Reducing the Environmental Impacts of Desalination Reject Brine Using Modified Solvay Process Based on Calcium Oxide
Abstract
:1. Introduction
2. Materials and Method
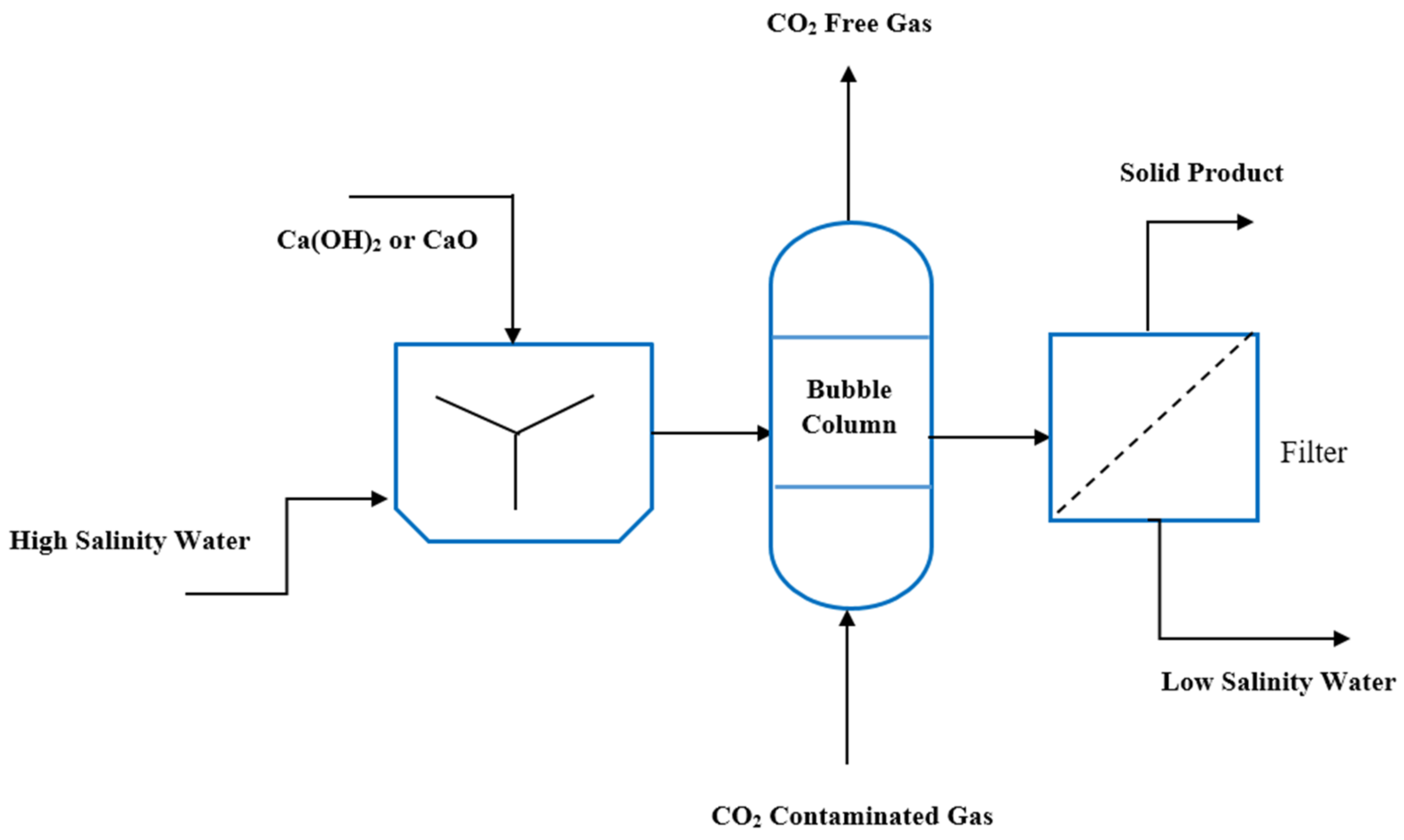
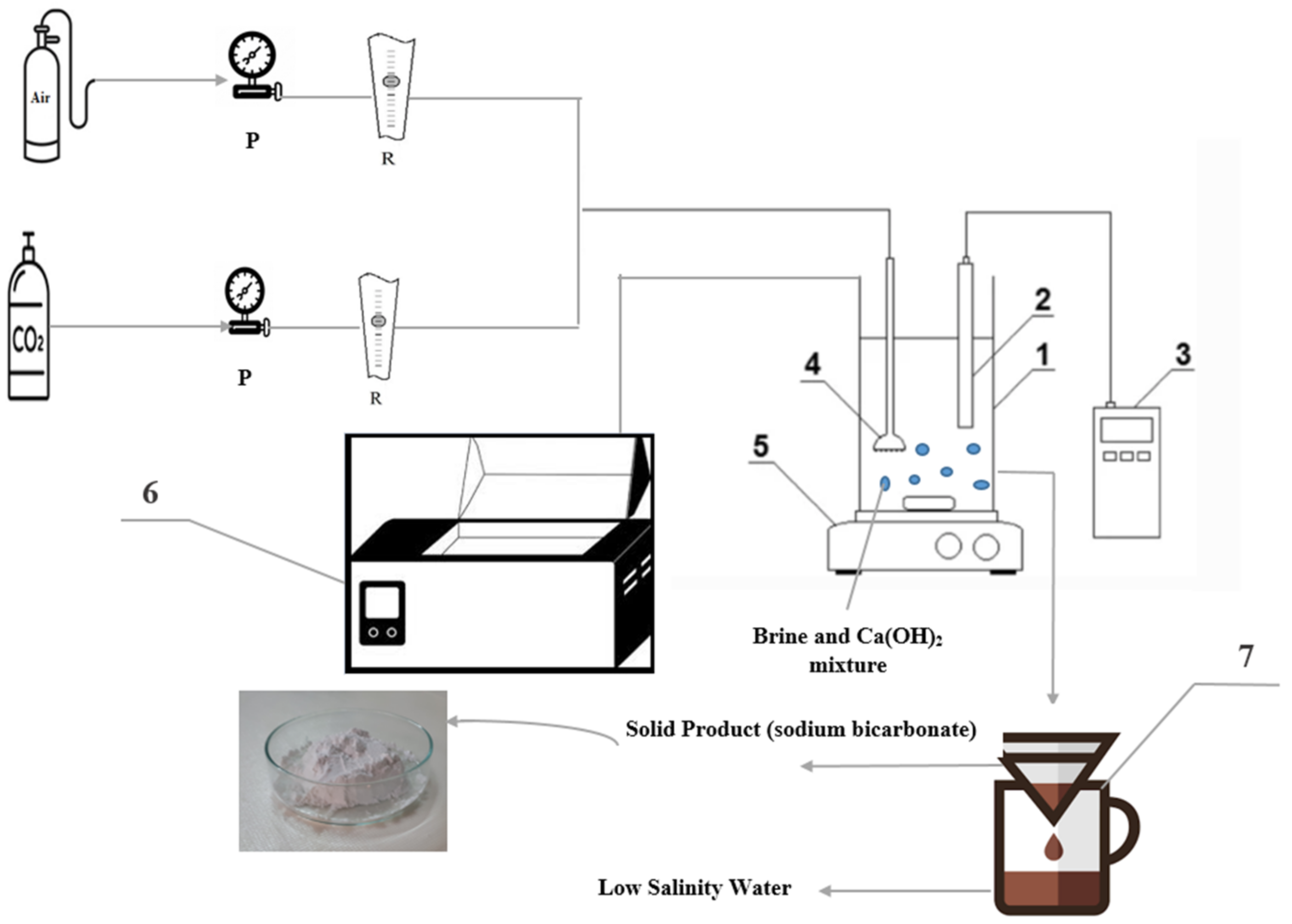
2.1. Experimental Setup
2.2. Experimental Materials
2.3. Design of Experiment for Modified Solvay Process
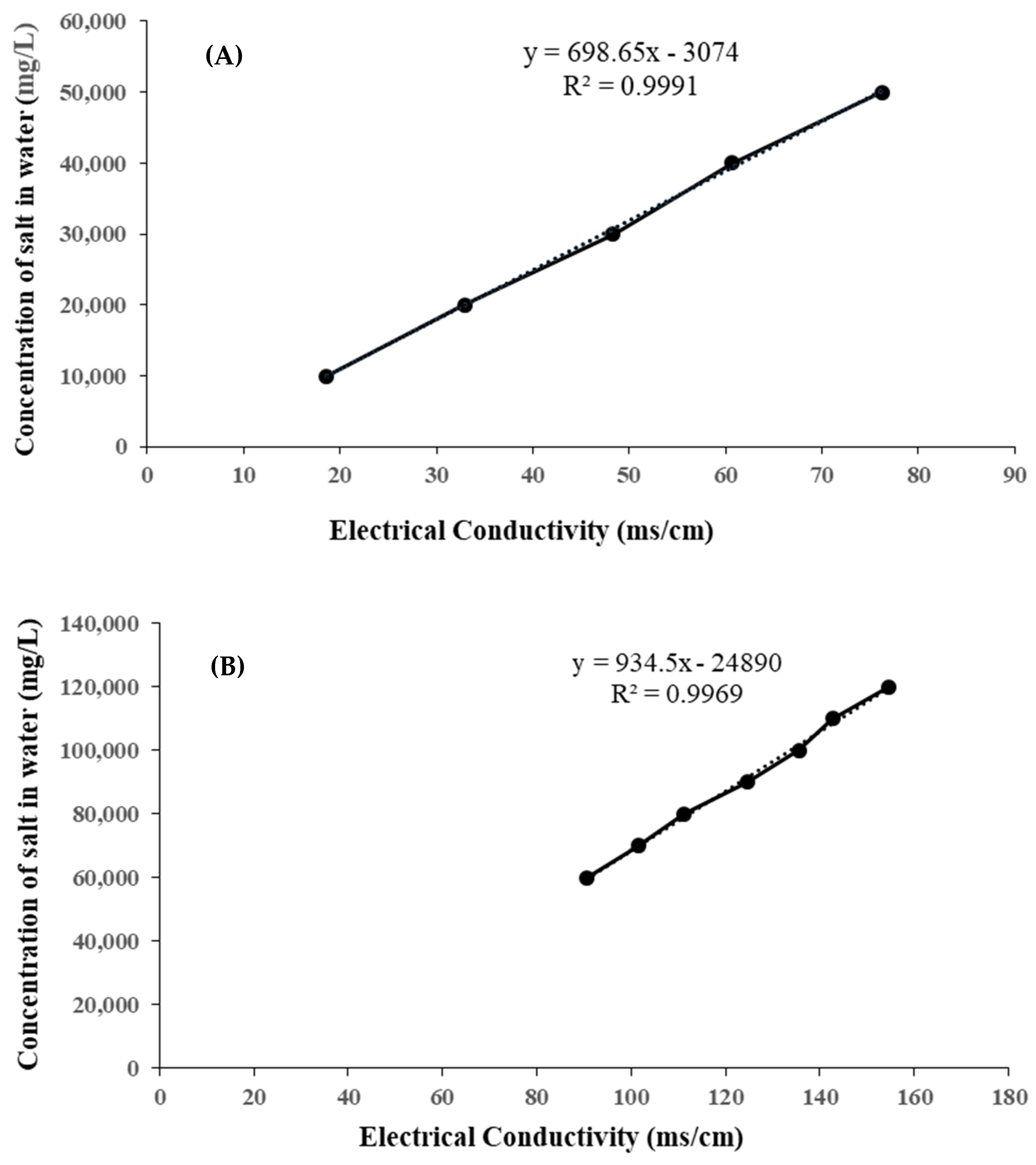
