Catalytic Performance of Cow-Dung Sludge in Water Treatment Mitigation and Conversion of Ammonia Nitrogen into Nitrate
Abstract
1. Introduction
2. Ammonia Nitrogen in the Water and Discharge Limit
3. Available Techniques and Demerits on Mass-Scale Treatment
4. Focus on New Research: Catalytic Effect of Cow-Dung Sludge
5. CowDung as a Bioresource of Catalytic Enzymes and Nitrifying Bacterial Mass
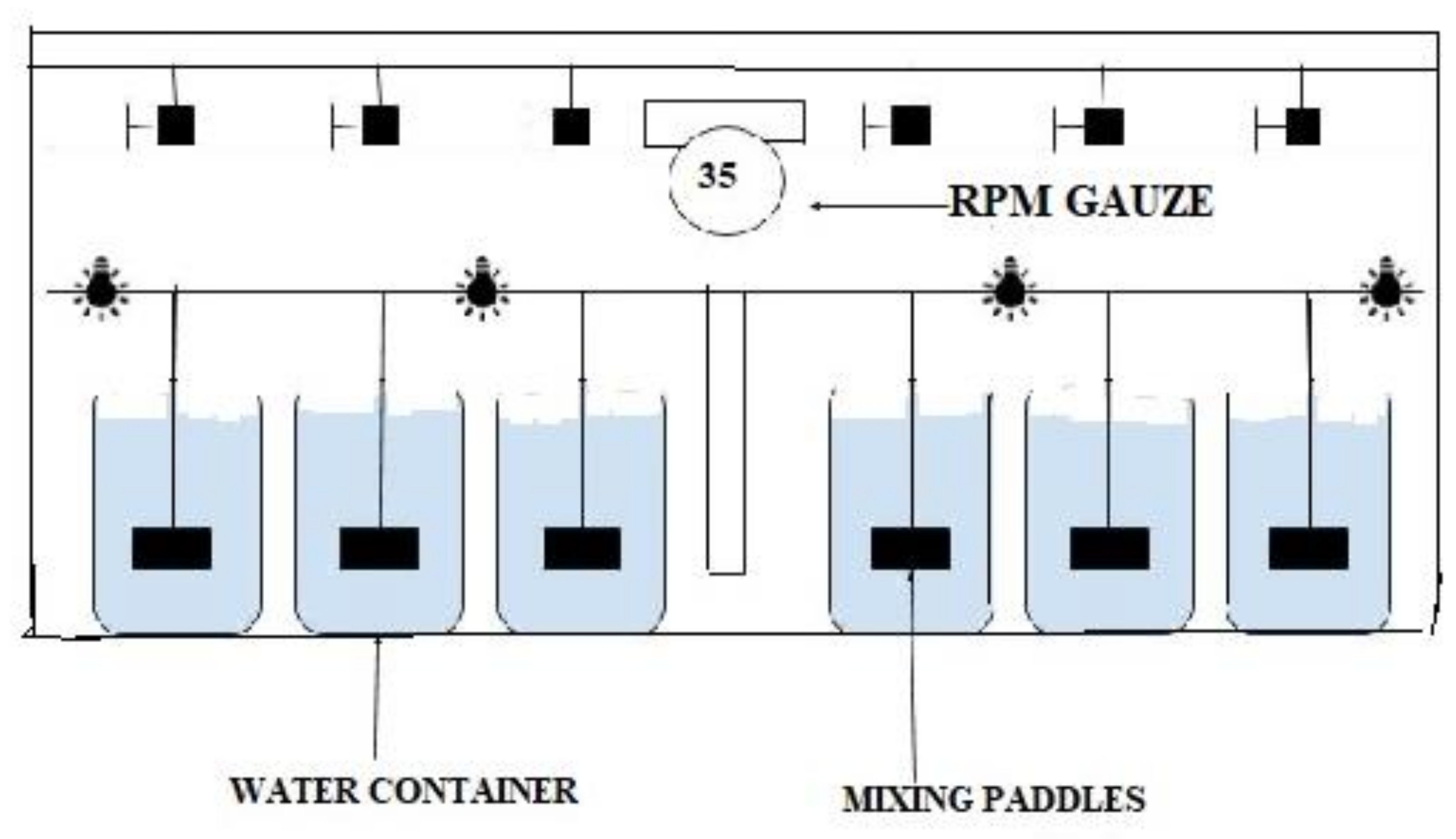
6. Materials and Methods
6.1. Collection of Samples and Testing
6.2. Preparation of Yucca Extract
6.3. Preparation of Indian Cow Dung
6.4. Experimental Setup and Oxidation of Ammonia Nitrogen
7. Results and Discussions
7.1. Biological Oxidation of Ammonia Nitrogen
7.2. Biological Oxidation of Nitrite to Nitrate
7.3. Oxidation of Nitrite (NO2) into Nitrate (NO3)
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawson, C.E.; Lücker, S. Complete ammonia oxidation: An important control on nitrification in engineered ecosystems? Curr. Opin. Biotechnol. 2018, 50, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Kaur, R.; Sharma, J. The efficiency of zeolites in water treatment for combating ammonia – An experimental study on Yamuna River water & treated sewage effluents. Inorg. Chem. Commun. 2021, 134, 108978. [Google Scholar]
- Grady, C.P.L.; Daigger, G.T.; Love, N.G.; Filipe, C.D.M. Biological Wastewater Treatment, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781420009637. [Google Scholar]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0471084980. [Google Scholar]
- Gray, N.F. Biology of Wastewater Treatment, 2nd ed.; Imperial College Press: London, UK; University of Dublin: Dublin, Ireland, 2004; ISBN 1-86094-328-4/1-86094-332-2. [Google Scholar]
- Chen, G.H.; van Loosdrecht, M.C.; Ekama, G.A.; Brdjanovic, D. (Eds.) Biological Wastewater Treatment Principles, Modelling and Design; IWA Publishing: London, UK, 2008; ISBN 9781843391883. [Google Scholar]
- Spellman, F.R. Mathematics Manual for Water and Wastewater Treatment Plant Operators; American Water Works Association: Denver, CO, USA, 2004; ISBN 1566706750. [Google Scholar]
- Gerardi, M.H. Wastewater Microbiology: Nitrification and Denitrification in the Activated Sludge Process; John Wiley & Sons: New York, NY, USA, 2002; ISBN 9780471065081. [Google Scholar]
- EPA. Ambient Water Quality Criteria for Endosulfan; EPA: Washington, DC, USA, 1980. [Google Scholar]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous Ammonia Equilibrium Calculations: Effect of pH and Temperature. J. Fish. Res. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- The Environment (Protection) Rules. General standards for discharge of environment pollutants: Effluent. Gaz. Notif. MoEF 1986, 2, 545–560. [Google Scholar]
