Drying of Carrot Strips in Indirect Solar Dehydrator with Photovoltaic Cell and Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
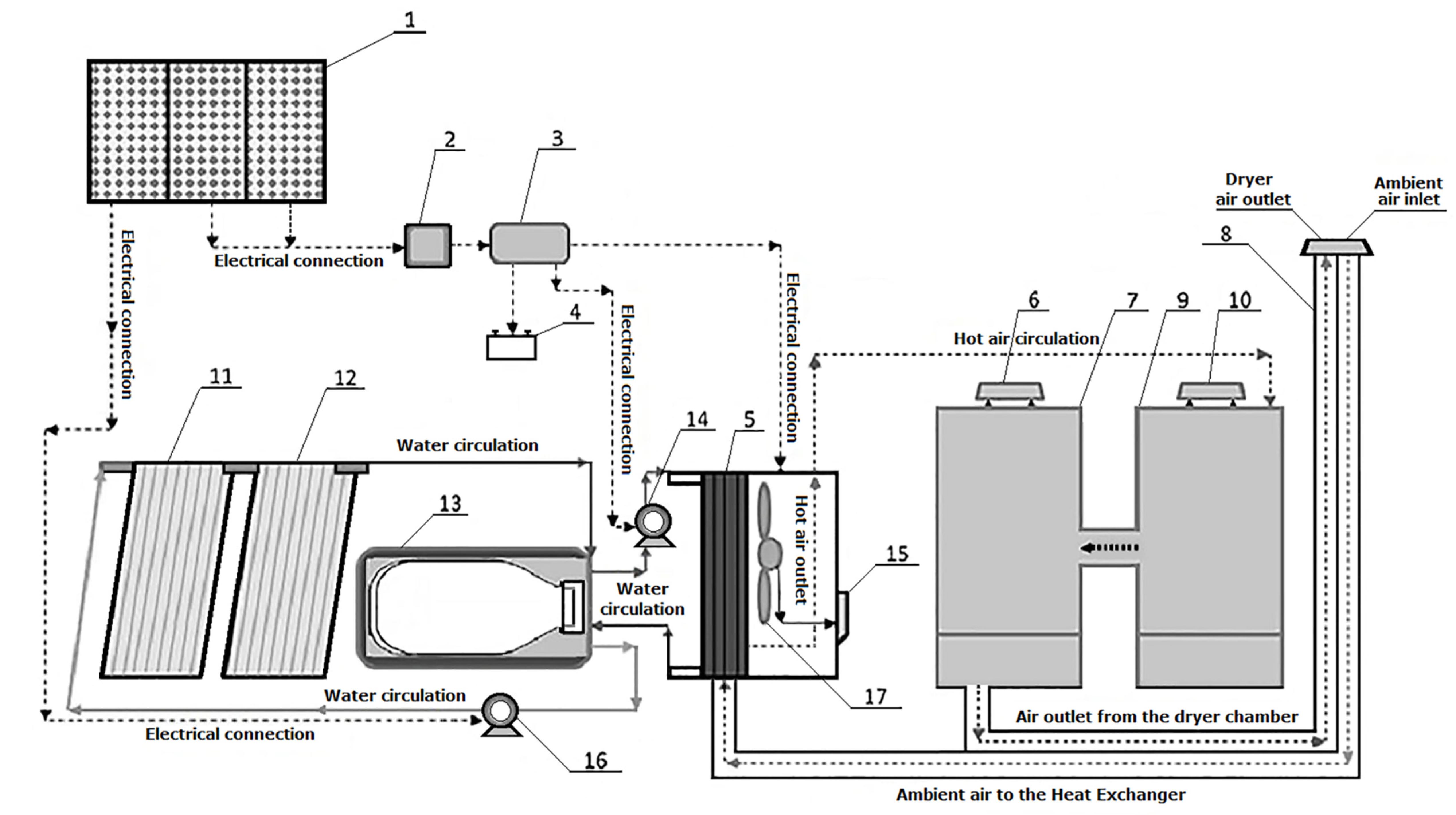
2.1. Solar Drier Equipment and Complementary Equipment
2.2. Experimental Procedure
2.3. Drying Curves and Mathematical Modeling
Calculations for Making Drying Curves and Determining the Drying Ratios
2.4. Color Analysis
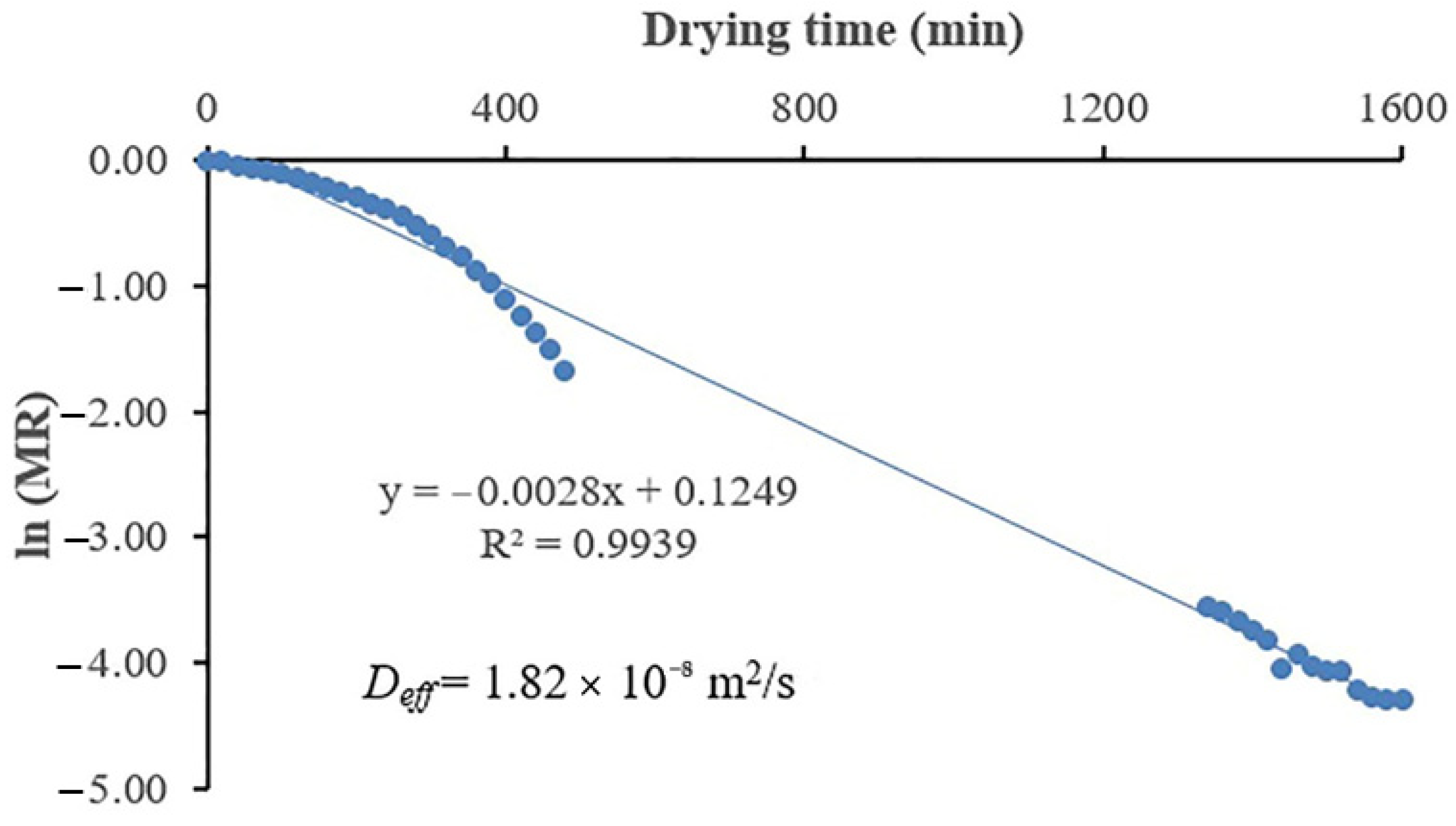
2.5. Calculation of Effective Diffusivity Coefficient
2.6. Statistical Analysis
3. Results and Discussion
3.1. Drying Characteristics
3.1.1. Drying Curve
3.1.2. Drying Rate Curve
3.2. Modeling of Drying Curves Statistical Parameters
3.3. Model Validation
3.4. Chromatic Coordinates
3.5. Estimation of the Effective Moisture Diffusivity (Deff)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva Dias, J.C. Nutritional and health benefits of carrots and their seed extracts. Food Nutr. Sci. 2014, 5, 2147–2156. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Comparison of World Crops Production Quantities 2017. Food and Agriculture Organization Corporate Statistical Database. 2017. Available online: http://www.fao.org/faostat (accessed on 15 September 2021).