2.4. Drawing a Calibration Chart and Converting Electrical Conductivity to Total Soluble Solids
2.5. Determination of the Optimal Reaction Conditions
3. Results and Discussion
3.1. Analysis of the Central Composite Design (CCD)
−0.000064 X2 × X2 − 0.04721 X4 × X4 + 0.04582 X1 × X4
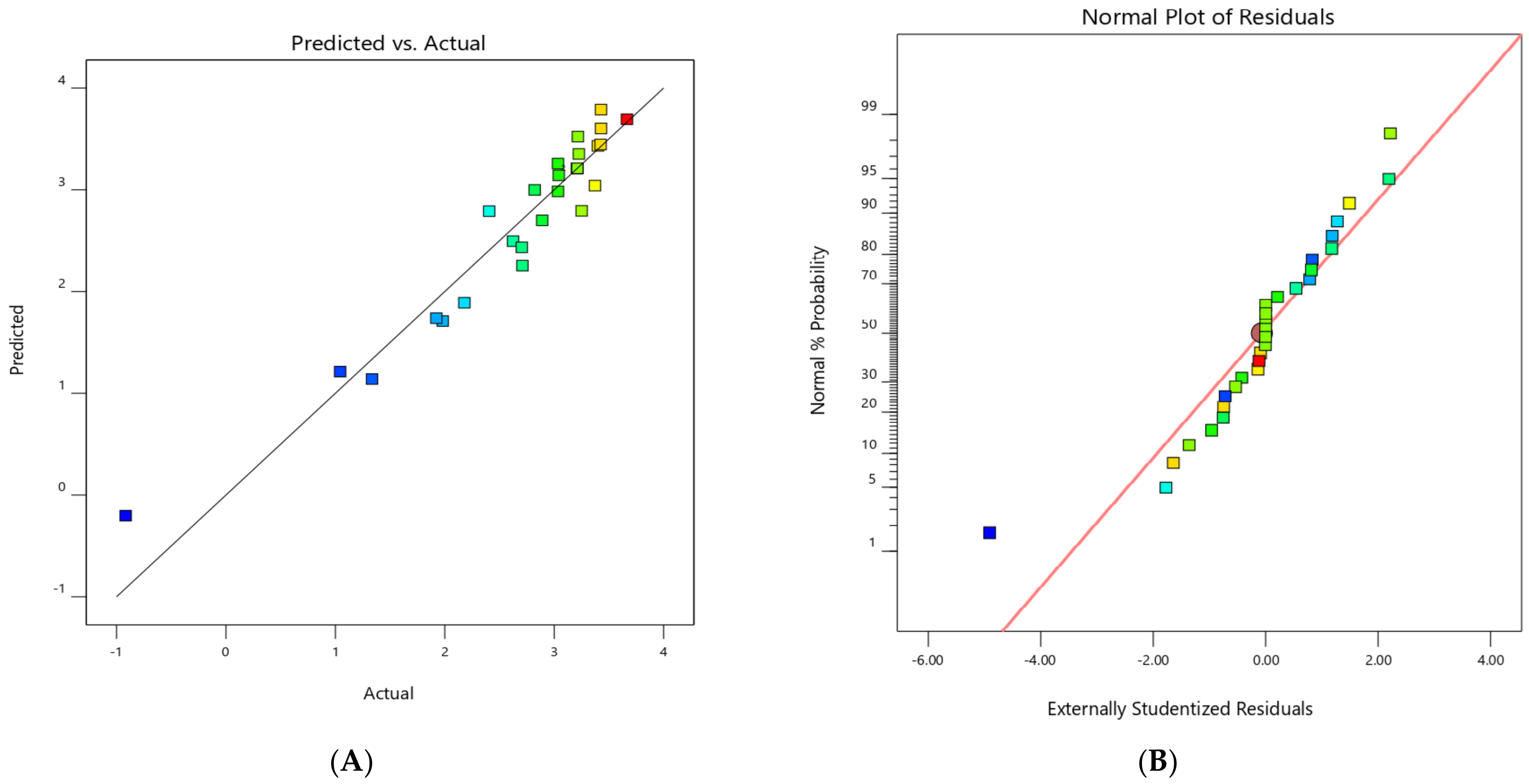
3.2. Evaluating the Suggested Model’s Precision and Validity
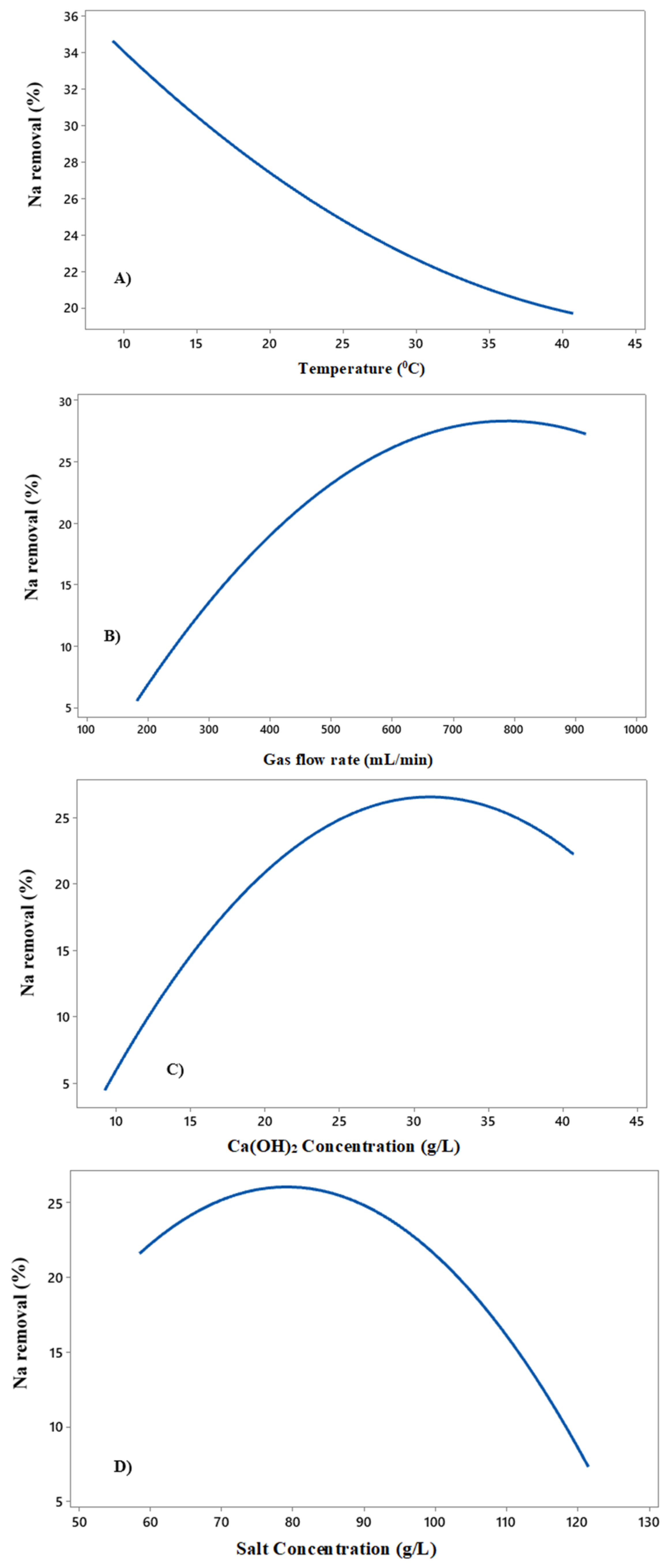
3.3. The Influence of Main Factors in the Modified Solvay Process
