Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification
Abstract
:1. Introduction
2. Modeling and Simulation
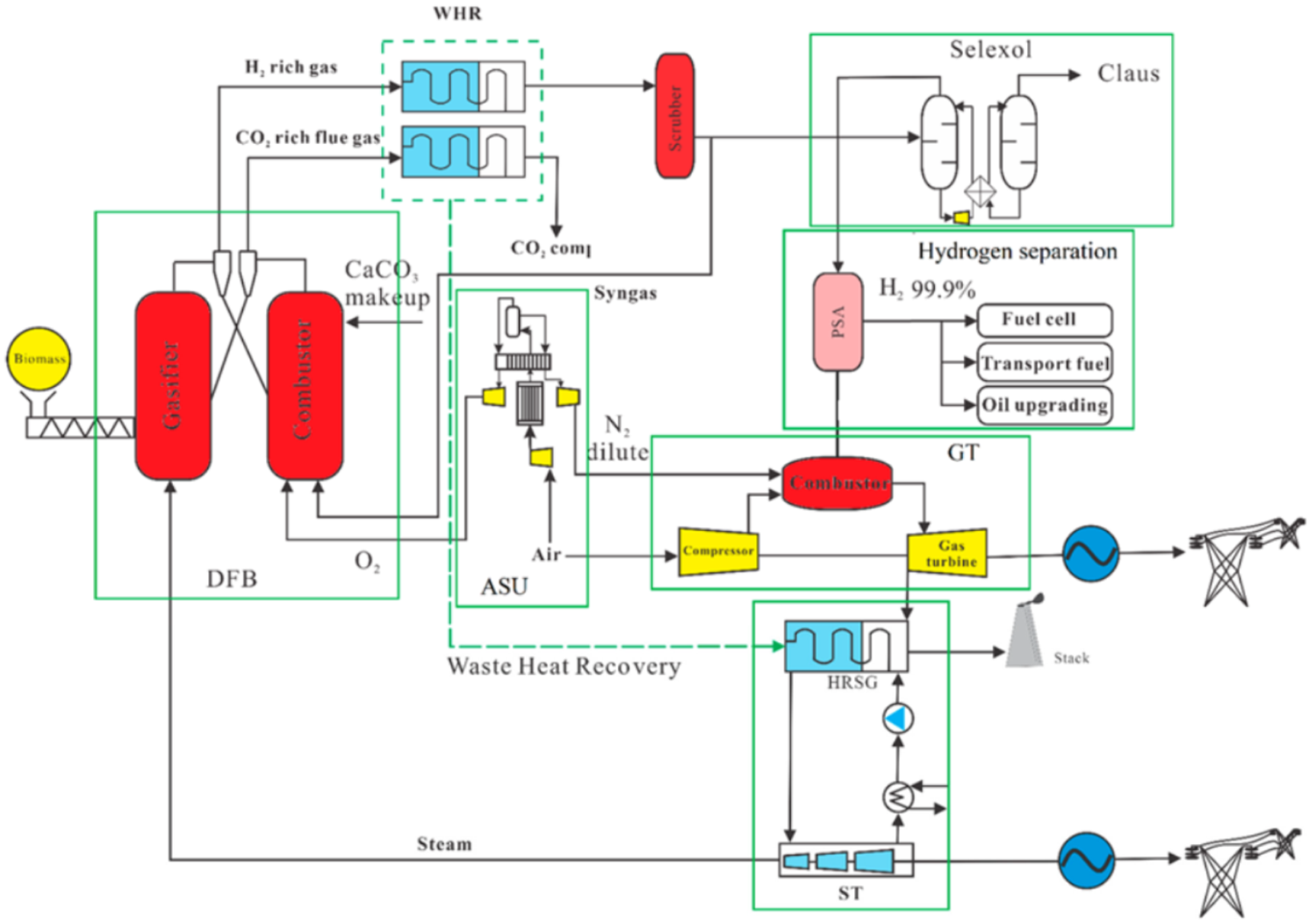
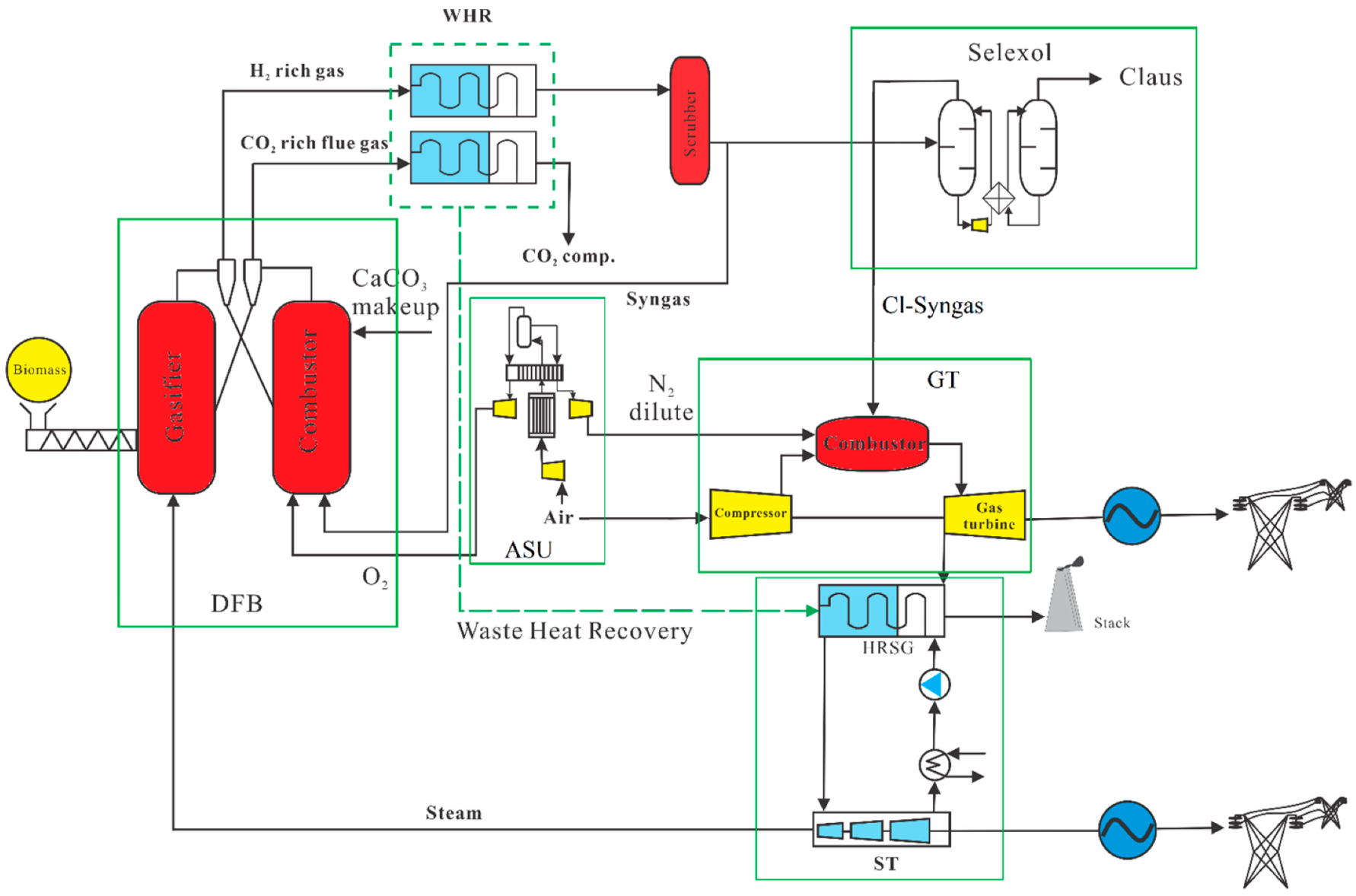
2.1. Hydrogen and Electricity Production by Calcium Looping Gasification Process
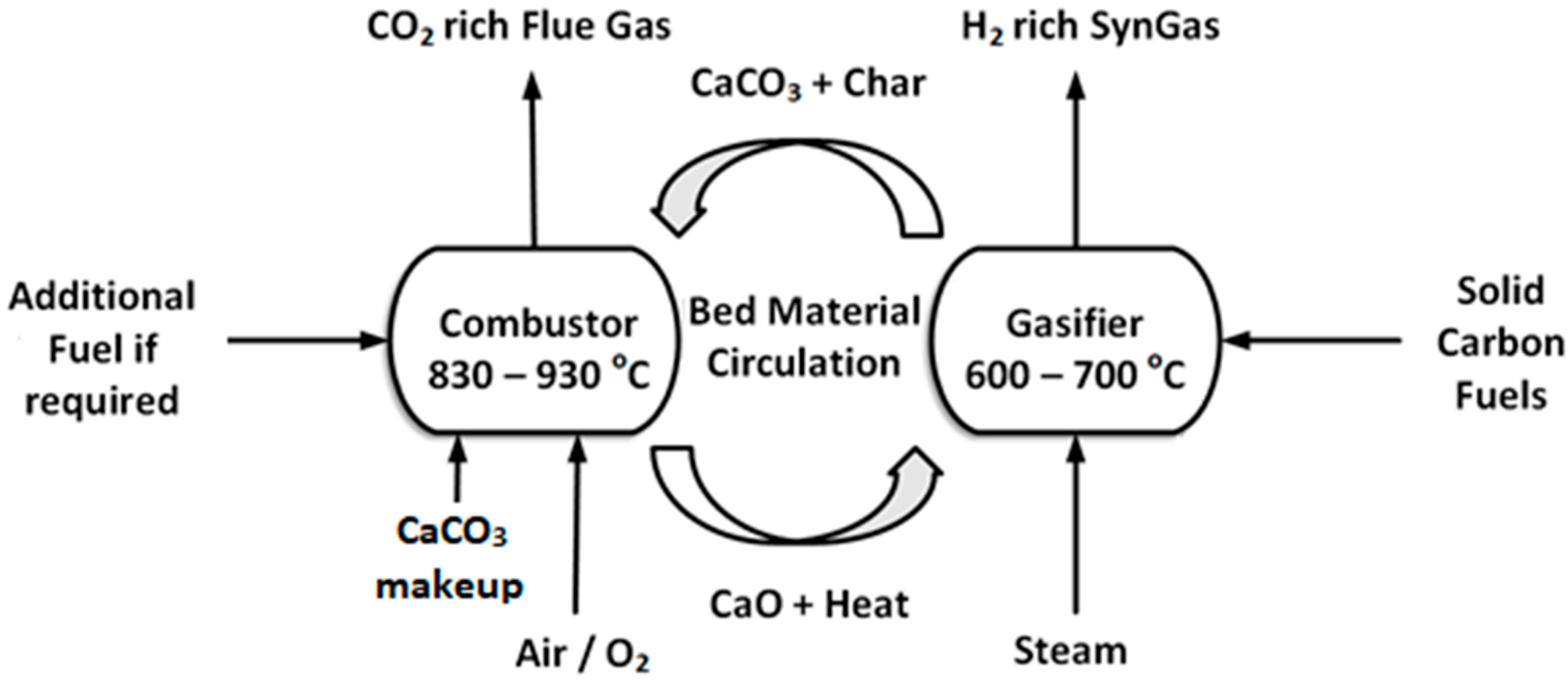
2.1.1. Dual Fluidized Bed Calcium Looping Gasification
2.1.2. Air Separation Unit
2.1.3. Heat Recovery and Water Scrubbing
2.1.4. Selexol Unit
2.1.5. Pressure Swing Adsorption (PSA) Unit
2.1.6. The Gas Turbine (GT) and Steam Turbine (ST)
3. Techno-Economic Evaluation
3.1. Thermodynamic Evaluation
3.2. Economic Evaluation
4. Results and Discussion
4.1. System Validation and Optimization
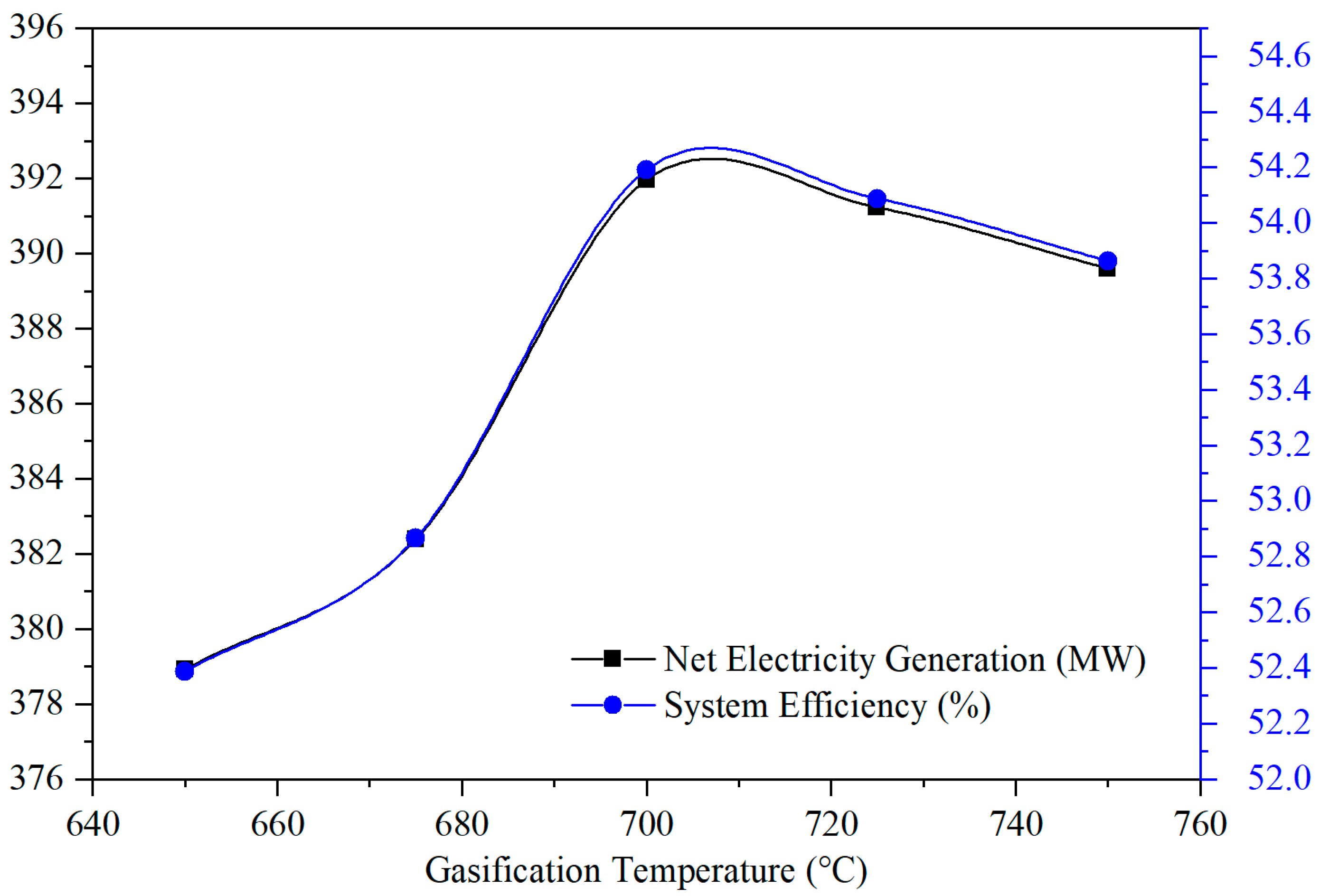
