Development of a Bio-Digital Interface Powered by Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
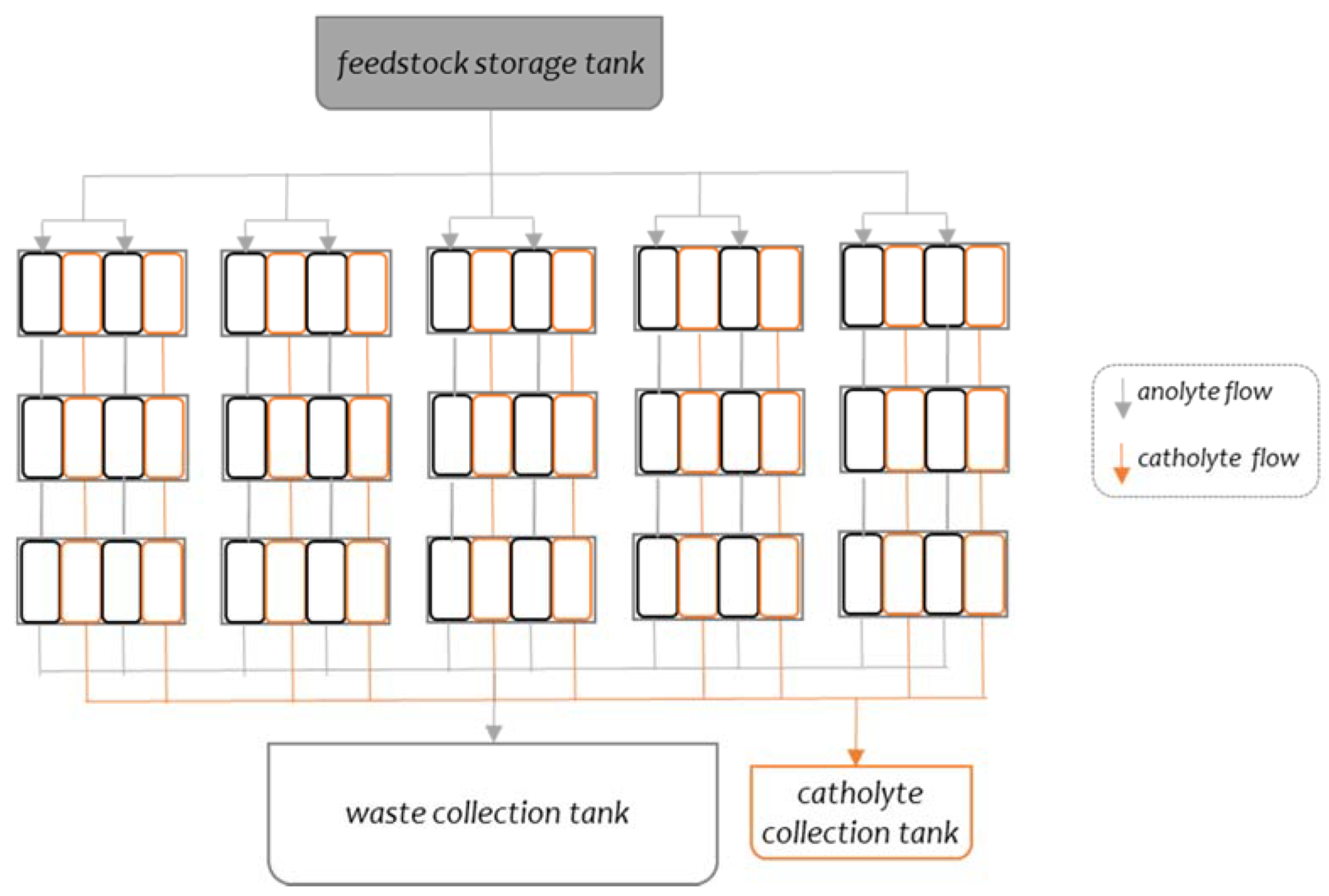
2.1. System Design, Operation and Performance Measurement
2.2. Electrical Infrastructure
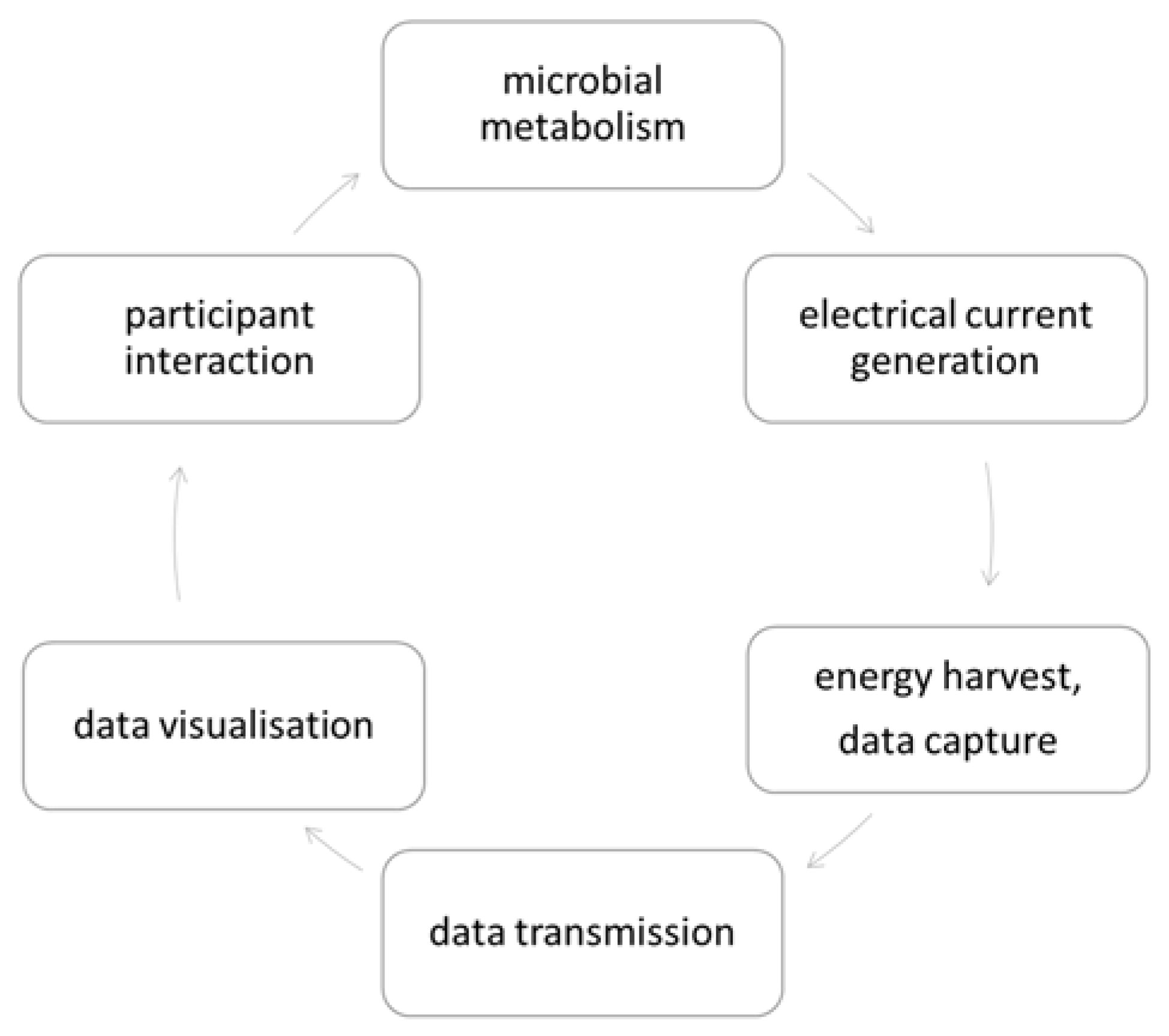
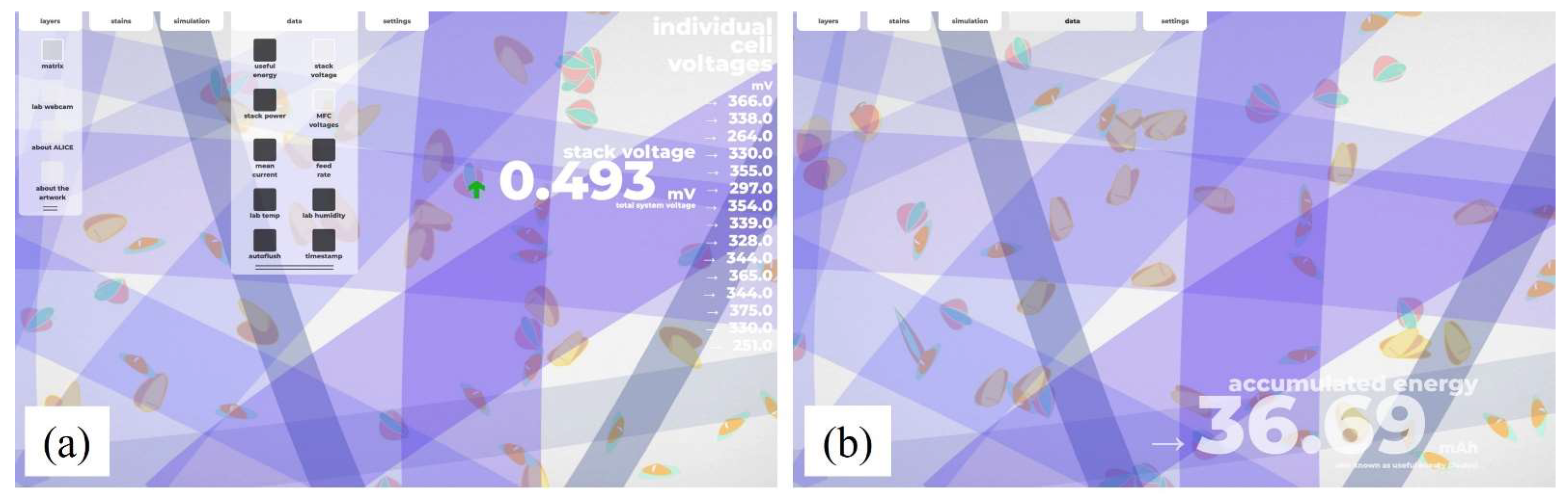
2.3. Bio-Digital Interface
3. Results and Discussion
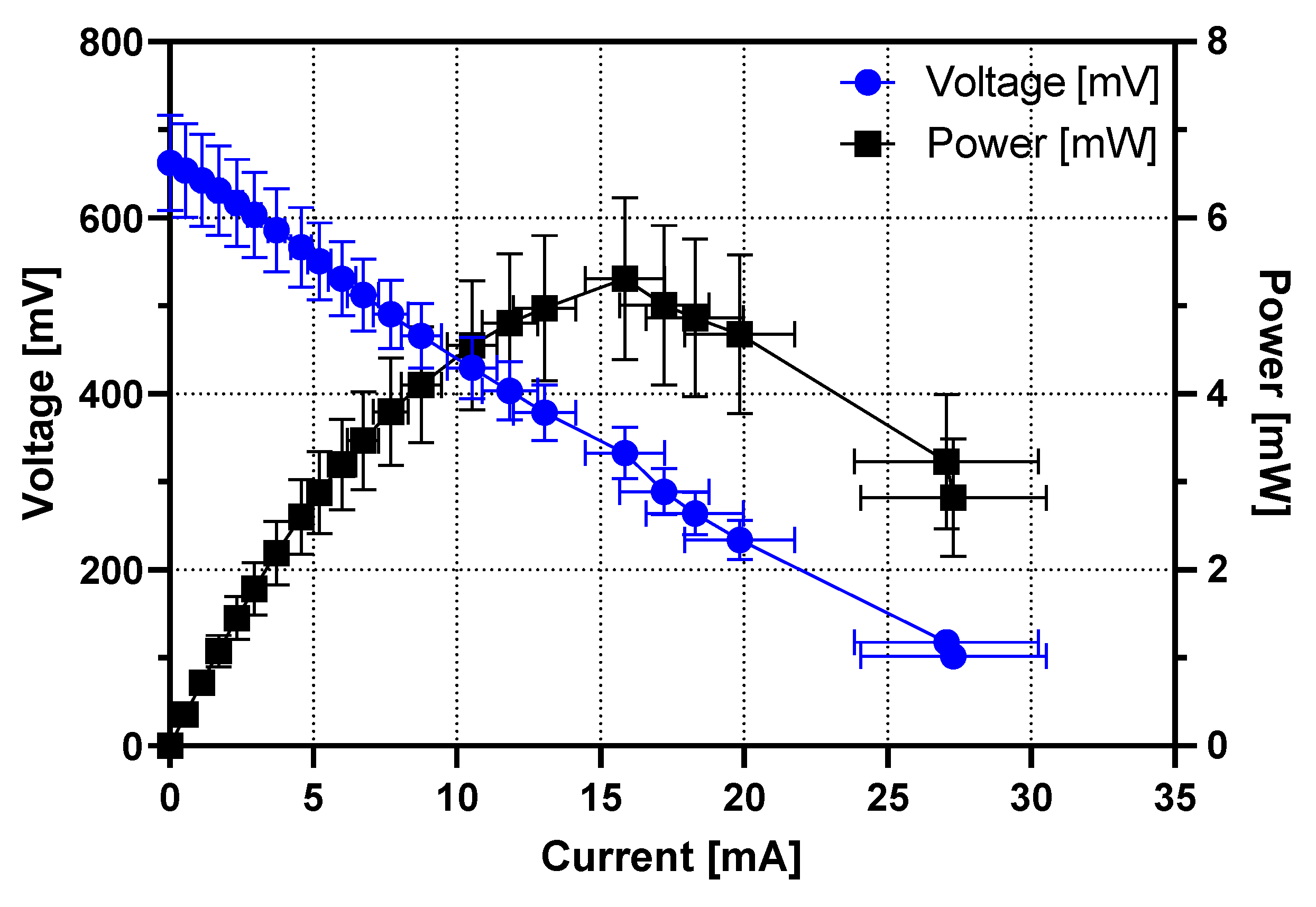
3.1. Individual MFC Performance
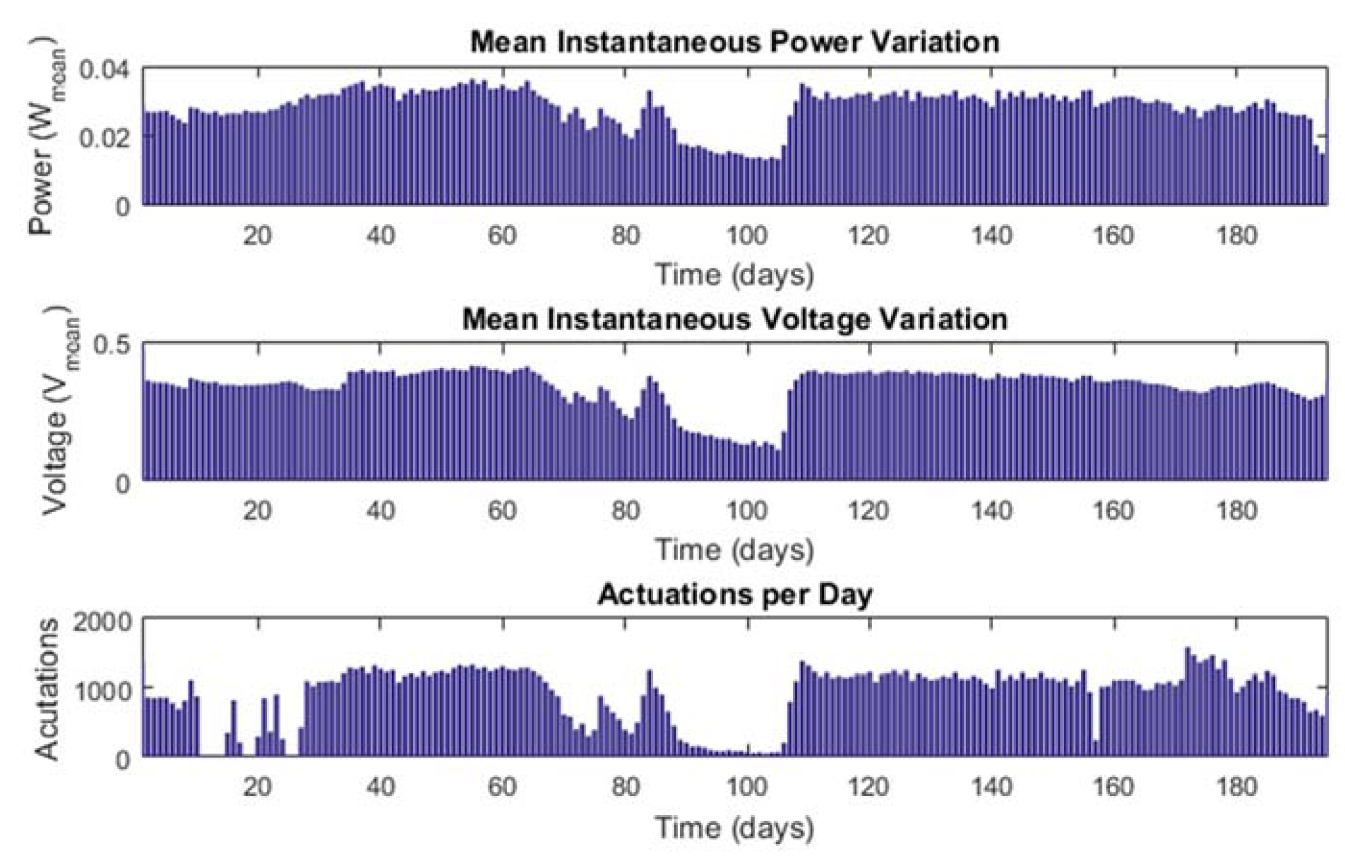
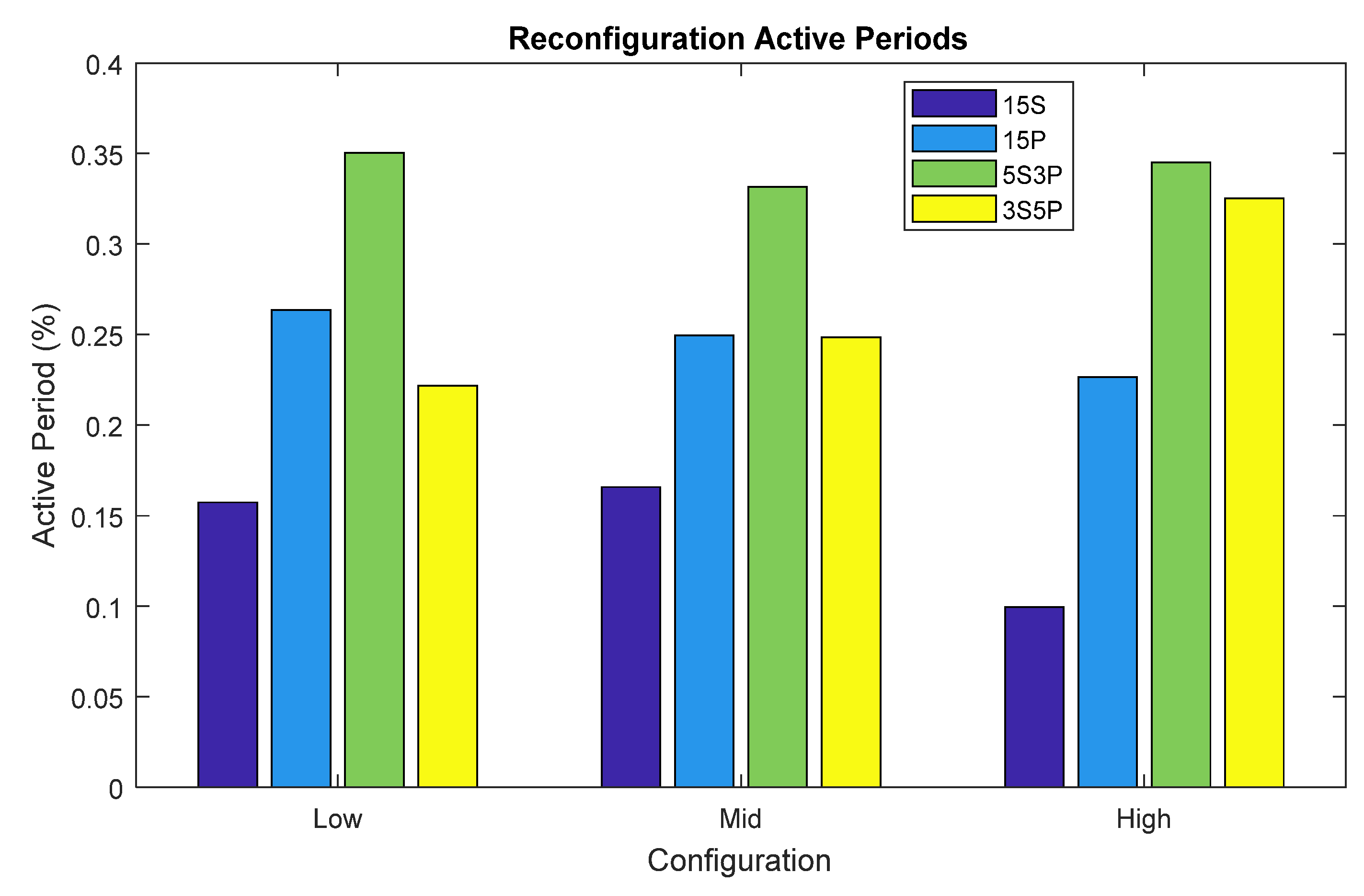
3.2. MFC Stack Performance as a Digital Data Generator
3.3. Bio-Digital Interface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276. [Google Scholar] [CrossRef]
- You, J.; Greenman, J.; Ieropoulos, I.A. Microbial fuel cells in the house: A study on real household wastewater samples for treatment and power. Sustain. Energy Technol. Assess. 2021, 48, 101618. [Google Scholar] [CrossRef]