- Hiranvarachat, B.; Devahastin, S.; Chiewchan, N. Effects of acid pretreatments on some physicochemical properties of carrot undergoing hot air drying. Food Bioprod. Process. 2011, 89, 116–127. [Google Scholar] [CrossRef]
- ODEPA. Oficina de Estudios y Políticas Agrarias. 2017. Available online: http://www.odepa.gob.cl/wp-content/files_mf/1501684642Antofagastajulio.pdf/ (accessed on 18 May 2020).
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Food Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doymaz, I. Convective air drying characteristics of thin layer carrots. J. Food Eng. 2004, 61, 359–364. [Google Scholar] [CrossRef]
- Anonymous. Estrategia Regional de Innovación de la Región de Antofagasta. 2017. Available online: https://www.opia.cl/static/website/601/articles-77269_archivo_03.pdf (accessed on 27 August 2019).
- Ferrada, P.; Araya, F.; Marzo, A.; Fuentealba, E. Performance analysis of photovoltaic systems of two different technologies in a coastal desert climate zone of Chile. Sol. Energy 2015, 114, 356–363. [Google Scholar] [CrossRef]
- Lee, G.H. A Study for the Use of Sol Energy for Agricultural Industry—Solar Drying System Using Evacuated Tubular Solar Collector and Auxiliary Heater. J. Biosyst. Eng. 2013, 38, 41–47. [Google Scholar] [CrossRef]
- Urbano, J.B.; González, F.H.T.; Perilla, P.E.V.; Contreras, J.U.C. Estudio para el uso de la tecnología solar fotovoltaica. Ing. Solidar. 2011, 6, 69–81. [Google Scholar]
- Ekechukwu, O.V.; Norton, B. Design and measured performance of a solar chimney for natural-circulation solar-energy dryers. Renew. Energ. 1997, 10, 81–90. [Google Scholar] [CrossRef]
- Oliveros, C.E.; Sanz, J.R.; Ramirez, C.A.; Peñuela, A.E. Aprovechamiento eficiente de la energía en el secado mecánico del café. Av. TéCnicos Cenicafé. 2009, 380, 1–8. [Google Scholar]
- Sharma, A.; Chen, C.R.; Lan, N.V. Solar-energy drying systems: A review. Renew. Sustain. Energy Rev. 2009, 13, 1185–1210. [Google Scholar] [CrossRef]
- Mezquita, P.C.; López, A.Á.; Muñoz, W.B. Effect of Drying on Lettuce leaves using Indirect Solar Dryer Assisted with Photovoltaic Cells and Thermal Energy Storage. Processes 2020, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Charvát, P.; Klimeš, L.; Ostrý, M. Numerical and experimental investigation of a PCM-based thermal storage unit for solar air systems. Energy Build. 2014, 68, 488–497. [Google Scholar] [CrossRef]
- Doymaz, İ. Drying kinetics, rehydration and colour characteristics of convective hot-air drying of carrot slices. Heat Mass Transf. 2017, 53, 25–35. [Google Scholar] [CrossRef]
- Vishwanathan, K.H.; Giwari, G.K.; Hebbar, H.U. Infrared assisted dry-blanching and hybrid drying of carrot. Food Bioprod. Process. 2013, 91, 89–94. [Google Scholar] [CrossRef]
- Lin, T.M.; Durance, T.D.; Scaman, C.H. Characterization of vacuum microwave, air and freeze dried carrot slices. Food Res. Int. 1998, 31, 111–117. [Google Scholar] [CrossRef]