3.3.1. The Effect of Temperature on the Reaction
3.3.2. The Influence of the Rate of Gas Flow on the Reaction
3.3.3. The Effect of Calcium Hydroxide Concentration on the Reaction
3.3.4. The Effect of Sodium Chloride Concentration
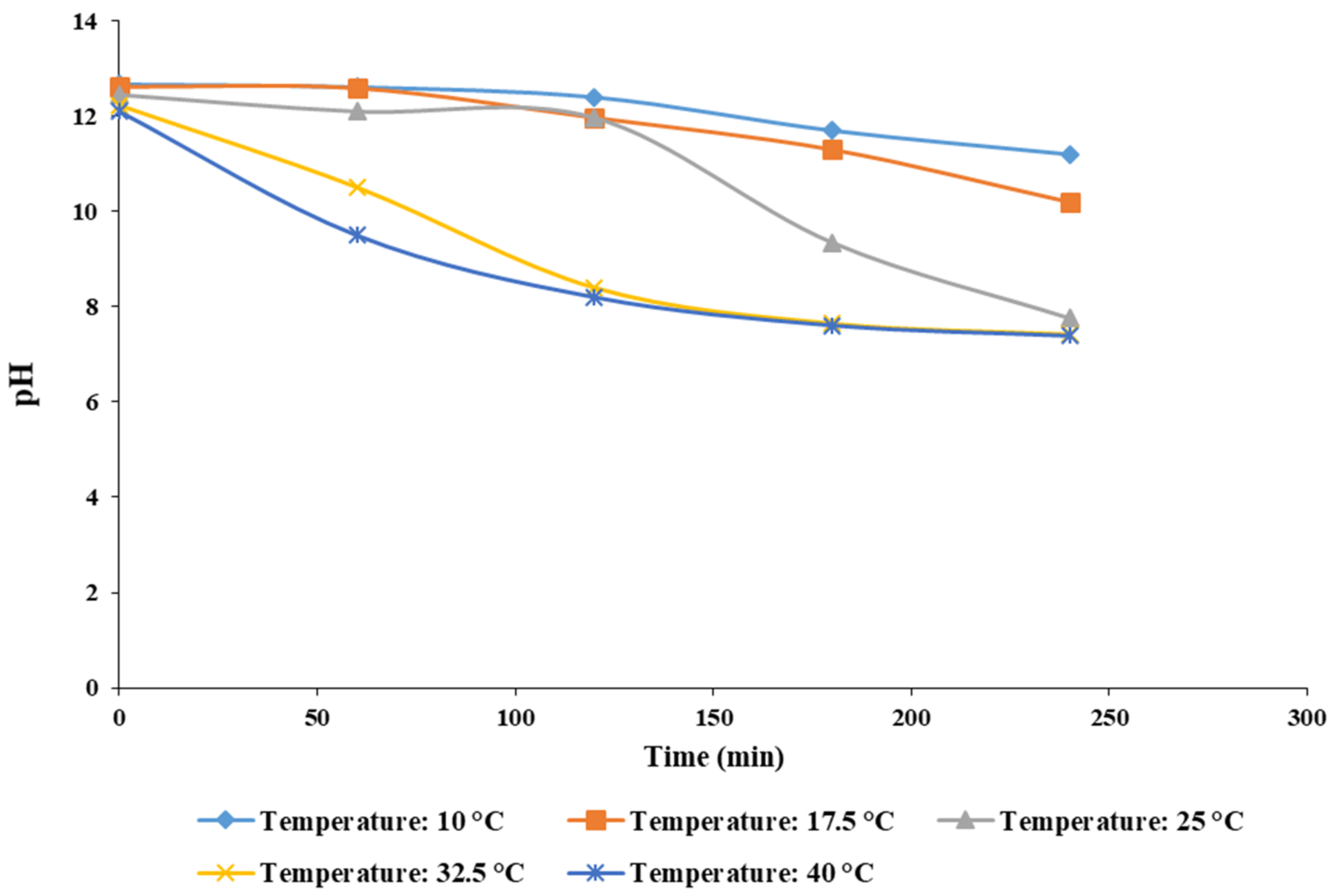
3.3.5. Effect of Reaction Temperature on pH
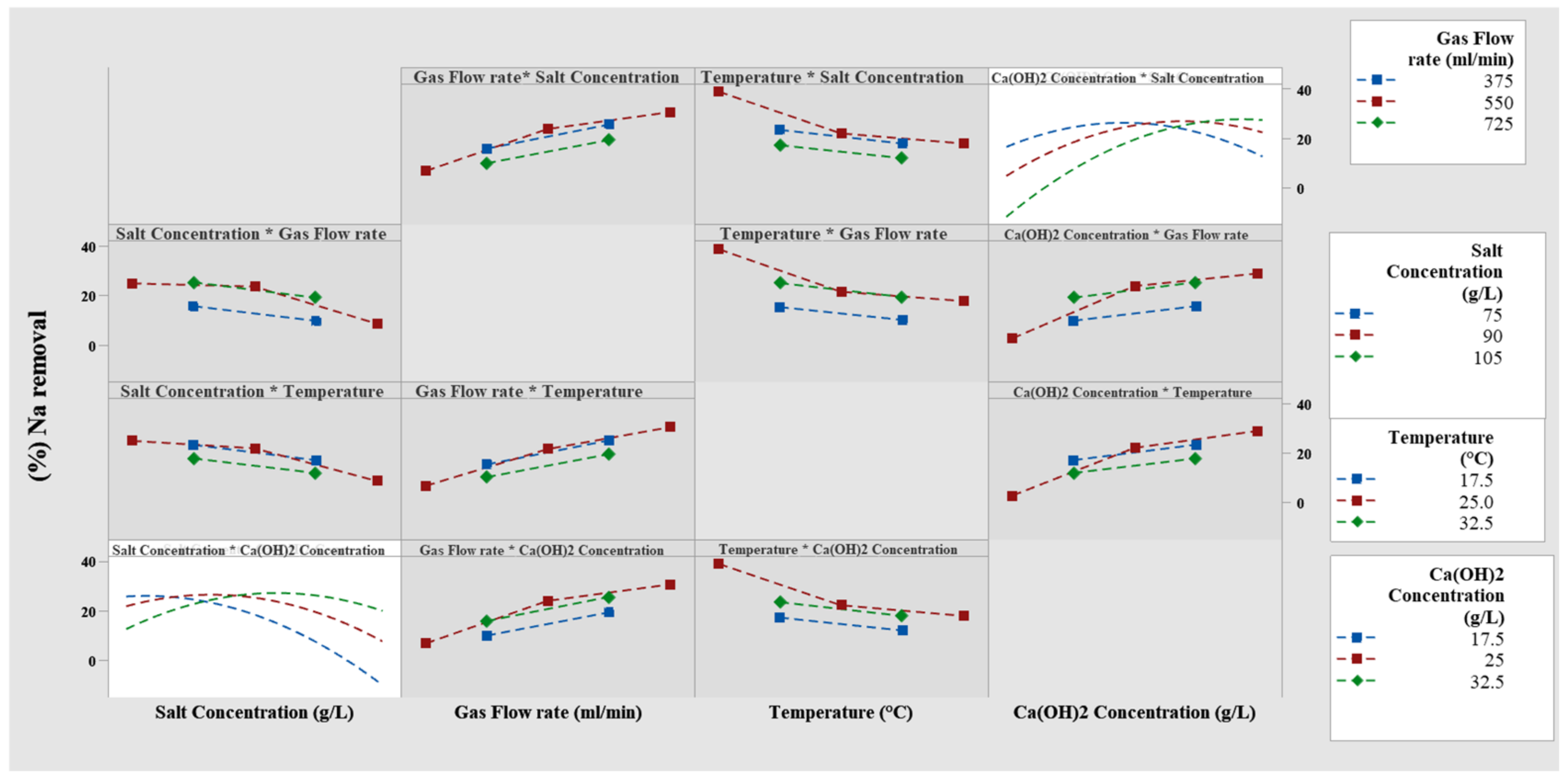
3.4. The Effect of Interaction between Factors in the Reaction