- Khandaker, S.; Das, S.; Hossain, M.T.; Islam, A.; Miah, M.R.; Awual, M.R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation—A comprehensive review. J. Mol. Liq. 2021, 344, 117795. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulos, I. PEE POWER® urinal II—Urinal scale-up with microbial fuel cell scale-down for improved lighting. J. Power Sources 2018, 392, 150–158. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal—Microbial fuel cell technology field trials in the context of sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Staddon, C.; Cook, A.; Walker, J.; Boulton, J.; Powell, W.; Ieropoulos, I. Multidimensional Benefits of Improved Sanitation: Evaluating ‘PEE POWER®’ in Kisoro, Uganda. Int. J. Environ. Res. Public Health 2020, 17, 2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bixio, D.; Thoeye, C.; De Koning, J.; Joksimovic, D.; Savic, D.; Wintgens, T.; Melin, T. Wastewater reuse in Europe. Desalination 2006, 187, 89–101. [Google Scholar] [CrossRef]
- Lee, H.; Tan, T.P. Singapore’s experience with reclaimed water: NEWater. Int. J. Water Resour. Dev. 2016, 32, 611–621. [Google Scholar] [CrossRef]
- ALICE Consortium ALICE Interface—Active Living Infrastructure: Controlled Environment. Available online: https://alice-interface.eu/ (accessed on 16 September 2020).
- LIAR Consortium Living Architecture—Transform Our Habitats from Inert Spaces into Programmable Sites. Available online: https://livingarchitecture-h2020.eu/ (accessed on 10 September 2019).
- Gajda, I.; You, J.; Santoro, C.; Greenman, J.; Ieropoulos, I.A. A new method for urine electrofiltration and long term power enhancement using surface modified anodes with activated carbon in ceramic microbial fuel cells. Electrochim. Acta 2020, 353, 136388. [Google Scholar] [CrossRef]
- Walter, X.A.; Greenman, J.; Ieropoulos, I.A. Microbial fuel cells directly powering a microcomputer. J. Power Sources 2020, 446, 227328. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wallis, L.; Radisavljevic, N.; Pasternak, G.; Sglavo, V.M.; Hanczyc, M.M.; Greenman, J.; Ieropoulos, I. A Comprehensive Study of Custom-Made Ceramic Separators for Microbial Fuel Cells: Towards “Living” Bricks. Energies 2019, 12, 4071. [Google Scholar] [CrossRef] [Green Version]