4.2. Effect of Gasifier Temperature on Hydrogen Yield and System Performance
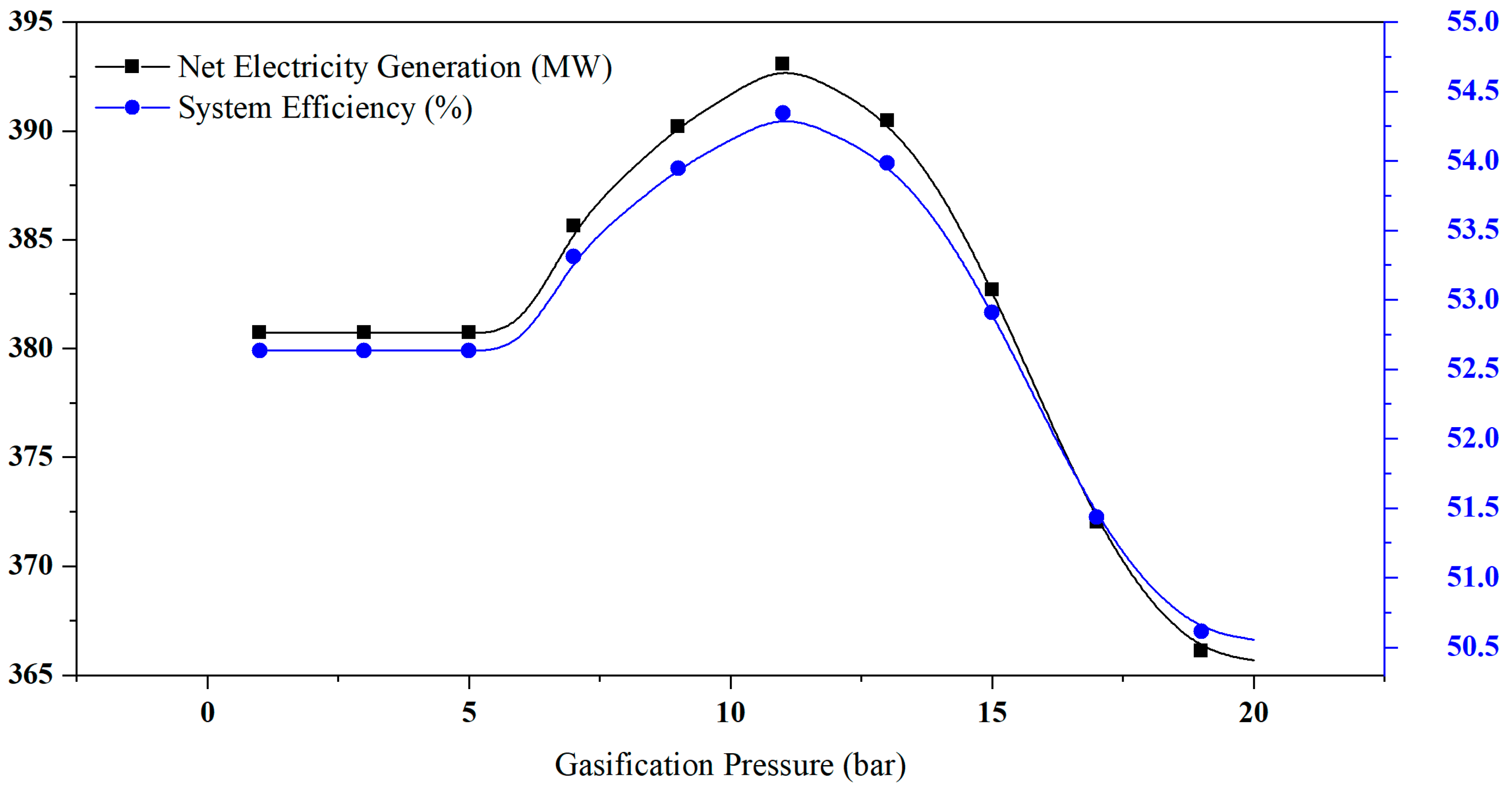
4.3. Effect of Gasifier Pressure on Hydrogen Yield and System Performance
4.4. System Energy Conversion Efficiency
4.5. Economic Analyses
4.6. Sensitivity Analysis
4.7. Uncertainty Sources of Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CLGCC−H | Calcium looping gasification with the combined cycle, for hydrogen |
| CLGCC | Calcium looping gasification with the combined cycle |
| IGCC | Integrated gasification with combined cycle |
| DFB | Dual fluidized bed |
| IRR | Internal rate of return |
| CCS | Carbon capture and storage |
| CLR | Chemical looping reform |
| ATR | Autothermal reform |
| SMR | Steam methane reform |
