Effect of Thymol and Nanostructured Lipid Carriers (NLCs) Incorporated with Thymol as Antimicrobial Agents in Sausage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Formulation of NLCs
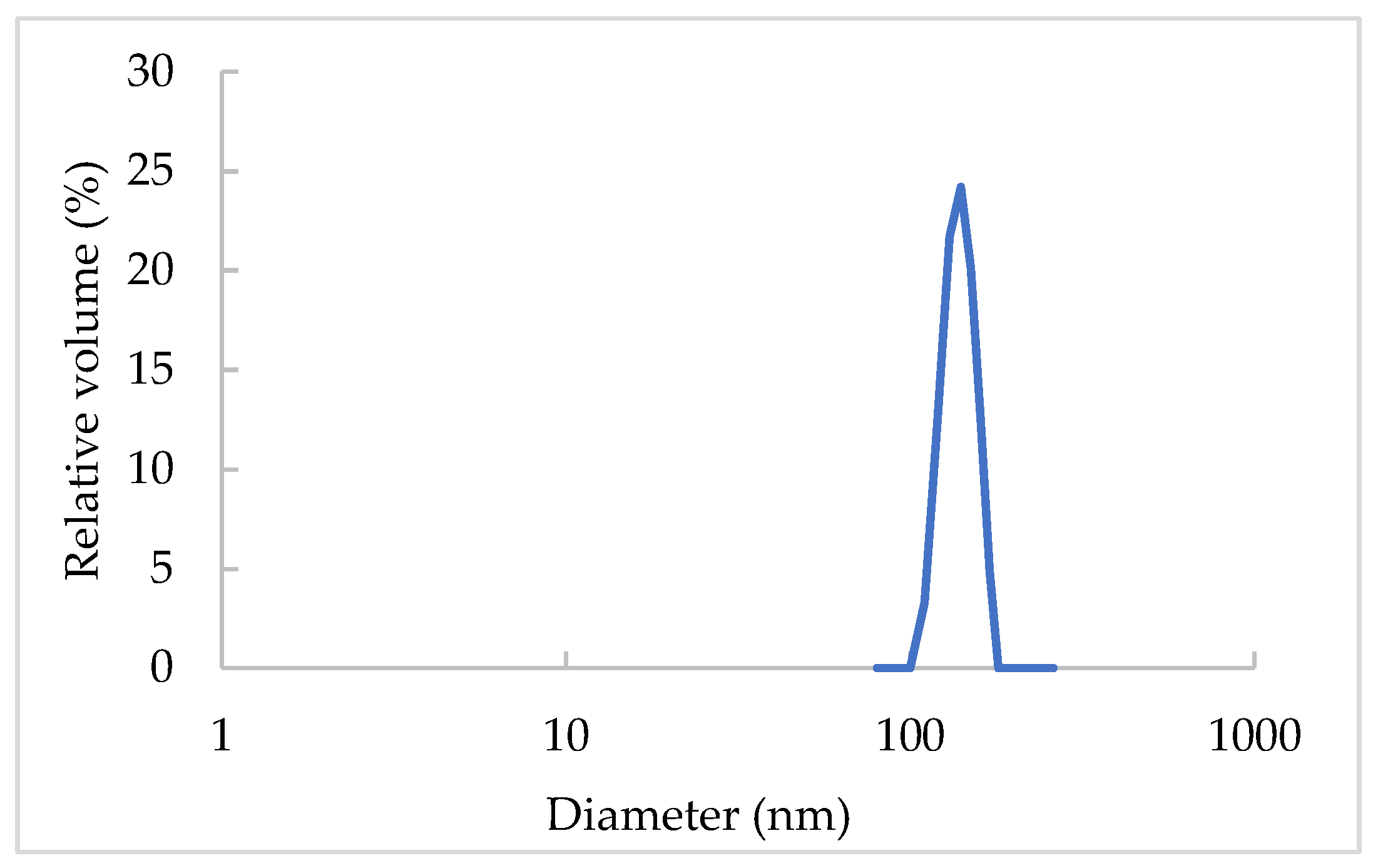
2.3. Particle Size Distribution
2.4. Encapsulation Efficiency
2.5. Determination of Minimum Inhibitory Concentration (MIC)
2.6. Determination of Minimum Bactericidal Concentration (MBC)
2.7. Agar Diffusion Method
2.8. Sausage Preparation
2.9. The Microbial Counts of Sausages
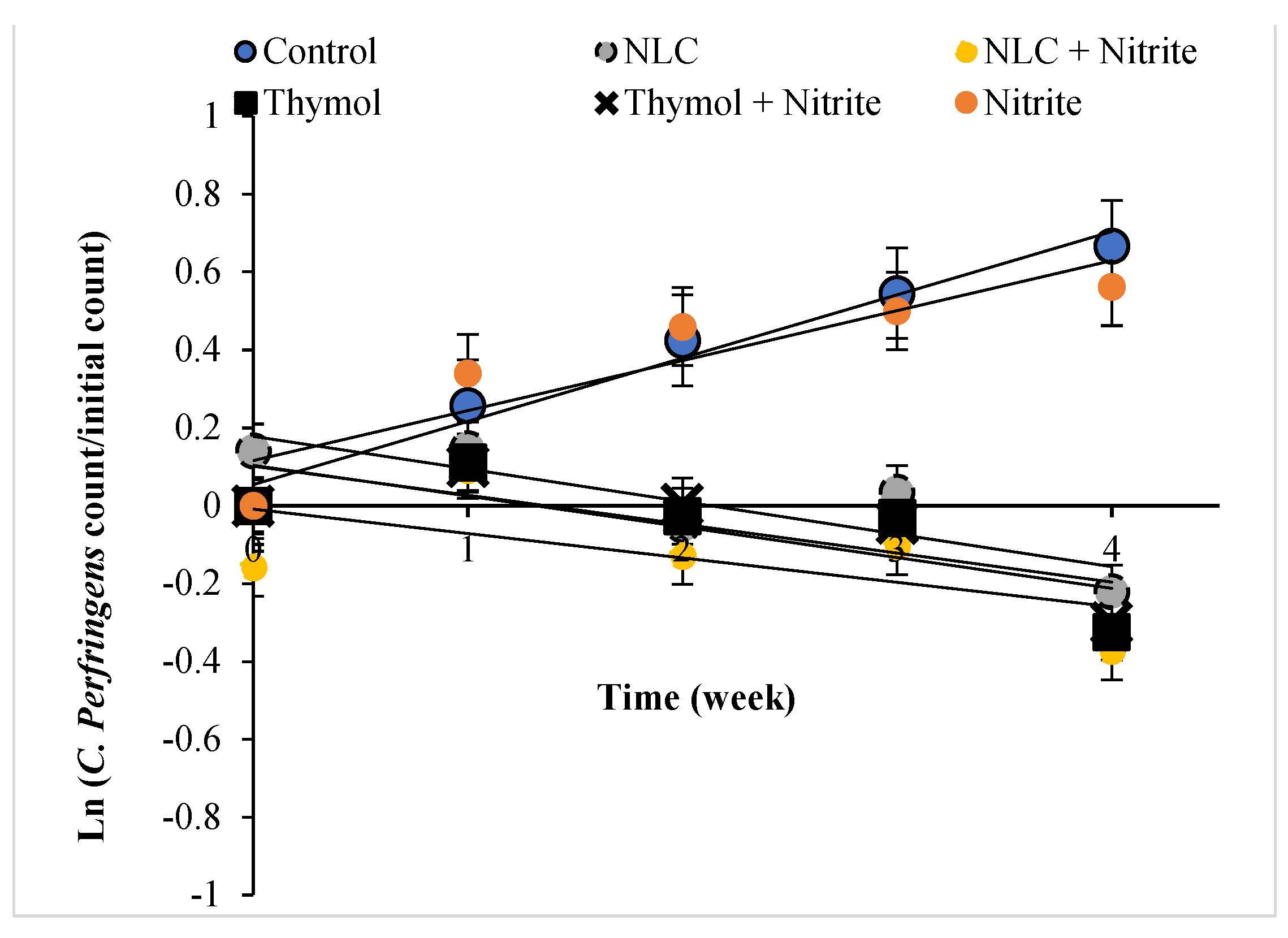
2.10. Mathematical Modeling
2.11. Statistical Analysis
3. Results and Discussion
3.1. NLC Characterization
3.2. MIC, MBC, and Agar Well Diffusion Test Results
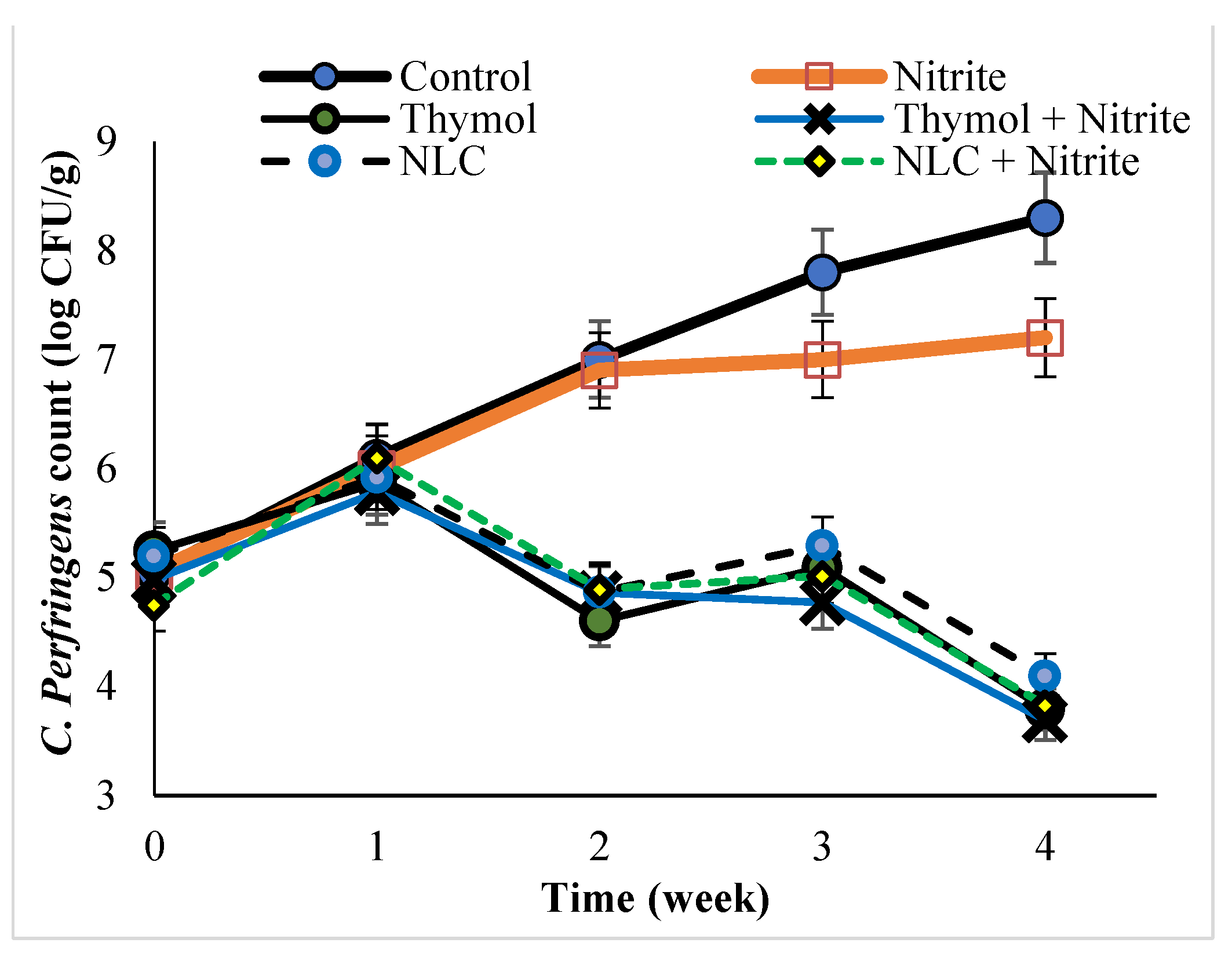
3.3. The Antimicrobial Activity of Thymol and Thymol-Loaded NLC in the Sausage Product
3.4. The Growth Kinetic of E. coli, S. aureus, and C. perfringens in the Sausage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayadi, M.; Amiri, S.; Radi, M. Active packaging nanocomposite gelatin-based films as a carrier of nano TiO2 and cumin essential oil: The effect on quality parameters of fresh chicken. J. Food Meas. Charact. 2021, 16, 420–430. [Google Scholar] [CrossRef]
- Almasi, L.; Radi, M.; Amiri, S. The release rate and antimicrobial activity of calcium-alginate films containing self-microemulsifying Thymus vulgaris essential oil against Escherichia coli and Staphylococcus aureus. J. Food Saf. 2020, 40, e12828. [Google Scholar] [CrossRef]
- Sepahvand, S.; Amiri, S.; Radi, M.; Akhavan, H.-R. Antimicrobial Activity of Thymol and Thymol-Nanoemulsion Against Three Food-Borne Pathogens Inoculated in a Sausage Model. Food Bioprocess Technol. 2021, 14, 1936–1945. [Google Scholar] [CrossRef]
- Ding, Z.; Johanningsmeier, S.D.; Price, R.; Reynolds, R.; Truong, V.-D.; Payton, S.C.; Breidt, F. Evaluation of nitrate and nitrite contents in pickled fruit and vegetable products. Food Control 2018, 90, 304–311. [Google Scholar] [CrossRef]