- Brooks, T.; Keevil, C.W. A simple artificial urine for the growth of urinary pathogens. Lett. Appl. Microbiol. 1997, 24, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Melhuish, C.; Ieropoulos, I.; Greenman, J.; Horsfield, I. Energetically autonomous robots: Food for thought. Auton. Robots 2006, 21, 187–198. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C.; Horsfield, I. EcoBot-III: A robot with guts. In Proceedings of the Alife XII Conference, Odense, Denmark, 19–23 August 2010; pp. 733–740. [Google Scholar]
- You, J.; Greenman, J.; Ieropoulos, I. Novel analytical microbial fuel cell design for rapid in situ optimisation of dilution rate and substrate supply rate, by flow, volume control and anode placement. Energies 2018, 11, 2377. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, G.S.; Ghangrekar, M.M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J. Improving domestic wastewater treatment efficiency with constructed wetland microbial fuel cells: Influence of anode material and external resistance. Sci. Total Environ. 2018, 631–632, 1406–1414. [Google Scholar] [CrossRef]
- Grondin, F.; Perrier, M.; Tartakovsky, B. Microbial fuel cell operation with intermittent connection of the electrical load. J. Power Sources 2012, 208, 18–23. [Google Scholar] [CrossRef]
- Walter, X.A.; Greenman, J.; Ieropoulos, I.A. Intermittent load implementation in microbial fuel cells improves power performance. Bioresour. Technol. 2014, 172, 365–372. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Li, N.; Wan, L.; Zhou, L.; Du, Q.; Li, T.; Wang, X. Electric field induced salt precipitation into activated carbon air-cathode causes power decay in microbial fuel cells. Water Res. 2017, 123, 369–377. [Google Scholar] [CrossRef]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A mini review on biofouling on air cathode of single chamber microbial fuel cell; prevention and mitigation strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Faíña, A.; Nejatimoharrami, F.; Stoy, K.; Theodosiou, P.; Taylor, B.; Ieropoulos, I. EvoBot: An Open-Source, Modular Liquid Handling Robot for Nurturing Microbial Fuel