- EPA. Wastewater Technology Fact Sheet—Ammonia Stripping (EPA 832-F-00-019); EPA: Washington, DC, USA, 2000; pp. 1–7. [Google Scholar]
- De la Noue, J.; de Pauw, N. The potential of microalgal biotechnology: A review of production and uses of microalgae. Biotechnol. Adv. 1988, 6, 725–770. [Google Scholar] [CrossRef]
- Prajapati, J.C.; Syed, H.S.; Chauhan, J. Removal of ammonia from wastewater by ion exchange technology. Int. J. Innov. Res. Technol. 2014, 1, 6–11. [Google Scholar]
- Margeta, K.; Zabukovec, N.; Siljeg, M.; Farkas, A. Natural Zeolites in Water Treatment—How Effective is Their Use. Water Treat. 2013, 5, 81–112. [Google Scholar]
- Mitch, W.A.; Sedlak, D.L. Formation of N-nitrosodimethylamine (NDMA) from dimethylamine during chlorination. Environ. Sci. Technol. 2002, 36, 588–595. [Google Scholar] [CrossRef]
- Siciliano, A.; Curcio, G.M.; Limonti, C. Experimental analysis and modeling of nitrate removal through zero-valent magnesium particles. Water 2019, 11, 1276. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W.; Park, J.; Kim, M. Substrate uptake, loss, and reserve in ammonia-oxidizing bacteria (AOB) under different substrate availabilities. Process Biochem. 2020, 91, 303–310. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W. Experimental investigation of substrate shock and environmental ammonium concentration on the stability of ammonia-oxidizing bacteria (AOB). Water 2020, 12, 223. [Google Scholar] [CrossRef]
- Ramavandi, B.; Mortazavi, S.B.; Moussavi, G.; Khoshgard, A.; Jahangiri, M. Experimental investigation of the chemical reduction of nitrate ion in aqueous solution by Mg/Cu bimetallic particles. React. Kinet. Mech. Catal. 2011, 102, 313–329. [Google Scholar] [CrossRef]
- Ahmadi, M.; Rahmani, H.; Ramavandi, B.; Kakavandi, B. Removal of nitrate from aqueous solution using activated carbon modified with Fenton reagents. Desalination Water Treat. 2017, 76, 265–275. [Google Scholar] [CrossRef]
- Kumar, L. Study of double breakpoints during chlorination of river Yamuna water, Delhi, India. Int. J. Water Resour. Environ. Eng. 2013, 5, 370–379. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Water Treatment Manuals: Coagulation, Flocculation & Clarification; EPA: Washington, DC, USA, 2002; ISBN 1840950900. [Google Scholar]
- Cheeke, P.R.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of yucca schidigera: A review. J. Inflamm. 2006, 3, 2–8. [Google Scholar] [CrossRef]
- Santacruz-Reyes, R.A.; Chien, Y.H. Efficacy of Yucca schidigera extract for ammonia reduction in freshwater: Effectiveness analysis and empirical modeling approach. Aquaculture 2009, 297, 106–111. [Google Scholar] [CrossRef]