| CLC | Chemical looping combustion |
| OC | Oxygen carrier |
| ASU | Air separation unit |
| HRSG | Heat recovery steam generator |
| GT and ST | Gas turbine and steam turbine |
| PSA | Pressure swing absorption |
| FCI | Fixed capital investment |
| CEPCI | Chemical engineering plant cost index |
| NPV | Net present value. |
Nomenclature
| ɳ and Ɛ | System efficiency and exergy |
| Net power generation (MW) | |
| Power generation of steam turbines (MW) | |
| Power generation of gas turbines (MW) | |
| Power consumption of auxiliary equipment (MW) | |
| Lower calorific value of biomass (MW) | |
| FCI | Fixed capital investment (106 USD) |
| Si | Production capacity index |
| Ct | Cash flows of the year t (106 USD) |
| CRF | Average annual investment ratio |
| OAM | Annual operation and management cost ratio |
| Cp | Annual income of products (106 USD) |
| CM | Material cost (106 USD) |
| CF | Fuel cost (106 USD) |
References
- Yan, Y.; Manovic, V.; Anthony, E.J.; Clough, P.T. Techno-economic analysis of low-carbon hydrogen production by sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 226, 113530. [Google Scholar] [CrossRef]
- Yan, Y.; Thanganadar, D.; Clough, P.T.; Mukherjee, S.; Patchigolla, K.; Manovic, V.; Anthony, E.J. Process simulations of blue hydrogen production by upgraded sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 222, 113144. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen, IEA, Paris. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 10 July 2021).