- Prakash, S.; Jha, S.K.; Datta, N. Performance evaluation of dried blanched carrots by three different driers. J. Food Eng. 2004, 62, 305–313. [Google Scholar] [CrossRef]
- Béttega, R.; Rosa, J.G.; Corrêa, R.G.; Freire, J.T. Comparison of Carrot (Daucus carota) Drying in Microwave and in Vacuum Microwave. Braz. J. Chem. Eng. 2014, 31, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Raghavan, G.S.V.; Wang, N. Carrot volatiles monitoring and control in microwave drying. LWT—Food Sci. Technol. 2010, 43, 291–297. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Tuohy, M.G.; O’Donnell, C.P.; Brunton, N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason. Sonochem. 2011, 18, 1172–1179. [Google Scholar] [CrossRef]
- Zielinska, M.; Markowski, M. Air drying characteristics and moisture diffusivity of carrots. Chem. Eng. Process. 2010, 49, 212–218. [Google Scholar] [CrossRef]
- Romano, G.; Kocsis, L.; Farkas, I. Analysis of Energy and Environmental Parameters during Solar Cabinet Drying of Apple and Carrot. Dry. Technol. 2009, 27, 574–579. [Google Scholar] [CrossRef]
- Seshachalam, K.; Thottipalayam, A.; Selvaraj, V. Drying of carrot slices in a triple pass solar dryer. Therm. Sci. 2017, 21, S389–S398. [Google Scholar] [CrossRef]
- Mahapatra, A.; Tripathy, P.P. Modeling and simulation of moisture transfer during solar drying of carrot slices. J. Food Process Eng. 2018, 41, 1–15. [Google Scholar] [CrossRef]
- Kumar, N.; Sarkar, B.C.; Sharma, H.K. Effect of air velocity on kinetics of thin layer carrot pomace drying. Food Sci. Technol. Int. 2011, 17, 459–469. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. A Review of thin layer drying of foods: Theory, modeling, and experimental results. Crit. Rev. Food Sci. Nutr. 2010, 50, 441–464. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Kianmehr, M.H.; Khani, S.; Ghasemi, M. Mathematical modelling of thin-layer drying of carrot. Int. Agrophys. 2009, 23, 313–317. [Google Scholar]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin Layers. Master’s Thesis, Department of Mechanical Engineering, Prude University, Prude, IN, USA, 1949. [Google Scholar]
- Henderson, S.M.; Pabis, S. Grain drying theory. IV. The effect of air flow rate on the drying index. J. Agric. Eng. Res. 1961, 7, 85–89. [Google Scholar]
- Yaldiz, O.; Ertekin, C.; Uzun, H.I. Mathematical modeling of thin layer solar drying of sultana grapes. Energy 2001, 26, 457–465. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Henderson, S.M. Progress in developing the thin layer drying equation. Trans. ASAE 1974, 17, 1167–1172. [Google Scholar] [CrossRef]
- Thompson, T.L.; Peart, R.M.; Foster, G.H. Mathematical simulation of corn drying—A new model. Trans. ASAE 1968, 11, 582–586. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Color and Appearance; Aspen Publishers: Gaithersburg, MD, USA, 1999. [Google Scholar] [CrossRef]