- Yu, X.; Dimitriou, E.; Konstantinos, S.; Markogianni, V.; Politi, D. EFFECTS of yucca shidigera extract on the reduction of ammonia concentration in lake Koumoundourou. J. Ecol. Eng. 2015, 16, 1–7. [Google Scholar] [CrossRef][Green Version]
- Santacruz-Reyes, R.A.; Chien, Y.H. Yucca schidigera extract—A bioresource for the reduction of ammonia from mariculture. Bioresour. Technol. 2010, 101, 5652–5657. [Google Scholar] [CrossRef]
- Barot, N.S.; Bagla, H.K. Eco-friendly waste water treatment by cow dung powder (Adsorption studies of Cr(III), Cr(VI) and Cd(II) using tracer technique). Desalination Water Treat. 2012, 38, 104–113. [Google Scholar] [CrossRef]
- Umanu, G.; Nwachukwu, S.C.U.; Olasode, O.K. Effects of Cow Dung on Microbial Degradation of Motor Oil in Lagoon Water. Glob. J. Bio-Sci. Biotechnol. 2013, 2, 542–548. [Google Scholar]
- Quraishi, T.; Kenekar, A.; Ranadive, P.; Kamath, G. Evaluation of Performance of cow dung as Microbial Inoculum in Industrial Wastewater Treatment and its Environmental Implications. Indian J. Sci. Technol. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Randhawa, G.K.; Kullar, J.S. Bioremediation of Pharmaceuticals, Pesticides, and Petrochemicals with Gomeya/Cow Dung. ISRN Pharmacol. 2011, 2011, 362459. [Google Scholar] [CrossRef] [PubMed]
- Tanvi Godambe, M.H.F. Cow dung Bacteria offer an Effective Bioremediation for Hydrocarbon-Benzene. Int. J. Biotechnol. Trends Technol. 2016, 6, 13–20. [Google Scholar]
- Sawant, A.A.; Hegde, N.V.; Straley, B.A.; Donaldson, S.C.; Love, B.C.; Knabel, S.J.; Jayarao, B.M. Antimicrobial-resistant enteric bacteria from dairy cattle. Appl. Environ. Microbiol. 2007, 73, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Marler, L.; Allen, S.; Siders, J. Rapid enzymatic characterization of clinically encountered anaerobic bacteria with the API ZYM system. Eur. J. Clin. Microbiol. 1984, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Adebusoye, S.A.; Ilori, M.O.; Amund, O.O.; Teniola, O.D.; Olatope, S.O. Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World J. Microbiol. Biotechnol. 2007, 23, 1149–1159. [Google Scholar] [CrossRef]
- Akinde, S.B.; Obire, O. Aerobic heterotrophic bacteria and petroleum-utilizing bacteria from cow dung and poultry manure. World J. Microbiol. Biotechnol. 2008, 24, 1999–2002. [Google Scholar] [CrossRef]
- Singh, D.; Fulekar, M. Bioremediation of phenol using microbial consortium in bioreactor. Innov. Rom. Food Biotechnol. 2007, 1, 31–36. [Google Scholar]