- Esmaili, E.; Mostafavi, E.; Mahinpey, N. Economic assessment of integrated coal gasification combined cycle with sorbent CO2 capture. Appl. Energy 2016, 169, 341–352. [Google Scholar] [CrossRef]
- Sara, H.R.; Enrico, B.; Mauro, V.; Andrea, D.C.; Vincenzo, N. Techno-Economic Analysis of Hydrogen Production Using Biomass Gasification—A Small Scale Power Plant Study. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 806–813. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.P.; Gallachóir, B.P.Ó. The role of hydrogen in low carbon energy futures—A review of existing perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Capros, P.; Zazias, G.; Evangelopoulou, S.; Kannavou, M.; Fotiou, T.; Siskos, P.; De Vita, A.; Sakellaris, K. Energy-system modelling of the EU strategy towards climate-neutrality. Energy Policy 2019, 134, 110960. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Li, X.Y.; Tang, B.J. Incorporating the transport sector into carbon emission trading scheme: An overview and outlook. Nat. Hazards 2017, 88, 683–698. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Bourne, S. The future of fuel: The future of hydrogen. Fuel Cells Bull. 2012, 2012, 12–15. [Google Scholar] [CrossRef]
- Nazir, S.M.; Cloete, J.H.; Cloete, S.; Amini, S. Pathways to low-cost clean hydrogen production with gas switching reforming. Int. J. Hydrog. Energy 2020, 46, 20142–20158. [Google Scholar] [CrossRef]
- Spallina, V.; Pandolfo, D.; Battistella, A.; Romano, M.C.; Van Sint Annaland, M.; Gallucci, F. Techno-economic assessment of membrane assisted fluidized bed reactors for pure H2 production with CO2 capture. Energy Convers. Manag. 2016, 120, 257–273. [Google Scholar] [CrossRef]
- Spallina, V.; Shams, A.; Battistella, A.; Gallucci, F.; Annaland, M.V.S. Chemical Looping Technologies for H2 Production with CO2 Capture: Thermodynamic Assessment and Economic Comparison. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 419–428. [Google Scholar] [CrossRef]
- Khojasteh Salkuyeh, Y.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrog. Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Hydrogen production via chemical looping steam methane reforming process: Effect of cerium and calcium promoters on the performance of Fe2O3/Al2O3 oxygen carrier. Appl. Energy 2016, 165, 685–694. [Google Scholar] [CrossRef]
- Kathe, M.V.; Empfield, A.; Na, J.; Blair, E.; Fan, L.S. Hydrogen production from natural gas using an iron-based chemical looping technology: Thermodynamic simulations and process system analysis. Appl. Energy 2016, 165, 183–201. [Google Scholar] [CrossRef]
- Adiya, Z.I.S.G.; Dupont, V.; Mahmud, T. Steam reforming of shale gas in a packed bed reactor with and without chemical looping using nickel based oxygen carrier. Int. J. Hydrog. Energy 2018, 43, 6904–6917. [Google Scholar] [CrossRef]
- Adiya, Z.I.S.G.; Dupont, V.; Mahmud, T. Steam reforming of shale gas with nickel and calcium looping. Fuel 2019, 237, 142–151. [Google Scholar] [CrossRef]
- Chisalita, D.A.; Cormos, C.C. Techno-economic assessment of hydrogen production processes based on various natural gas chemical looping systems with carbon capture. Energy 2019, 181, 331–344. [Google Scholar] [CrossRef]
- Saithong, N.; Authayanun, S.; Patcharavorachot, Y.; Arpornwichanop, A. Thermodynamic analysis of the novel chemical looping process for two-grade hydrogen production with CO2 capture. Energy Convers. Manag. 2019, 180, 325–337. [Google Scholar] [CrossRef]
- Udomsirichakorn, J.; Salam, P.A. Review of hydrogen-enriched gas production from steam gasification of biomass: The prospect of CaO-based chemical looping gasification. Renew. Sustain. Energy Rev. 2014, 30, 565–579. [Google Scholar] [CrossRef]
- Ii, T.A.A.; Barton, P.I. Combining coal gasification and natural gas reforming for efficient polygeneration. Fuel Process. Technol. 2010, 92, 639–655. [Google Scholar] [CrossRef]
- Ahmed, U.; Kim, C.; Zahid, U.; Lee, C.-J.; Han, C. Integration of IGCC and methane reforming process for power generation with CO2 capture. Chem. Eng. Process Process Intensif. 2016, 111, 14–24. [Google Scholar] [CrossRef]
- Ahmed, U.; Zahid, U.; Lee, Y. Process simulation and integration of IGCC systems for H2 /syngas/electricity generation with control on CO2 emissions. Int. J. Hydrog. Energy 2019, 44, 7137–7148. [Google Scholar] [CrossRef]
- Cormos, C.C. Economic evaluations of coal-based combustion and gasification power plants with post-combustion CO2 capture using calcium looping cycle. Energy 2014, 78, 665–673. [Google Scholar] [CrossRef]
- Cormos, C.C. Biomass direct chemical looping for hydrogen and power co-production: Process configuration, simulation, thermal integration and techno-economic assessment. Fuel Process. Technol. 2015, 137, 16–23. [Google Scholar] [CrossRef]
- Jiang, P.; Berrouk, A.S.; Dara, S. Biomass Gasification Integrated with Chemical Looping System for Hydrogen and Power. Coproduction Process—Thermodynamic and Techno-Economic Assessment. Chem. Eng. Technol. 2019, 42, 1153–1168. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Potential for improving the energy efficiency of cryogenic air separation unit (ASU) using binary heat recovery cycles. Appl. Therm. Eng. 2015, 81, 223–231. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Atsonios, K.; Panopoulos, K.D.; Karellas, S.; Kakaras, E.; Karl, J. Modelling and assessment of acid gas removal processes in coal-derived SNG production. Appl. Therm. Eng. 2015, 74, 128–135. [Google Scholar] [CrossRef]
- Sun, L.; Smith, R. Rectisol wash process simulation and analysis. J. Clean. Prod. 2012, 39, 321–328. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Liu, F.; Weng, Y.; Ma, S.; Zhang, Y. Thermodynamic analysis and techno-economic assessment of synthetic natural gas production via ash agglomerating fluidized bed gasification using coal as fuel. Int. J. Hydrog. Energy 2020, 45, 27359–27368. [Google Scholar] [CrossRef]
- Li, K.; Wang, Q.; Fang, M.; Shaikh, A.R.; Xie, G.; Luo, Z. Techno-economic analysis of coal staged conversion polygeneration system for power and chemicals production. Chem. Eng. Technol. 2018, 42, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Piéplu, A.; Saur, O.; Lavalley, J.-C.; Legendre, O.; Nédez, C. Claus Catalysis and H2S Selective Oxidation. Catal. Rev. Sci. Eng. 1998, 40, 409–450. [Google Scholar] [CrossRef]
- Eow, J.S. Recovery of sulfur from sour acid gas: A review of the technology. Environ. Prog. 2002, 21, 143–162. [Google Scholar] [CrossRef]
- Riboldi, L.; Bolland, O. Evaluating Pressure Swing Adsorption as a CO2 separation technique in coal-fired power plants. Int. J. Greenh. Gas Control. 2015, 39, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.S.; Wang, L.Q. Energy and exergy analysis of gas production from biomass intermittent gasification. J. Renew. Sustain. Energy 2013, 5, 063141. [Google Scholar] [CrossRef]
- Seyitoglu, S.S.; Dincer, I.; Kilicarslan, A. Assessment of an IGCC based trigeneration system for power, hydrogen and synthesis fuel production. Int. J. Hydrog. Energy 2016, 41, 8168–8175. [Google Scholar] [CrossRef]
- Sweny, J.W. Synthetic Fuel Gas Purification by the SELEXOL Process. In Proceedings of the 65. Meeting of the American Chemical Society; Dallas, TX, USA, 8 April 1973. Available online: https://www.osti.gov/biblio/7346050 (accessed on 10 July 2021).