- Amiri, S.; Akhavan, H.; Zare, N.; Radi, M. Effect of Gelatin-Based Edible Coatings Incorporated with Aloe Vera and Green Tea Extracts on the Shelf-Life of Fresh-Cut Apple. Ital. J. Food Sci. 2018, 30, 61–74. [Google Scholar]
- Pakfetrat, S.; Amiri, S.; Radi, M.; Abedi, E.; Torri, L. The influence of green tea extract as the steeping solution on nutritional and microbial characteristics of germinated wheat. Food Chem. 2020, 332, 127288. [Google Scholar] [CrossRef]
- Amiri, S.; Nicknam, Z.; Radi, M.; Sayadi, M.; Bagheri, F.; Khorrami, N.K.; Abedi, E. Postharvest quality of orange fruit as influenced by salicylic acid, acetic acid, and carboxymethyl cellulose coating. J. Food Meas. Charact. 2021, 15, 3912–3930. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.-H.; Liu, Y.; Wang, Z.; Zhang, Y.-T.; Feng, N.P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar]
- Almasi, L.; Radi, M.; Amiri, S.; Torri, L. Fully dilutable Thymus vulgaris essential oil:acetic or propionic acid microemulsions are potent fruit disinfecting solutions. Food Chem. 2020, 343, 128411. [Google Scholar] [CrossRef]
- Almasi, L.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of antimicrobial biopolymer films containing essential oil-loaded microemulsions or nanoemulsions. Food Hydrocoll. 2021, 117, 106733. [Google Scholar] [CrossRef]
- Karam, L.; Roustom, R.; Abiad, M.G.; El-Obeid, T.; Savvaidis, I.N. Combined effects of thymol, carvacrol and packaging on the shelf-life of marinated chicken. Int. J. Food Microbiol. 2018, 291, 42–47. [Google Scholar] [CrossRef]
- Khorrami, N.K.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of alginate-based films functionalized with nanostructured lipid carriers. Int. J. Biol. Macromol. 2021, 182, 373–384. [Google Scholar] [CrossRef]
