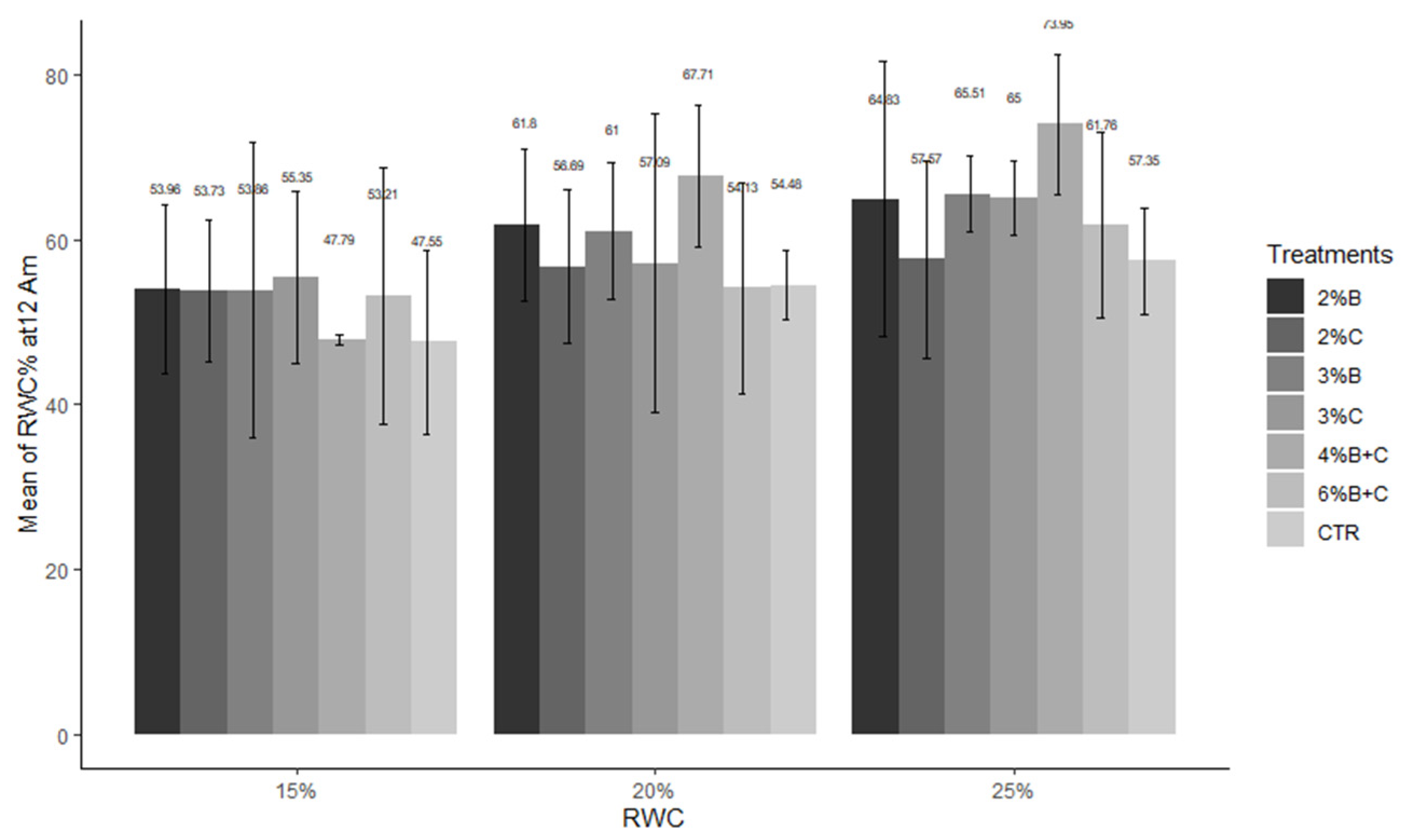
3.3. Effect of Agricultural Biochar on Relative Water Content (RWC)
3.4. Effect of Agricultural Waste on Leaf Stomatal Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Carrow, R.N. Drought Resistance Aspects of Turfgrasses in the Southeast: Evapotranspiration and Crop Coefficients. Crop Sci. 1995, 35, 1685–1690. [Google Scholar] [CrossRef]
- Dean, D.E.; Devitt, D.A.; Verchick, L.S.; Morris, R.L. Turfgrass Quality, Growth, and Water Use Influenced by Salinity and Water Stress. Agron. J. 1996, 88, 844–849. [Google Scholar] [CrossRef]
- Morley, J. Compost and Charcoal. Natl. Greenskeeper 1929, 3, 8–26. [Google Scholar]
- Vaughn, S.F.; Dinelli, F.D.; Jackson, M.A.; Vaughan, M.M.; Peterson, S.C. Biochar-organic amendment mixtures added to simulated golf greens under reduced chemical fertilization increase creeping bentgrass growth. Ind. Crops Prod. 2018, 111, 667–672. [Google Scholar] [CrossRef]
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and Mineral-Nutrition Properties of Sand-Based Turfgrass Root Zones Amended with Biochar. Agron. J. 2010, 102, 1627–1631. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J.; Zitomer, D.H. Biochar from pyrolysis of biosolids for nutrient adsorption and turfgrass cultivation. Water Environ. Res. 2015, 87, 2098–2106. [Google Scholar] [CrossRef] [Green Version]
- Pompeiano, A.; Giannini, V.; Gaetani, M.; Vita, F.; Guglielminetti, L.; Bonari, E.; Volterrani, M. Response of warm–season grasses to N fertilization and salinity. Sci. Hortic. 2014, 177, 92–98. [Google Scholar] [CrossRef]
- Pompeiano, A.; Di Patrizio, E.; Volterrani, M.; Scartazza, A.; Guglielminetti, L. Growth responses and physiological traits of seashore paspalum subjected to short-term salinity stress and recovery. Agric. Water Manag. 2016, 163, 57–65. [Google Scholar] [CrossRef]
- Fetjah, D.; Ainlhout, L.F.E.; Ihssane, B.; Houari, A.; Idardare, Z.; Bouqbis, L. Biological, Physico-Chemical and Morphological Analyses of Four Biochars Derived from Agricultural Waste. J. Ecol. Eng. 2021, 22, 36–46. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Fetjah, D.; Ainlhout, L.F.E.; Ihssane, B.; Bouqbis, L. Effect of Banana Waste Biochar on Physiological Responses and Growth of Seashore Paspalum. J. Ecol. Eng. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Nikpour-Rashidabad, N.; Tavasolee, A.; Torabian, S.; Farhangi-Abriz, S. The effect of biochar on the physiological, morphological and anatomical characteristics of mung bean roots after exposure to salt stress. Arch. Biol. Sci. 2019, 71, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Sairam, R.K.; Rao, K.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Fetjah, D.; Ihssane, B.; Idardare, Z.; Ainlhout, L.F.E.; Bouqbis, L. Limiting the Hurtful Oxidative Stress and Seasonal Physiological Adaptations in Seashore Paspalum through the Use of Banana Waste Biochar and Compost Mixtures. J. Ecol. Eng. 2022, 23, 216–227. [Google Scholar] [CrossRef]
- Monir, M.U.; Aziz, A.A.; Kristanti, R.A.; Yousuf, A. Co-gasification of empty fruit bunch in a downdraft reactor: A pilot scale approach. Bioresour. Technol. Rep. 2018, 1, 39–49. [Google Scholar] [CrossRef]
- Monir, M.U.; Aziz, A.A.; Kristanti, R.A.; Yousuf, A. Gasification of lignocellulosic biomass to produce syngas in a 50 kW downdraft reactor. Biomass Bioenerg. 2018, 119, 335–345. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Huang, J.; Williams, P.T. Pyrolysis/gasification of cellulose, hemicellulose and lignin for hydrogen production in the presence of various nickel-based catalysts. Fuel 2013, 106, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, B.P.; Cowie, A. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Clemente, J.S.; Beauchemin, S.; Thibault, Y.; Mackinnon, T.; Smith, D. Differentiating Inorganics in Biochars Produced at Commercial Scale Using Principal Component Analysis. ACS Omega 2018, 3, 6931–6944. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Mohapatra, S.; Singh, S.K. Nutrient-rich biomass and effluent sludge waste co-utilization for production of biochar fertilizer through different thermal treatments. J. Clean. Prod. 2019, 228, 570–579. [Google Scholar] [CrossRef]
- Cornelissen, G.; Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.D.; Rutherford, D.W.; Sparrevik, M.; Hale, S.E.; Obia, A.; Mulder, J. Biochar Effect on Maize Yield and Soil Characteristics in Five Conservation Farming Sites in Zambia. Agronomy 2013, 3, 256–274. [Google Scholar] [CrossRef] [Green Version]
- De Melo Carvalho, M.T.; de Holanda Nunes Maia, A.; Madari, B.E.; Bastiaans, L.; van Oort, P.A.J.; Heinemann, A.B.; Soler da Silva, M.A.; Petter, F.A.; Marimon, B.H., Jr.; Meinke, H. Biochar increases plant-available water in a sandy loam soil under an aerobic rice crop system. Solid Earth 2014, 5, 939–952. [Google Scholar] [CrossRef] [Green Version]