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill Book Company: New York, NY, USA, 2008. [Google Scholar]
- CEPCI August 2020. Available online: https://www.scribd.com/document/488333013/CEPCI-august-2020 (accessed on 16 July 2021).
- Cormos, A.M.; Cormos, C.C. Techno-economic assessment of combined hydrogen & power co-generation with carbon capture: The case of coal gasification. Appl. Therm. Eng. 2019, 147, 29–39. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Q.; Fang, M.; Luo, Z.; Cen, K. Thermodynamic and economic analysis of polygeneration system integrating atmospheric pressure coal pyrolysis technology with circulating fluidized bed power plant. Appl. Energy 2014, 113, 1301–1314. [Google Scholar] [CrossRef]
- Park, C.S. Contemporary Engineering Economics, 6th ed.; Pearson Education: London, UK, 2015. [Google Scholar]
- Ye, C.; Wang, Q.; Zheng, Y.; Li, G.; Zhang, Z.; Luo, Z. Techno-economic analysis of methanol and electricity poly-generation system based on coal partial gasification. Energy 2019, 185, 624–632. [Google Scholar] [CrossRef]
- Shi, B.; Xu, W.; Wu, W.; Kuo, P.-C. Techno-economic analysis of oxy-fuel IGCC power plants using integrated intermittent chemical looping air separation. Energy Convers. Manag. 2019, 195, 290–301. [Google Scholar] [CrossRef]
- Turton, R. Analysis, Synthesis, and Design of Chemical Processes, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 2013. [Google Scholar]
- Towler, G.; Sinnott, R.K. Chemical Engineering Design—Principles, Practice and Economics of Plant and Process Design, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Ulrich, G.D. A Guide to Chemical Engineering Process Design and Economics; John Wiley Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Fout, T.; Zoelle, A.; Keairns, D.; Turner, M.; Woods, M.; Kuehn, N.; Shah, V.; Chou, V.; Pinkerton, L. Cost and Performance Baseline for Fossil Energy Plants; National Energy Technology Laboratory: Pittsburgh, PA, USA; Morgantown, WV, USA, 2015. [Google Scholar]
- Exchange Rates. US Dollar to Chinese Yuan Spot Exchange Rates for 2019. Available online: https://www.exchangerates.org.uk/USD-CNY-spot-exchange-rates-history-2019.html (accessed on 16 July 2021).
- Han, L.; Wang, Q.; Luo, Z.; Rong, N.; Deng, G. H2 rich gas production via pressurized fluidized bed gasification of sawdust with in situ CO2 capture. Appl. Energy. 2013, 109, 36–43. [Google Scholar] [CrossRef]
- Müller, S.; Fuchs, J.; Schmid, J.C.; Benedikt, F.; Hofbauer, H. Experimental development of sorption enhanced reforming by the use of an advanced gasification test plant. Int. J. Hydrog. Energy 2017, 42, 29694–29707. [Google Scholar] [CrossRef]
- Udomsirichakorn, J.; Basu, P.; Abdul Salam, P.; Acharya, B. CaO-based chemical looping gasification of biomass for hydrogen-enriched gas production with in situ CO2 capture and tar reduction. Fuel Process. Technol. 2014, 127, 7–12. [Google Scholar] [CrossRef]
- Pfeifer, C.; Puchner, B.; Hofbauer, H. Comparison of dual fluidized bed steam gasification of biomass with and without selective transport of CO2. Chem. Eng. Sci. 2009, 64, 5073–5083. [Google Scholar] [CrossRef]
- Hanaoka, T.; Yoshida, T.; Fujimoto, S.; Kamei, K.; Harada, M.; Suzuki, Y.; Hatano, H.; Yokoyama, S.Y.; Minowa, T. Hydrogen production from woody biomass by steam gasification using a CO2 sorbent. Biomass Bioenergy 2005, 28, 63–68. [Google Scholar] [CrossRef]
- Han, L.; Wang, Q.; Yang, Y.; Yu, C.; Fang, M.; Luo, Z. Hydrogen production via CaO sorption enhanced anaerobic gasification of sawdust in a bubbling fluidized bed. Int. J. Hydrog. Energy 2011, 36, 4820–4829. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Lin, K.; Jia, X.; Zhang, H.; Zhong, Y.; Wang, Q.; Li, Z. Developing a Novel CaO-Based Sorbent for Promoted CO2 Capture and Tar Reduction. Energy Fuels 2017, 31, 5306–5317. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kajiwara, D.; Kuramoto, K.; Sharma, A.; Suzuki, Y. Factors affecting steam gasification rate of low rank coal char in a pressurized fluidized bed. Fuel Process. Technol. 2009, 90, 895–900. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wires Rev. Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Shangyi, Y.; Baosheng, J.; Wenqi, Z.; Yong, L.; Yingjuan, S.; Hao, L. Experimental research of gas-solid flow behaviors in pressurized circulating fluidized bed—I: Solid holdup distribution. J. Southeast Univ. 2012, 42, 308–312. [Google Scholar]