- APHA; AWWA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Gulhane, H.; Nakanekar, A.; Mahakal, N.; Bhople, S.; Salunke, A. Gomutra (Cow Urine): A Multidimensional Drug Review Article. Int. J. Res. Ayurveda Pharm. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Dhama, K.; Rathore, R.; Chauhan, R.S.; Tomar, S. Panchgavya (Cowpathy): An Overview. Int. J. Cow Sci. 2005, 1, 1–17. [Google Scholar]
- Munshi, S.K.; Roy, J.; Noor, R. Microbiological investigation and determination of the antimicrobial potential of cow dung samples. Stamford J. Microbiol. 2018, 8, 34–37. [Google Scholar] [CrossRef]
- Thakare, A.; Ahmad, M.; Pande, K.; Metkari, S.; Engineering, C.; College, D.Y.P. Purification of Water by using Cow Dung Ash. Int. J. Eng. Technol. 2019, 6, 393–397. [Google Scholar]
- Ojedokun, A.T.; Bello, O.S. Sequestering heavy metals from wastewater using cow dung. Water Resour. Ind. 2016, 13, 7–13. [Google Scholar] [CrossRef]
- Verma, L. Indigenous Technology Knowledge for Watershed Management in Upper North-West Himalayas of India (PWMTA, 1998); FAO (UN): Kathmandu, Nepal, 1998. [Google Scholar]




| Microbial Enumeration of the cow dung Sample | |
|---|---|
| Parameters | Results |
| Total viable count | 2.29 × 108 cell/ml |
| Total Fungal count | 1.16 × 107 cell/ml |
| Yeast count | 7.5 × 106 cfu/ml |
| Physico-Chemical Characterization of a Cow Dung Sample | |
| pH | 7.3 |
| Dissolved Oxygen | 6.4 mg/L |
| Temperature | 25.9 °C |
| % Organic Carbon | 0.67% |
| Biological Oxygen Demand | 19.83 mg/L |
| Chemical Oxygen Demand | 195.2 mg/L |
| Phosphorous | 0.23 mg/L |
| Sulphate | 38.5 mg/L |
| Concentration of Phenol | Degradation % | Time Duration |
|---|---|---|
| 100 mg/L | 98.59 | 24 h |
| 250 mg/L | 99.4 | 72 h |
| 500 mg/L | 99.6 | 96 h |
| 1000 mg/L | Not degraded | up-to 168 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, L.; Sharma, J.; Kaur, R. Catalytic Performance of Cow-Dung Sludge in Water Treatment Mitigation and Conversion of Ammonia Nitrogen into Nitrate. Sustainability 2022, 14, 2183. https://doi.org/10.3390/su14042183
Kumar L, Sharma J, Kaur R. Catalytic Performance of Cow-Dung Sludge in Water Treatment Mitigation and Conversion of Ammonia Nitrogen into Nitrate. Sustainability. 2022; 14(4):2183. https://doi.org/10.3390/su14042183
Chicago/Turabian StyleKumar, Lokesh, Jaigopal Sharma, and Raminder Kaur. 2022. "Catalytic Performance of Cow-Dung Sludge in Water Treatment Mitigation and Conversion of Ammonia Nitrogen into Nitrate" Sustainability 14, no. 4: 2183. https://doi.org/10.3390/su14042183
APA StyleKumar, L., Sharma, J., & Kaur, R. (2022). Catalytic Performance of Cow-Dung Sludge in Water Treatment Mitigation and Conversion of Ammonia Nitrogen into Nitrate. Sustainability, 14(4), 2183. https://doi.org/10.3390/su14042183