- Amiri, S.; Abbasi, S.; Ezzatpanah, H.; Hosseini, E. Nanocapsulation of orange peel oil using microemulsion technique. Agro Food Ind. Hi Tech 2013, 24, 72–75. [Google Scholar]
- Radi, M.; Abbasi, S.; Hamidi, Z.; Azizi, M.H. Development of a new method for extraction of canola oil using lecithin based microemulsion systems. Agro Food Ind. Hi Tech 2013, 24, 70–72. [Google Scholar]
- Bagheri, F.; Radi, M.; Amiri, S. Evaluating the physical, mechanical and morphological properties of sodium alginate nanocomposite film containing solid lipid nano-particles. J. Food Sci. Technol. 2019, 16, 263–271. [Google Scholar]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2019, 90, 162–171. [Google Scholar] [CrossRef]
- Cinay, G.E.; Erkoc, P.; Alipour, M.; Hashimoto, Y.; Sasaki, Y.; Akiyoshi, K.; Kizilel, S. Nanogel-integrated pH-responsive composite hydrogels for controlled drug delivery. ACS Biomater. Sci. 2017, 3, 370–380. [Google Scholar] [CrossRef]
- Robledo, N.; López, L.; Bunger, A.; Tapia, C.; Abugoch, L. Effects of Antimicrobial Edible Coating of Thymol Nanoemulsion/Quinoa Protein/Chitosan on the Safety, Sensorial Properties, and Quality of Refrigerated Strawberries (Fragaria × ananassa) Under Commercial Storage Environment. Food Bioprocess Technol. 2018, 11, 1566–1574. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515. [Google Scholar] [CrossRef]
- Abdou, E.S.; Galhoum, G.F.; Mohamed, E.N. Curcumin loaded nanoemulsions/pectin coatings for refrigerated chicken fillets. Food Hydrocoll. 2018, 83, 445–453. [Google Scholar] [CrossRef]
- Radi, M.; Amiri, S. Comparison of the Rheological Behavior of Solutions and Formulated Oil-in-Water Emulsions Containing Carboxymethylcellulose (CMC). J. Dispers. Sci. Technol. 2013, 34, 582–589. [Google Scholar] [CrossRef]
- Mozaffar, S.; Radi, R.; Amiri, S.; McClements, D.J. A new approach for drying of nanostructured lipid carriers (NLC) by spray-drying and using sodium chloride as the excipient. J. Drug Deliv. Sci. Technol. 2021, 61, 102212. [Google Scholar] [CrossRef]