- Wang, Q.; Rong, N.; Fan, H.; Meng, Y.; Fang, M.; Cheng, L.; Cen, K. Enhanced hydrogen-rich gas production from steam gasification of coal in a pressurized fluidized bed with CaO as a CO 2 sorbent. Int. J. Hydrog. Energy 2014, 39, 5781–5792. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, J.; Caldeira, C.; Corrado, S.; Sala, S. Techno-economic and profitability analysis of food waste biorefineries at European level. Bioresour. Technol. 2018, 259, 244–252. [Google Scholar] [CrossRef] [PubMed]






| Unit/Component/System | Modeling Unit | Parameters |
|---|---|---|
| Gasification Reactor | RYield, RGibbs (Reactor) | Biomass flow rate = 131.16 ton/h |
| Temperature: 700 °C | ||
| Pressure: 12 bar | ||
| Combustor Reactor | RGibbs (Reactor) | Temperature: 1000 °C |
| Pressure: 12 bar | ||
| Air Separation Unit (ASU) | HeatX, Compr | O2 = 94.5% (vol) |
| Power consumption: 235 kW/t | ||
| H2S removal Section | RadFrac | Selexol solvent |
| H2S removal ~99.9% | ||
| Absorber and regeneration stages: 12 each with thermal regeneration. | ||
| PSA | Sep2 | Hydrogen separation ~99.99% |
| Power consumption: 5.63 kW/ft3/m | ||
| Pump Efficiency | Pump | 85% |
| Compressor Efficiency | Compr | 82% |
| Steam Turbine Eff (Isentropic) | Compr | 87.50% |
| Gas Turbine Eff (Adiabatic) | Compr | TIT (Turbine inlet temp) = ~1550 °C |
| TOT (Turbine outlet temp) = ~570 °C | ||
| Adiabatic efficiency = 85% | ||
| HRSG | HeatX, Compr | 124/40/20/0.069 (bars) |
| ΔTmin = 10 °C |
| Pine Sawdust | ||
|---|---|---|
| Ultimate analysis | C | 52.53 |
| H | 6.08 | |
| O | 39.04 | |
| N | 0.09 | |
| S | 0.55 | |
| A | 1.70 | |
| Proximate analysis | V | 85.15 |
| FC | 13.15 | |
| A | 1.70 | |
| LHV (kJ/kg) | 19,853 | |
| Moisture | 2.07 |
| Facility | Parameter | Unit | Value |
|---|---|---|---|
| Air Compressor | Outlet Pressure | bar | 21.2 |
| GT | Inlet Temperature | °C | 1550 |
| Outlet Temperature | °C | 570 | |
| Steam Turbine | Inlet Temperature of HP | °C | 520 |
| Inlet Pressure of HP | bar | 124 | |
| Reheating Temperature | °C | 520 | |
| Reheating Pressure | bar | 40 | |
| Inlet Temperature of IP | °C | 373.55 | |
| Inlet Pressure of IP | bar | 20 | |
| Outlet Pressure of LP | bar | 0.069 | |
| Pump | Outlet Pressure | bar | 124 |
| Components | Basic Cost (106 USD in 2019) * | Basic Scale | Scale Factor | Installation Factor | Reference |
|---|---|---|---|---|---|
| Biomass preparation (kg s−1) | 21.11 | 23.15 | 0.7 | 1 | [47] |
| Gasifier (dual fluidized bed) biomass input (MW) | 559.27 | 1198.03 | 0.6 | 1 | [28] |
| ASU (O2 kg s−1) | 134.93 | 53.36 | 0.6 | 1 | [47] |
| Selexol (t d−1 sulfur input) | 46.91 | 81 | 0.7 | 1 | [34] |
| Claus/SCOT (t d−1 sulfur input) | 32.51 | 81 | 0.7 | 1 | [34] |
| H2 PSA (kmol H2 h−1) | 17.79 | 16,616 | 0.65 | 2.56 | [44] |
| Heat exchanger (MWth) | 13.34 | 138 | 0.6 | 5 | [48,49,50] |
| Power island | |||||
| Gas turbine (net MW) | 118.36 | 276 | 0.75 | 1 | [44] |
| Steam turbine and cycle (gross MW) | 57.54 | 275 | 0.67 | 2 | [48,49,50] |
| CO2 compression unit (m3 h−1) | 76.76 | 430,850.77 | 0.67 | 1 | [51] |
| Facility | Parameter | CLGCC–H | CLGCC |
|---|---|---|---|
| Fuel input | |||
| Input pulverized biomass (kg/s) | 36.43 | 36.43 | |
| LHV (MJ/kg) | 19.85 | 19.85 | |
| Thermal input (MW) | 723.31 | 723.31 | |
| Gasifier | |||
| Outlet pressure (bar) | 12.00 | 12.00 | |
| Outlet temperature (°C) | 700.00 | 700.00 | |
| Steam (kg/s) | 26.25 | 26.25 | |
| Syngas mass flow (kg/s) | 23.21 | 23.21 | |
| Syngas LHV (MJ/kg) | 24.26 | 24.26 | |
| Thermal output (MW) | 562.98 | 562.98 | |
| Combustor | |||
| Outlet pressure (bar) | 12 | 12 | |
| Outlet temperature (°C) | 1000 | 1000 | |
| Oxygen mass flow (kg/s) | 14.91 | 14.91 | |
| CO2-rich mass flow (kg/s) | 53.79 | 53.79 | |
| Scrubber column | |||
| Pressure syngas (bar) | 11.7 | 11.7 | |
| Outlet temperature syngas (°C) | 45 | 45 | |
| H2S absorber | |||
| Outlet temperature syngas (°C) | 11.92 | 11.92 | |
| Mass flow syngas (kg/s) | 12.8 | 12.8 | |
| Syngas LHV (MJ/kg) | 41.13 | 41.13 | |