3.4.1. Investigation of the Charts of Interaction Effects between Factors
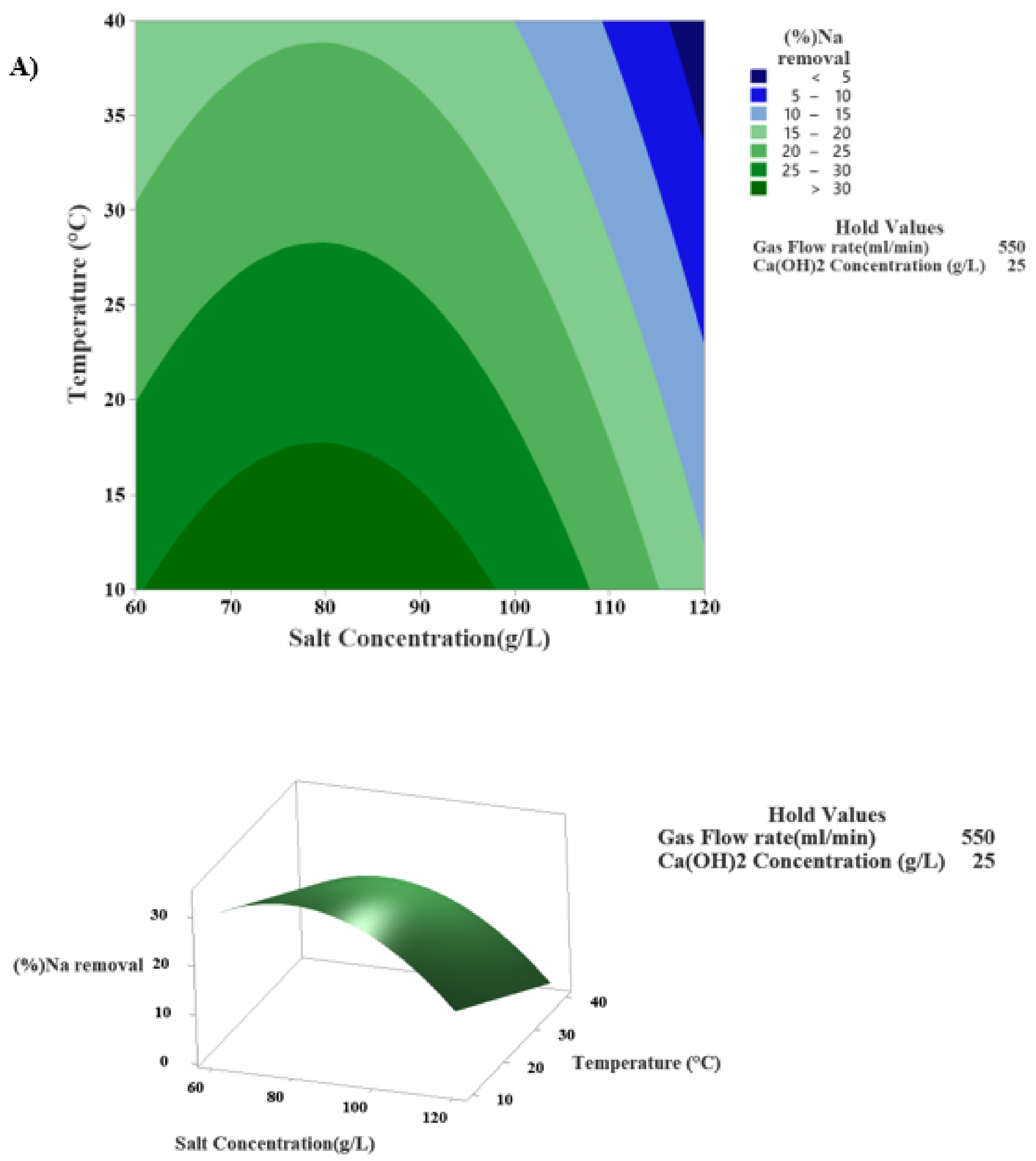
3.4.2. Investigation of the Interaction Effect of Temperature and Sodium Chloride Concentration in the Modified Solvay Process for Sodium Removal
3.4.3. The Interaction Effects of Temperature and the Rate of Gas Flow in the Modified Solvay Process on Sodium Removal