- Aditya, N.; Aditya, S.; Yang, H.-J.; Kim, H.W.; Park, S.O.; Lee, J.; Ko, S. Curcumin and catechin co-loaded water-in-oil-in-water emulsion and its beverage application. J. Funct. Foods 2015, 15, 35–43. [Google Scholar] [CrossRef]
- Bagheri, F.; Nejatian, M.; Abbaszadeh, S.; Taghdir, M. The effect of gelatin and thymol-loaded nanostructured lipid carrier on physicochemical, rheological, and sensory properties of sesame paste/date syrup blends as a snack bar. J. Texture Stud. 2020, 51, 501–510. [Google Scholar] [CrossRef]
- Akhavan, H.-R.; Hosseini, F.-S.; Amiri, S.; Radi, M. Cinnamaldehyde-Loaded Nanostructured Lipid Carriers Extend the Shelf Life of Date Palm Fruit. Food Bioprocess Technol. 2021, 14, 1478–1489. [Google Scholar] [CrossRef]
- Radi, M.; Abbasi, S. Optimization of Novel Oil Extraction Technique from Canola Seeds: Lecithin-Based Microemulsion. Eur. J. Lipid Sci. Technol. 2018, 120, 1700267. [Google Scholar] [CrossRef]
- Pivettaa, T.P.; Simõesb, S.; Araújoa, M.M.; Carvalhoc, T.; Arrudad, C.; Marcatoa, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Amiri, S.; Niakousari, M. Shelf life of unpasteurized sour orange juice in Iran. Fruits 2008, 63, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Radi, M.; Ahmadi, H.; Amiri, S. Effect of Cinnamon Essential Oil-Loaded Nanostructured Lipid Carriers (NLC) Against Penicillium Citrinum and Penicillium Expansum Involved in Tangerine Decay. Food Bioprocess Technol. 2021, 11, 1566–1574. [Google Scholar] [CrossRef]
- Piran, P.; Kafil, H.S.; Ghanbarzadeh, S.; Safdari, R.; Hamishehkar, H. Formulation of menthol-loaded nanostructured lipid carriers to enhance its antimicrobialactivity for food preservation. Adv. Pharm. Bull. 2017, 7, 261–268. [Google Scholar] [CrossRef]