| PSA | |||
| Hydrogen (kg/h) | 10,284.57 | - | |
| Hydrogen LHV (MJ/kg) | 119.90 | - | |
| Hydrogen energy output (MW) | 342.54 | - | |
| Power block | |||
| Air compressor discharge (bar) | 20 | 20 | |
| Combustion chamber (bar) | 19.02 | 19.02 | |
| Gas turbine inlet temperature (°C) | 1502 | 1502 | |
| Gas turbine pressure ratio | 18.65 | 18.65 | |
| Gas turbine power output (MWe) | 107.82 | 317.76 | |
| Steam cycle power output (MWe) | 39.73 | 95.17 | |
| Power output | |||
| Gross power output (MW) | 490.09 | 412.93 | |
| Auxiliary load (MW) | 21.76 | 20.97 | |
| Net power output (MW) | 468.33 | 391.96 | |
| Net efficiency (%) | 64.75 | 54.19 | |
| Exergy efficiency (%) | 60.79 | 51.22 | |
| ηCCS (%) | 83.12 | 83.12 |
| Component | Basis |
|---|---|
| (A) Raw material | Pine sawdust 65.12 USD/t |
| (B) Utilities | Water 0.7 USD/t; electricity 0.07 USD/kWh |
| (C) Operating and maintenance | |
| (1) Operating labor + welfare | 200 laborers (45,000 USD/laborer/year) + 10% operating labor |
| (2) Direct supervisory and clerical labor | 20% of operating labor |
| (3) Maintenance and repairs | 2% of fixed capital investment |
| (4) Operating supplies | 0.7% of fixed capital investment |
| (5) Laboratory charge | 15% of operating labor |
| (D) Depreciation | Salvage value: 4%, life period: 30 years |
| (E) Interest and surcharge | According the landing schedule |
| (F) Plant overhead cost | 60% of (1) + (2) + (3) |
| (G) Administrative cost | 2% of total production cost |
| (H) Distribution and selling cost | 2% of total production cost |
| (I) Total production cost | (A) + (B) + (C) + (D) + (E) + (F) + (G) + (H) |
| Items | Value | Price | Value |
|---|---|---|---|
| Discount rate | 8% | Biomass | 65.12 USD/t |
| Plant life | 30 years | Water | 0.76 USD/t |
| Yearly running time | 8000 h | Electricity | 0.07 USD/kWh |
| Establishment period | 3 years | Sulfur | 115 USD/t |
| Tax | 25% | Limestone | 19.97 USD/t |
| Interest | 7.53% |
| Items | CLGCC–H | CLGCC |
|---|---|---|
| Fuel and material consumption (106 t/y) | ||
| Biomass | 1.05 | 1.05 |
| Water | 1.24 | 1.24 |
| Limestone (t/y) | 6299 | 6299 |
| Product | ||
| Sulfur (t/y) | 5681.83 | 5681.83 |
| Hydrogen (99.99 vol% purity) (kg/y) | 8.74 × 107 | - |
| Electricity (kWh/y) | 1.18 × 109 | 3.30 × 109 |
| CO2 | 1.55 × 106 | 1.55 × 106 |
| Capital cost (106) | ||
| Fuel preparation | 29 | 29 |
| DFB (including gasifier, combustor, cyclones, fans, other facilities) | 413.18 | 413.18 |
| Air separation unit (ASU) | 73.32 | 73.32 |
| Selexol unit | 15.76 | 15.76 |
| H2 PSA unit | 21.14 | - |
| Claus/SCOT unit | 10.92 | 10.92 |
| Heat exchangers | 26.03 | 30.38 |
| Power island (GT+ST) | 89.97 | 188.08 |
| CO2 compression | 10.35 | 10.35 |
| Fixed capital cost | 689.66 | 770.99 |
| Working capital | 137.93 | 154.20 |
| Total capital investment | 827.60 | 925.18 |
| Raw material | 68.46 | 68.46 |
| Utilities | 15.87 | 15.55 |
| Operating and maintenance | 30.86 | 33.09 |
| Depreciation | 23.29 | 26.07 |
| Intrest and surcharges | 47.24 | 54.08 |
| Plant overhead cost | 14.81 | 15.80 |
| Administrative cost | 3.26 | 3.38 |
| Distribution and selling cost | 3.26 | 3.38 |
| Total production cost | 207.05 | 219.80 |
| Hydrogen production cost a (USD/kg) | 2.37 | - |
| Revenue b (106 USD] | 290.56 | 231.74 |
| Profit before tax c (106 USD) | 83.51 | 11.94 |
| Tax rate | 25% | 25% |
| Profit after tax (106 USD/year) | 62.63 | 8.96 |
| IRR (%) | 17.43 | 11.45 |
| NPV (106 USD) | 677.67 | 267.42 |
| Payback Period (y) | 7.35 | 9.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, A.R.; Wang, Q.; Han, L.; Feng, Y.; Sharif, Z.; Li, Z.; Cen, J.; Kumar, S. Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. Sustainability 2022, 14, 2189. https://doi.org/10.3390/su14042189
Shaikh AR, Wang Q, Han L, Feng Y, Sharif Z, Li Z, Cen J, Kumar S. Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. Sustainability. 2022; 14(4):2189. https://doi.org/10.3390/su14042189
Chicago/Turabian StyleShaikh, Abdul Rahim, Qinhui Wang, Long Han, Yi Feng, Zohaib Sharif, Zhixin Li, Jianmeng Cen, and Sunel Kumar. 2022. "Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification" Sustainability 14, no. 4: 2189. https://doi.org/10.3390/su14042189