3.4.4. The Interaction Effect of Sodium Chloride Concentration and Gas Flow Rate in Modified Solvay Process on Sodium Removal
3.4.5. The Interaction Effect of Sodium Chloride and Calcium Hydroxide Concentrations in the Modified Solvay Process on Sodium Removal
3.4.6. The Interaction Effect of Calcium Hydroxide Concentration and Temperature in the Modified Solvay Process on Sodium Removal
3.4.7. The Interaction Effect of Calcium Hydroxide Concentration and Gas Flow Rate in the Modified Solvay Process on Sodium Removal
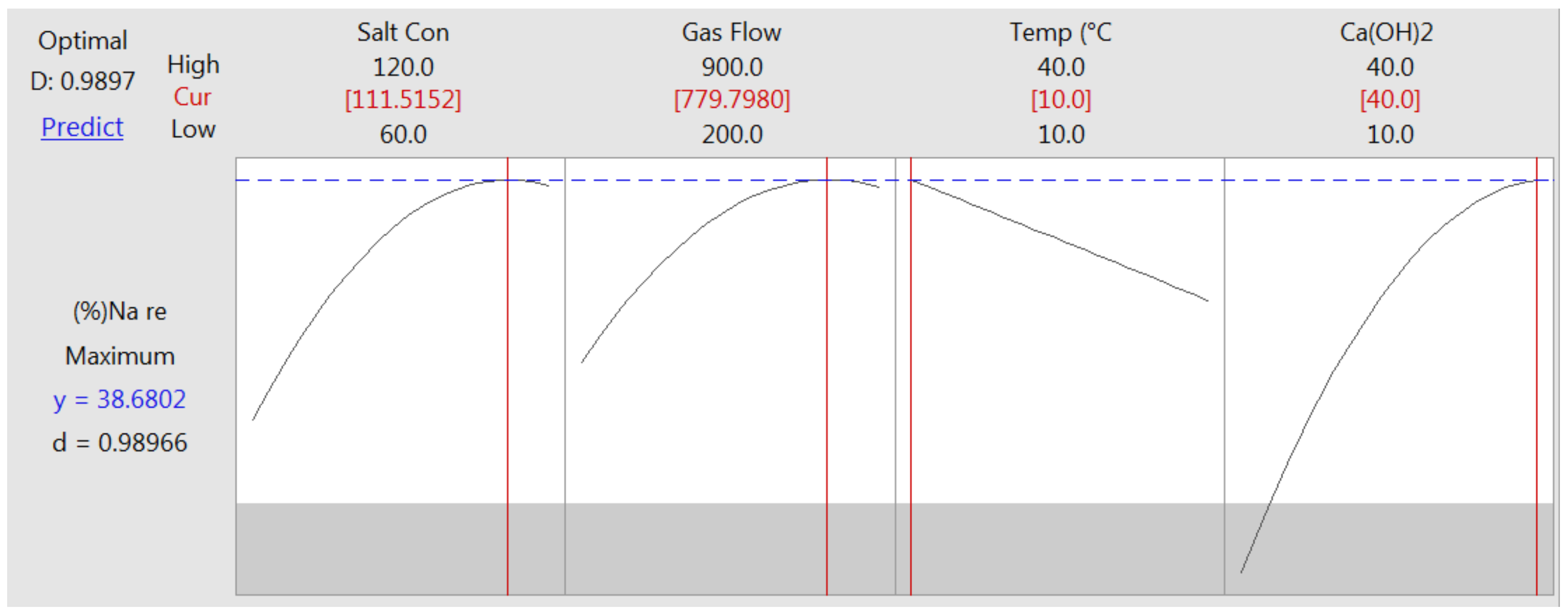
3.5. Determining the Optimal Conditions for More Effective Removal of Sodium
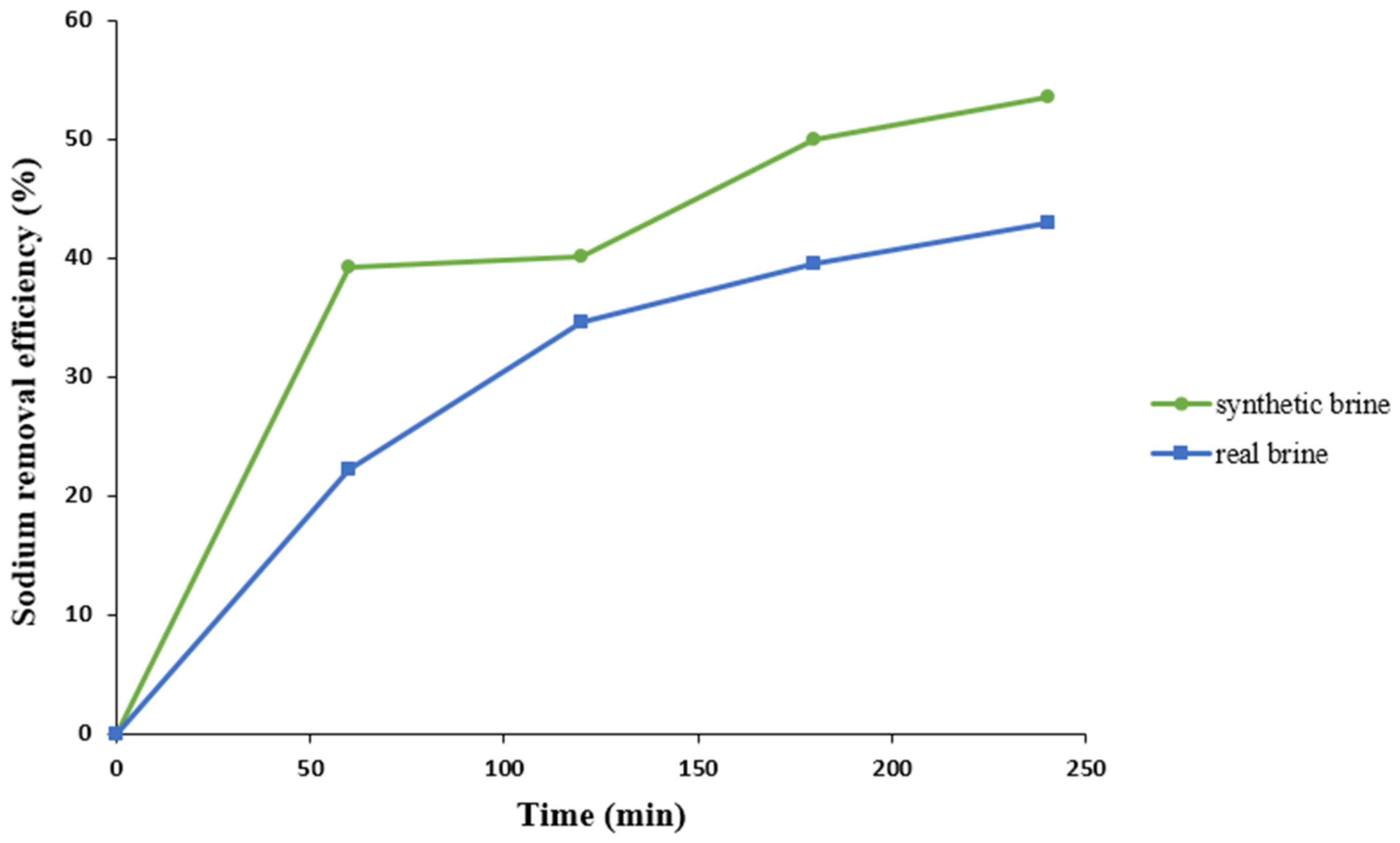
3.5.1. Sodium Removal Efficiency in Synthetic Brine under Optimum Operating Conditions
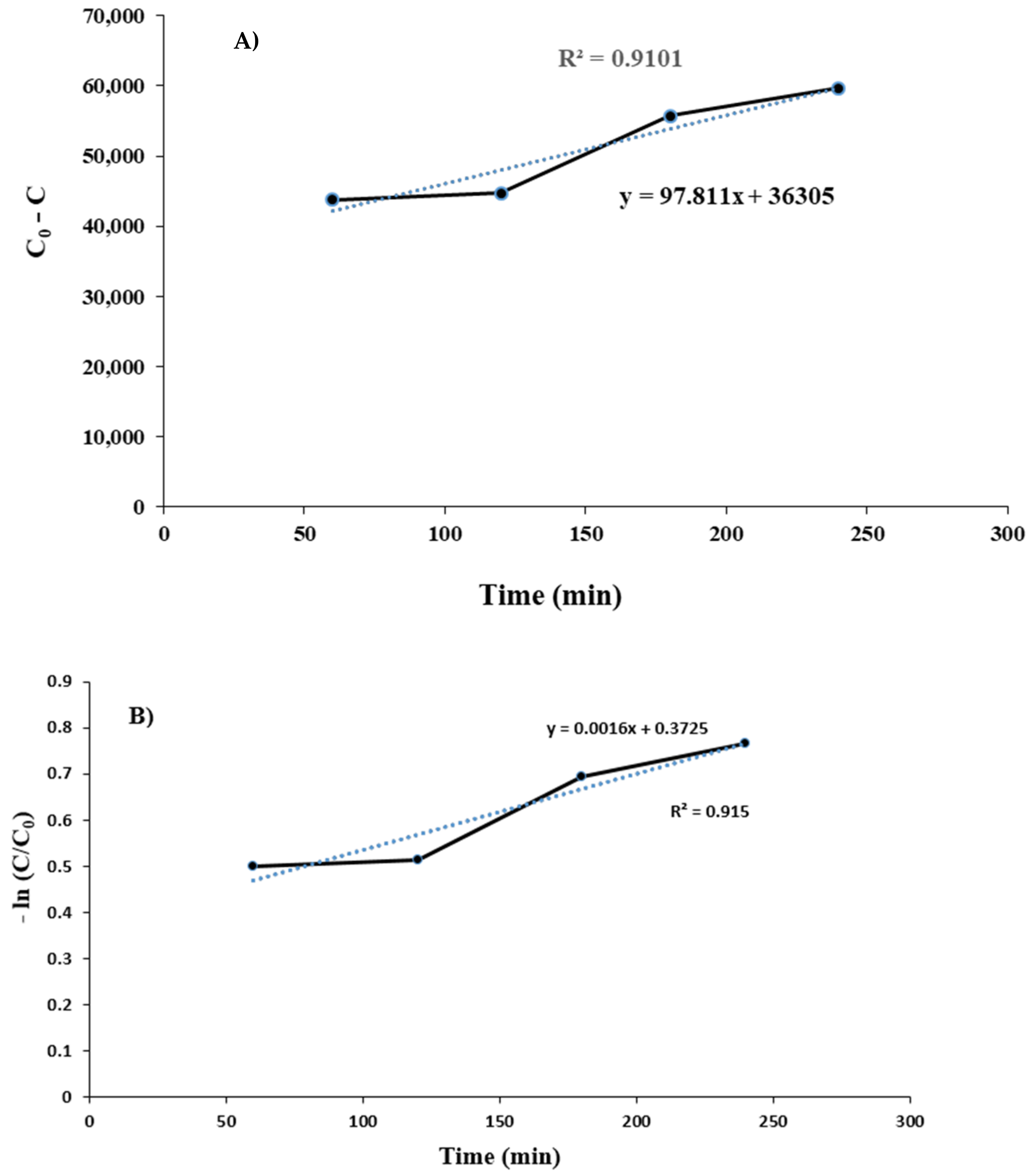
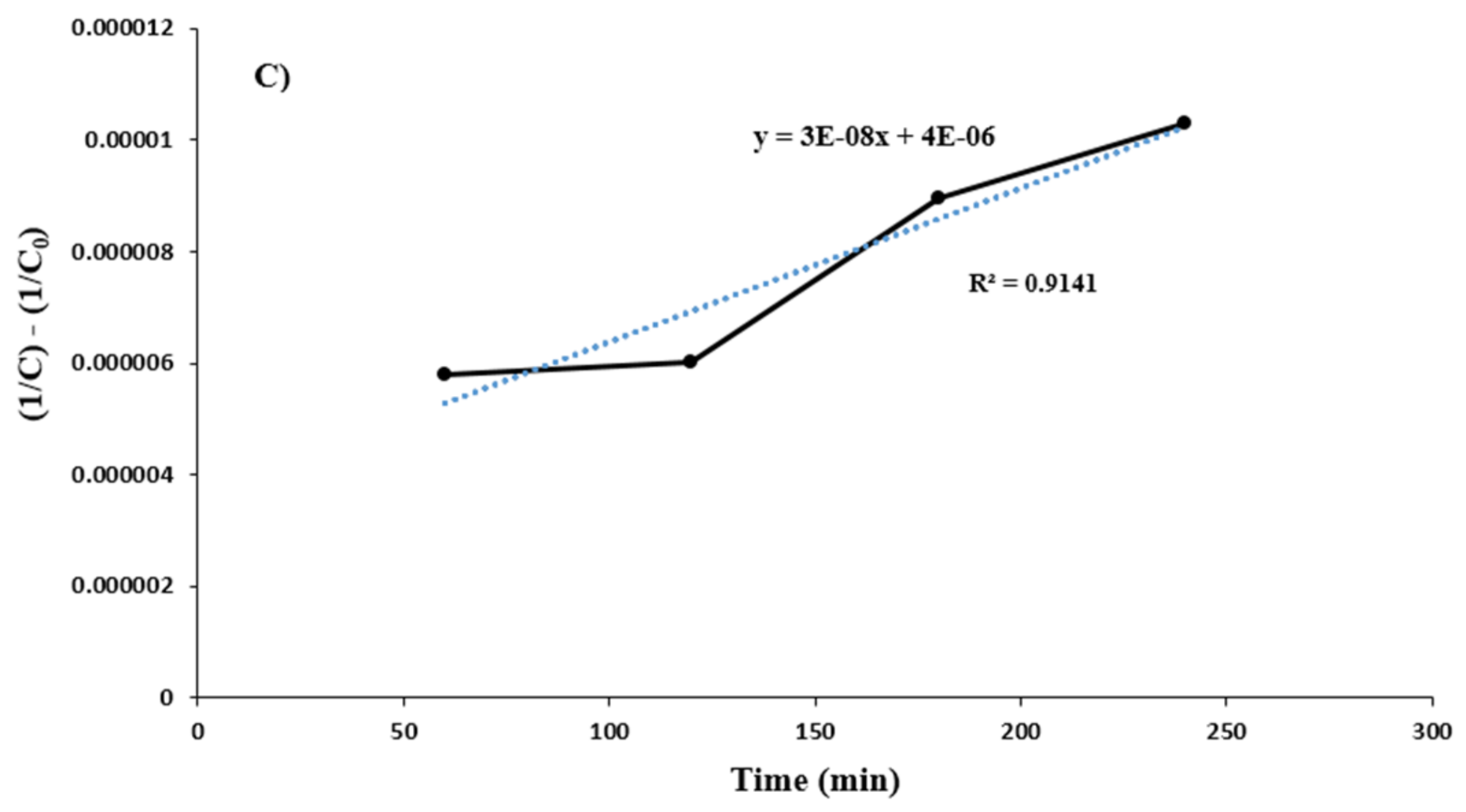
Investigation of Process Kinetics in the Presence of Synthetic Brine under Optimal Conditions