Cells; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Papaharalabos, G.; Greenman, J.; Stinchcombe, A.; Horsfield, I.; Melhuish, C.; Ieropoulos, I. Dynamic electrical reconfiguration for improved capacitor charging in microbial fuel cell stacks. J. Power Sources 2014, 272, 34–38. [Google Scholar] [CrossRef]
- Papaharalabos, G.; Stinchcombe, A.; Horsfield, I.; Melhuish, C.; Greenman, J.; Ieropoulos, I. Autonomous energy harvesting and prevention of cell reversal in MFC stacks. J. Electrochem. Soc. 2017, 164, H3047–H3051. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, R.C.; Blalock, T.N. Microelectronic Circuit Design; McGraw-Hill: New York, NY, USA, 1997; ISBN 0070324824. [Google Scholar]
- Brabazon, A.; O’Neill, M.; McGarraghy, S. Chapter 11. Bacterial Foraging Algorithms. In Natural Computing Algorithms; Springer: Berlin/Heidelberg, Germany, 2015; pp. 187–199. ISBN 978-3-662-43630-1. [Google Scholar]
- Freeman, J.; Wiggins, G.; Starks, G.; Sandler, M. A concise taxonomy for describing data as an art material. Leonardo 2018, 51, 75–79. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.; Mendis, A.; Greenman, J.; Freeman, J.; Wolff, S.; Armstrong, R.; Hughes, R.; Ieropoulos, I.A. Development of a Bio-Digital Interface Powered by Microbial Fuel Cells. Sustainability 2022, 14, 1735. https://doi.org/10.3390/su14031735
You J, Mendis A, Greenman J, Freeman J, Wolff S, Armstrong R, Hughes R, Ieropoulos IA. Development of a Bio-Digital Interface Powered by Microbial Fuel Cells. Sustainability. 2022; 14(3):1735. https://doi.org/10.3390/su14031735
Chicago/Turabian StyleYou, Jiseon, Arjuna Mendis, John Greenman, Julie Freeman, Stephen Wolff, Rachel Armstrong, Rolf Hughes, and Ioannis A. Ieropoulos. 2022. "Development of a Bio-Digital Interface Powered by Microbial Fuel Cells" Sustainability 14, no. 3: 1735. https://doi.org/10.3390/su14031735
APA StyleYou, J., Mendis, A., Greenman, J., Freeman, J., Wolff, S., Armstrong, R., Hughes, R., & Ieropoulos, I. A. (2022). Development of a Bio-Digital Interface Powered by Microbial Fuel Cells. Sustainability, 14(3), 1735. https://doi.org/10.3390/su14031735