- Katuwal, K.B.; Tishchenko, V.; Jespersen, D. Assessing drought resistance in seashore paspalum genotypes using leaf gas exchange, osmotic adjustment, and rooting characteristics. Crop Sci. 2021, 61, 2121–2134. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Adrees, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Qayyum, M.F.; Nawaz, R. Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Imran, M.; Naveed, M.; Khan, M.Y.; Ahmad, M.; Zahir, Z.A.; Crowley, D.E. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J. Sci. Food Agric. 2017, 97, 5139–5145. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.M.; Omara, A.E.D.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol. Plant. Ppl. 2020, 13261. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Effect of biochar on growth and ion contents of bean plant under saline condition. Environ. Sci. Pollut. Res. Int. 2018, 25, 11556–11564. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H.-W. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil. 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Fu, Q.S.; Yang, R.C.; Wang, H.S.; Zhao, B.; Zhou, C.L.; Ren, S.X.; Guo, Y.D. Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica 2013, 51, 109–114. [Google Scholar] [CrossRef]
- Xu, K.; Zou, G.; Zhao, Y. Effects of soil water stress and shading on growth characteristics of ginger. Ying Yong Sheng Tai Xue Bao. 2003, 14, 1645–1648. [Google Scholar] [PubMed]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetjah, D.; Ainlhout, L.F.Z.; Idardare, Z.; Ihssane, B.; Bouqbis, L. Effect of Banana-Waste Biochar and Compost Mixtures on Growth Responses and Physiological Traits of Seashore Paspalum Subjected to Six Different Water Conditions. Sustainability 2022, 14, 1541. https://doi.org/10.3390/su14031541
Fetjah D, Ainlhout LFZ, Idardare Z, Ihssane B, Bouqbis L. Effect of Banana-Waste Biochar and Compost Mixtures on Growth Responses and Physiological Traits of Seashore Paspalum Subjected to Six Different Water Conditions. Sustainability. 2022; 14(3):1541. https://doi.org/10.3390/su14031541
Chicago/Turabian StyleFetjah, Dounia, Lalla Fatima Zohra Ainlhout, Zaina Idardare, Bouchaib Ihssane, and Laila Bouqbis. 2022. "Effect of Banana-Waste Biochar and Compost Mixtures on Growth Responses and Physiological Traits of Seashore Paspalum Subjected to Six Different Water Conditions" Sustainability 14, no. 3: 1541. https://doi.org/10.3390/su14031541