- Lai, F.; Wissing, S.A.; Müller, R.H.; Fadda, A.M. Artemisia arborescens essential oil-loaded solid lipid nanoparticles for potential agricultural application: Preparation and characterization. AAPS Pharm. Sci. Tech. 2006, 7, E10–E18. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The antibacterial mechanism of carvacrol and thymol againstEscherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [Green Version]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2013, 54, 625–644. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Mohammadpourfard, I.; Khanjari, A.; Basti, A.A.; Herrero-Latorre, C.; Shariatifar, N.; Hosseini, H. Evaluation of microbiological, chemical, and sensory properties of cooked probiotic sausages containing different concentrations of astaxanthin, thymol, and nitrite. Food Sci. Nutr. 2020, 9, 345–356. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Liu, L.; Liu, Y.; Wu, X. Impacts of chitosan nanoemulsions with thymol or thyme essential oil on volatile compounds and microbial diversity of refrigerated pork meat. Meat Sci. 2022, 185, 108706. [Google Scholar] [CrossRef]


| Samples | E. coli | S. aureus | C. perfringens | |||
|---|---|---|---|---|---|---|
| MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | |
| Thymol | 180 ± 0.0 a | 406 ± 0.0 a | 110 ± 0.0 a | 200 ± 0.0 a | 196.67 ± 0.0 a | 406.6 ± 0.0 a |
| Thymol-loaded NLC | 406.67 ± 0.0 b | 833.3 ± 0.0 b | 216.6 ± 0.0 b | 406.6 ± 0.0 b | 393.3 ± 0.0 b | 806.6 ± 0.0 b |
| NLC without thymol | Not effective | Not effective | Not effective | Not effective | Not effective | Not effective |
| Samples | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Concentration | E. coli | S. aureus | C. perfringens | |
| Gentamicin | 10 μg | 22 ± 1.00 b | 30.66 ± 1.00 a | 26.00 ± 1.00 b |
| Thymol (mg/L) | 100 | 11.3 ± 0.5 d | 12.6 ± 1.0 de | 10.35 ± 0.5 de |
| 50 | 10.3 ± 0.57 de | 12.0 ± 1.0 d | 9.30 ± 0.5 ef | |