- Chandran, J.; Nisha, P.; Singhal, R.S.; Pandit, A.B. Degradation of colour in beetroot (Beta vulgaris L.): A kinetics study. J. Food Sci. Technol. 2014, 51, 2678–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palou, E.; Lopez-Malo, A.; Barbosa-Canovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; p. 414. ISBN 0-19-853411-6. [Google Scholar]
- Erenturk, S.; Erenturk, K. Comparison of genetic algorithm and neural network approaches for the drying process of carrot. J. Food Eng. 2007, 78, 905–912. [Google Scholar] [CrossRef]
- Ayensu, A. Dehydration of food crops using a solar dryer with convective heat flow. Sol. Energy 1997, 59, 121–126. [Google Scholar] [CrossRef]
- Urrea, D.; Eim, V.; Roselló, C. Modelos cinéticos de degradación de carotenoides, polifenoles y actividad antioxidante durante el secado convectivo de zanahoria (Daucus carota V. Nantesa). Aliment. Hoy 2012, 21, 68–101. [Google Scholar]
- Lawaetz, A.J.; Christensen, S.M.U.; Clausen, S.K.; Jørnsgaard, B.; Rasmussen, S.K.; Andersen, S.B.; Rinnan, Å. Fast, cross cultivar determination of total carotenoids in intact carrot tissue by Raman spectroscopy and Partial Least Squares calibration. Food Chem. 2016, 204, 7–13. [Google Scholar] [CrossRef]
- Cui, Z.-W.; Xu, S.-Y.; Sun, D.-W. Effect of microwave-vacuum drying on the carotenoids retention of carrot slices and chlorophyll retention of Chinese chive leaves. Dry. Technol. 2004, 22, 563–575. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Kinetic models of β-carotene degradation during air drying of carrots. Dry. Technol. 2010, 28, 752–761. [Google Scholar] [CrossRef]
- Sun, X.F.; Zhu, W.X.; Li, X.L.; Fan, J.L. Effects of heat pump drying temperature and dietary fat on carrot β-carotene bioaccessibility. Int. J. Agric. Biol. Eng. 2017, 10, 234–242. [Google Scholar] [CrossRef]
- Zielinska, M.; Markowski, M. Color Characteristics of Carrots: Effect of Drying and Rehydration. Int. J. Food Prop. 2012, 15, 450–466. [Google Scholar] [CrossRef]
- Lam Ip, R.W.L.; Cheong, W.C.I. The New Use of Diffusion Theories for the design of heat setting process in fabric Drying. In Advances in Modeling of Fluid Dynamics; Liu, C., Ed.; Copyright InTech: Croatia, Brazil, 2012; Chapter 7; pp. 143–170. [Google Scholar] [CrossRef] [Green Version]
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Prediction of porosity of food materials during drying: Current challenges and directions. Crit. Rev. Food Sci. Nutr. 2018, 58, 2896–2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamboa-Santos, J.; Soria, A.C.; Fornari, T.; Villamiel, M.; Montilla, A. Optimisation of convective drying of carrots using selected processing and quality indicators. Int. J. Food Sci. Technol. 2013, 48, 1998–2006. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, H.; Banakar, A.; Zarein, M. Mathematical Modeling and Thin Layer Drying Kinetics of Carrot Slices. Glob. J. Sci. Front. Res. Math. Decis. Sci. 2012, 12, 57–64. [Google Scholar]