| 25 | 9.00 ± 1.0 ef | 11.0 ± 1.00 de | 9.30 ± 0.5 ef | |
| 12.5 | 7.60 ± 0.57 fg | 9.3 ± 0.57 fg | 8.30 ± 0.5 fg | |
| 6.25 | 6.70 ± 0.5 g | 8.0 ± 1.0 g | 7.60 ± 0.6 gh | |
| Thymol-loaded NLC (mg/L) | 100 | 10.67 ± 0.57 de | 11.33 ± 0.58 de | 11 ± 1.52 cd |
| 50 | 10.66 ± 0.57 de | 11.33 ± 0.58 de | 9.66 ± 0.57 de | |
| 25 | 9.33 ± 0.57 ef | 10.33 ± 0.58 ef | 9.33 ± 0.57 e | |
| 12.5 | 8.33 ± 0.57 fg | 8.33 ± 0.58 fg | 8.67 ± 0.57 fg | |
| 6.25 | 7.33 ± 0.57 fg | 7.66 ± 0.57 g | 6.66 ± 0.57 h | |
| NLC without thymol | - | 0.00 ± 0.00 i | 0.00 ± 0.00 i | 0.00 ± 0.00 i |
| Treatments | C. perfringens | E. coli | S. aureus | |||
|---|---|---|---|---|---|---|
| R2 | k (week−1) | R2 | k (week−1) | R2 | k (week−1) | |
| 0.97 | 0.162 a | 0.98 | 0.118 a | 0.97 | 0.128 a | Control |
| 0.82 | 0.124 b | 0.90 | 0.092 b | 0.84 | 0.094 b | Nitrite |
| 0.99 | −0.447 e | 0.92 | −0.107 c | 0.88 | −0.15 d | NLC |
| 0.95 | −0.116 c | 0.89 | −0.115 a | 0.96 | −0.128 a | Thymol |
| 0.91 | −0.115 c | 0.97 | −0.114 a | 0.91 | −0.127 a | Thymol + Nitrite |
| 0.98 | −0.229 d | 0.97 | −0.153 d | 0.86 | −0.11 c | NLC + Nitrite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepahvand, S.; Amiri, S.; Radi, M.; Amiri, M.J. Effect of Thymol and Nanostructured Lipid Carriers (NLCs) Incorporated with Thymol as Antimicrobial Agents in Sausage. Sustainability 2022, 14, 1973. https://doi.org/10.3390/su14041973
Sepahvand S, Amiri S, Radi M, Amiri MJ. Effect of Thymol and Nanostructured Lipid Carriers (NLCs) Incorporated with Thymol as Antimicrobial Agents in Sausage. Sustainability. 2022; 14(4):1973. https://doi.org/10.3390/su14041973
Chicago/Turabian StyleSepahvand, Somayeh, Sedigheh Amiri, Mohsen Radi, and Mohammad Javad Amiri. 2022. "Effect of Thymol and Nanostructured Lipid Carriers (NLCs) Incorporated with Thymol as Antimicrobial Agents in Sausage" Sustainability 14, no. 4: 1973. https://doi.org/10.3390/su14041973