- Raut, S.; Md Saleh, R.; Kirchhofer, P.; Kulig, B.; Hensel, O.; Sturm, B. Investigating the Effect of Different Drying Strategies on the Quality Parameters of Daucus carota L. Using Dynamic Process Control and Measurement Techniques. Food Bioprocess Technol. 2021, 14, 1067–1088. [Google Scholar] [CrossRef]
- Akpinar, E.K. Determination of suitable thin layer drying curve model for some vegetables and fruits. J. Food Eng. 2006, 73, 75–84. [Google Scholar] [CrossRef]
- Doymaz, İ. Infrared Drying Kinetics and Quality Characteristics of Carrot Slices. J. Food Process. Pres. 2015, 39, 2738–2745. [Google Scholar] [CrossRef]
- Yilmaz, B.; Cakmak, H.; Tavman, S. Ultrasonic pretreatment of carrot slices: Effects of sonication source on drying kinetics and product quality. An. Da Acad. Bras. De CiÊNcias 2019, 91, e20180447. [Google Scholar] [CrossRef]
- Markowski, M.; Stankiewicz, I.; Zapotoczny, P.; Borowska, J. Effect of Variety on Drying Characteristics and Selected Quality Attributes of Dried Carrots. Dry. Technol. 2006, 24, 1011–1018. [Google Scholar] [CrossRef]
- Koca, N.; Burdurlu, H.S.; Karadeniz, F. Kinetics of colour changes in dehydrated carrots. J. Food Eng. 2007, 78, 449–455. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, M.; Adhikari, B.; Yang, Z. Effect of microwave air spouted drying arranged in two and three-stages on the drying uniformity and quality of dehydrated carrot cubes. J. Food Eng. 2016, 177, 80–89. [Google Scholar] [CrossRef]
- Kowalski, S.J.; Szadzińska, J.; Pawłowski, A. Ultrasonic-assisted osmotic dehydration of carrot followed by convective drying with continuous and intermittent heating. Dry. Technol. 2015, 33, 1570–1580. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Miao, S.; Chong, C.; Sun, Y. Effect of a modified atmosphere on drying and quality characteristics of carrots. Food Bioprocess. Technol. 2014, 7, 2549–2559. [Google Scholar] [CrossRef]
- Demiray, E.; Tulek, Y. Color degradation kinetics of carrot (Daucus carota L.) slices during hot air drying. J. Food Process. Preserv. 2015, 39, 800–805. [Google Scholar] [CrossRef]
- Lavelli, V.; Zanoni, B.; Zaniboni, A. Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chem. 2007, 104, 1705–1711. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-López, J.A. Production of a red–purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Hong, S.; Han, J.; Krochta, J. Optical and surface properties of whey protein isolate coatings on plastic films as influenced by substrate, protein concentration, and plasticizer type. J. Appl. Polym. Sci. 2004, 92, 335–343. [Google Scholar] [CrossRef]
- Chen, Z.G.; Guo, X.Y.; Wu, T. A novel dehydration technique for carrot slices implementing ultrasound and vacuum drying methods. Ultrason. Sonochem. 2016, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Batu, A. Determination of acceptable firmness and colour values of tomatoes. J. Food Eng. 2004, 61, 471–475. [Google Scholar] [CrossRef]
- Abano, E.E.; Amoah, R.S.; Opoku, E.K. Temperature, microwave power and pomace thickness impact on the drying kinetics and quality of carrot pomace. J. Agric. Eng. 2019, 50, 28–37. [Google Scholar] [CrossRef]
- Abano, E.E.; Sam-Amoah, L.K.; Bart-Plange, A. Variation in ultrasonic frequency and time as pre-treatments to air-drying of carrot. J. Agric. Eng. 2013, 43, 149–158. [Google Scholar] [CrossRef]
- Wiktor, A.; Witrowa-Rajchert, D. Drying kinetics and quality of carrots subjected to microwave-assisted drying preceded by combined pulsed electric field and ultrasound treatment. Dry. Technol. 2019, 38, 176–188. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Hossien, M.; Arabhosseini, A.; Nazghelichi, T. Modelling the Carrot Thin-Layer Drying in a Semi-Industrial Continuous Band Dryer. Czech. J. Food Sci. 2011, 29, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Kurian, J.; Gariepy, Y.; Raghavan, V. Optimization of microwave-assisted fluidized-bed drying of carrot slices. Dry. Technol. 2017, 35, 1234–1248. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. A review on solar energy use in industries. Renew. Sustain. Energy Rev. 2011, 15, 1777–1790. [Google Scholar] [CrossRef]
- Parrado, C.; Girard, A.; Simon, F.; Fuentealba, E. 2050 LCOE (Levelized Cost of Energy) projection for a hybrid PV (photovoltaic)-CSP (concentrated solar power) plant in the Atacama Desert, Chile. Energy 2016, 94, 422–430. [Google Scholar] [CrossRef]


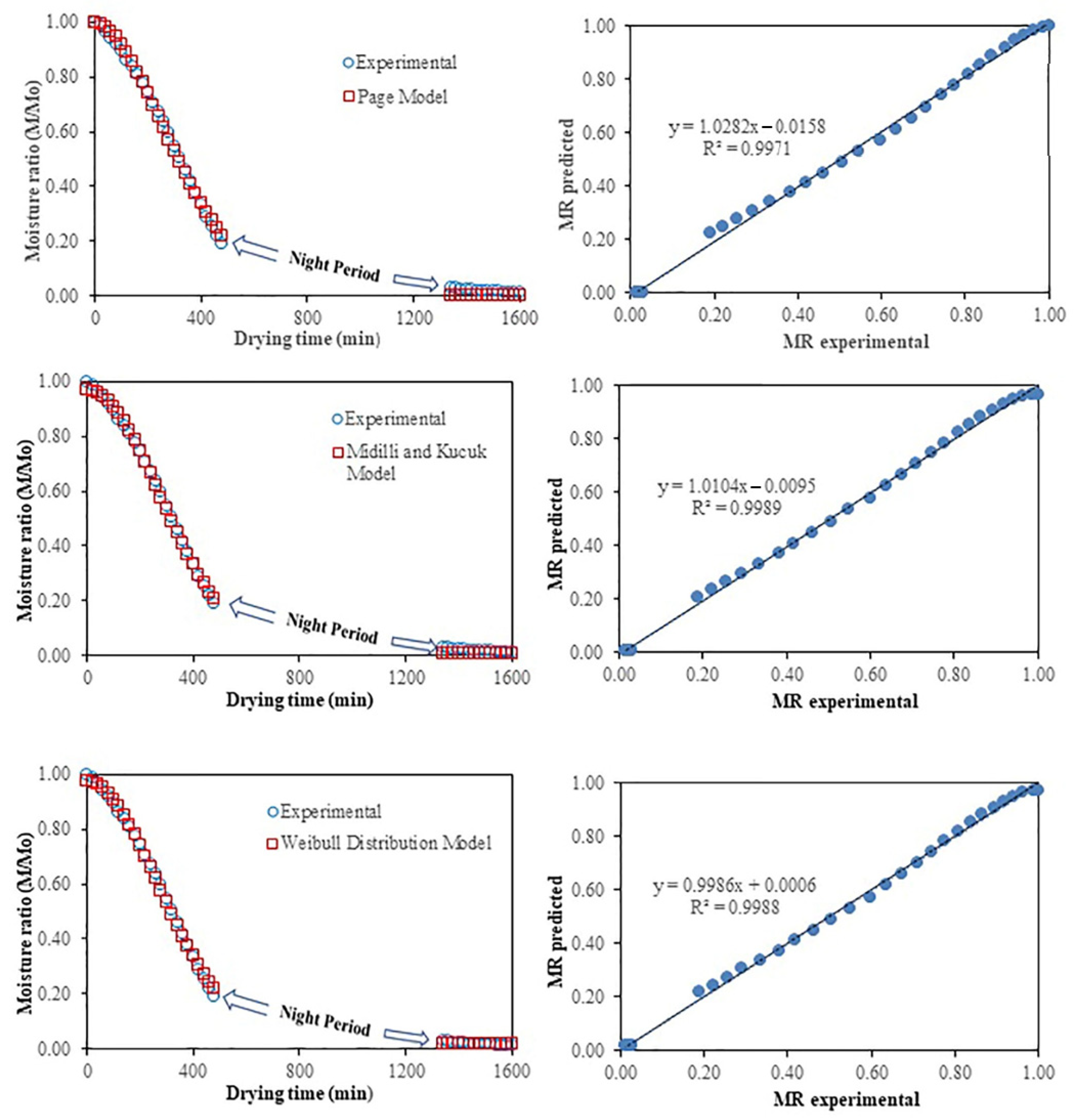
| N | Model Name | Model Equation | Ref. |
|---|---|---|---|
| Models derived from Newton’s law of cooling | |||
| 1 | Newton | MR = exp (−kt) | [30] |
| 2 | Page | MR = exp (−ktn) | [31] |
| 3 | Modified Page | MR = exp – (kt)n | [30] |
| Models derived from Fick’s second law of diffusion | |||
| 4 | Henderson and Pabis | MR = a exp (−kt) | [32] |
| 5 | Modified Henderson and Pabis | MR = a exp (−kt) + b exp (−gt )+ c exp (−ht) | [30] |
| 6 | Logarithmic | MR = a exp (−kt) + c | [30] |
| 7 | Approximation of diffusion | MR = a exp (−kt) + (1−a) exp (−kbt) | [33] |
| 8 | Midilli and Kucuk | MR = a exp – (–ktn) + bt | [34] |
| 9 | Two Term | MR = a exp (−k0 t)+ b exp (−k1 t) | [35] |
| 10 | Two Term exponential | MR = a exp (−kt) + (1−a) exp (−k a t) | [30] |
| 11 | Aghbashlo | MR = exp[−((k1 t)/(1 + k2 t))] | [30] |
| 12 | Verma | MR = a exp (−k t) + (1−a) exp (−g t) | [30] |
| Empirical models | |||
| 13 | Wang and Sing | MR = 1 + at + bt2 | [30] |
| 14 | Thompson | MR = exp ((−a –(a2 + 4 ⋅ bt)0.5)/2b | [36] |
| 15 | Weibull distribution | MR = a−b exp [−(ktn)] | [30] |
| N | Model | Coefficients and Constants | R2 | χ2 | SSE | RMSE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Newton | k = 0.00222 | 0.9632 | 0.00493 | 0.00480 | 0.06930 | |||||
| 2 | Page | k = 1.836 × 10−5 | n = 1.83288 | 0.9971 | 0.00040 | 0.00037 | 0.01936 | ||||
| 3 | Modified Page | k = 0.033307 | n = 0.066613 | 0.9632 | 0.00506 | 0.00480 | 0.06930 | ||||
| 4 | Henderson and Pabis | a = 1.11853 | k = 0.00260 | 0.9761 | 0.00328 | 0.00312 | 0.05582 | ||||
| 5 | Modified Henderson and Pabis | a = 7.30508 | b = 0.00051 | c = −6.34683 | k = 0.00548 | g = −0.00232 | h = 0.00654 | 0.9953 | 0.00072 | 0.00061 | 0.02474 |
| 6 | Logarithmic | a = 1.13061 | c = −0.01778 | k = 0.00251 | 0.9767 | 0.00330 | 0.00304 | 0.05515 | |||
| 7 | Approximation of diffusion | a = 10.67910 | b = 1.11492 | k = 0.00546 | 0.9941 | 0.00084 | 0.00078 | 0.02787 | |||
| 8 | Midilli and Kucuk | a = 0.967416 | b = 0.000013 | n = 2.047330 | k = 0.000005 | 0.9989 | 0.00021 | 0.00019 | 0.01376 | ||
| 9 | Two Term | a = 0.93984 | b = 0.17859 | k = 0.00260 | k1 = 0.00260 | 0.9761 | 0.00347 | 0.00312 | 0.05582 | ||
| 10 | Two Term exponential | a = 2.08704 | k = 0.00418 | 0.9933 | 0.00092 | 0.00088 | 0.02962 | ||||
| 11 | Aghbashlo | k1 = 0.00175 | k2 = −0.00059 | 0.9791 | 0.00287 | 0.00273 | 0.05222 | ||||
| 12 | Verma | a = 23.18260 | k = 0.00562 | g = 0.00590 | 0.9941 | 0.00084 | 0.00078 | 0.02784 | |||
| 13 | Wang and Sing | a = −0.00181 | b = 7.733 × 10−7 | 0.9744 | 0.00352 | 0.00334 | 0.05780 | ||||
| 14 | Thompson | a = −342.165 | b = 0.44676 | 0.9760 | 0.01032 | 0.00979 | 0.09896 | ||||
| 15 | Weibull distribution | a = 0.01812 | b = −0.95691 | k = 7.87 × 10−6 | n = 1.9768 | 0.9988 | 0.00017 | 0.00016 | 0.01252 | ||
| Chromatic Coordinates and Other Parameters | Carrot Strips | |
|---|---|---|
| Before Drying | Dried | |
| L* | 51.71 ± 0.04 a | 48.71 ± 0.05 b |
| a* | 34.76 ± 0.07 a | 29.76 ± 0.07 b |
| b* | 46.47 ± 0.14 a | 33.62 ± 0.09 b |
| ΔE | - | 14.11 ± 0.14 |
| ho | 53.20 ± 0.14 a | 48.48 ± 0.14 b |
| C | 58.03 ± 0.07 a | 44.90 ± 0.04 b |
| 0.75 ± 0.01 a | 0.89 ± 0.01 b | |
| BI | 209.82 ± 0.62 a | 148.38 ± 0.26 b |
| WI | 24.5 ± 0.11 a | 31.8 ± 0.17 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerezal-Mezquita, P.; Bugueño-Muñoz, W. Drying of Carrot Strips in Indirect Solar Dehydrator with Photovoltaic Cell and Thermal Energy Storage. Sustainability 2022, 14, 2147. https://doi.org/10.3390/su14042147
Cerezal-Mezquita P, Bugueño-Muñoz W. Drying of Carrot Strips in Indirect Solar Dehydrator with Photovoltaic Cell and Thermal Energy Storage. Sustainability. 2022; 14(4):2147. https://doi.org/10.3390/su14042147
Chicago/Turabian StyleCerezal-Mezquita, Pedro, and Waldo Bugueño-Muñoz. 2022. "Drying of Carrot Strips in Indirect Solar Dehydrator with Photovoltaic Cell and Thermal Energy Storage" Sustainability 14, no. 4: 2147. https://doi.org/10.3390/su14042147
APA StyleCerezal-Mezquita, P., & Bugueño-Muñoz, W. (2022). Drying of Carrot Strips in Indirect Solar Dehydrator with Photovoltaic Cell and Thermal Energy Storage. Sustainability, 14(4), 2147. https://doi.org/10.3390/su14042147