3.5.2. Sodium Removal Efficiency in Real Brine during Optimal Operating Conditions
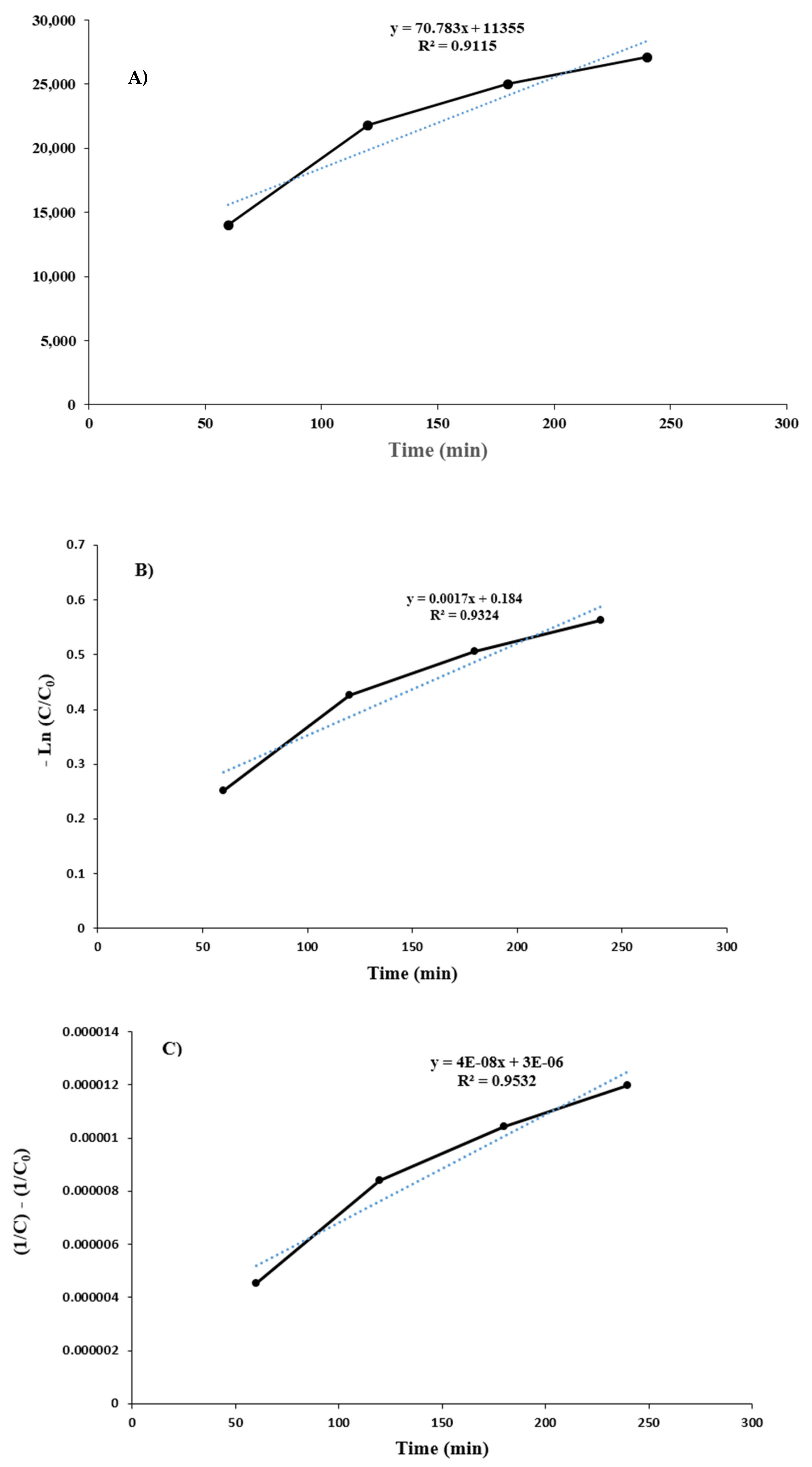
Investigation of Process Kinetics in the Presence of Real Brine under Optimal Conditions
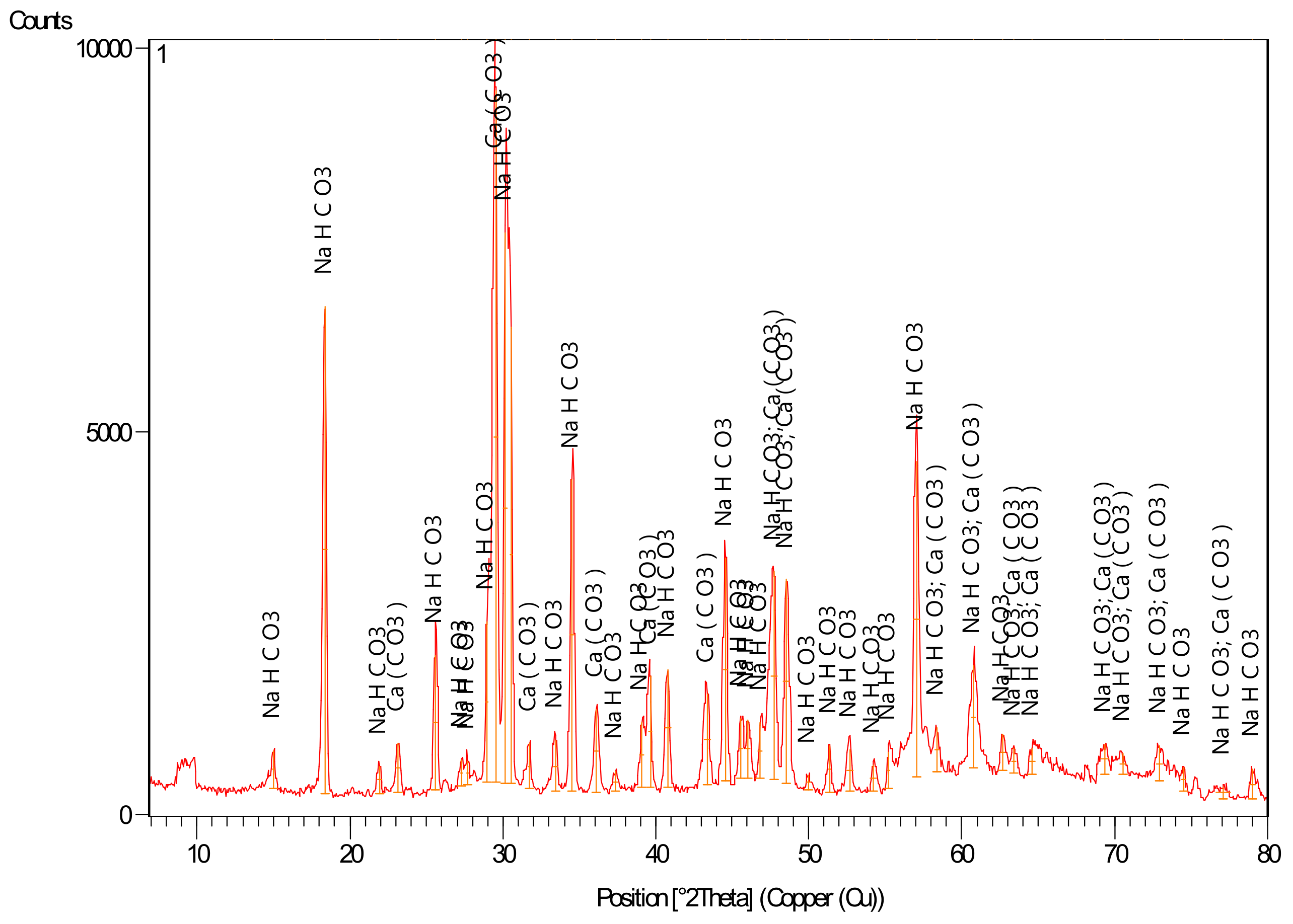
3.6. Production of Solid Sodium Bicarbonate
4. Conclusions
- Investigation and calculation of carbon dioxide capture under optimal conditions.
- Using lime for magnesium precipitation and sodium carbonate for calcium precipitation in the modified Solvay process input as a pretreatment.
- Analyzing the effect of reaction time on the shape and size of sodium bicarbonate crystals.
- Capturing the carbon dioxide output from the process and reusing it as an input gas.
- Determining the gas-liquid mass transfer model in the bubble column reactor.
- Accurate economic estimation to use this method on an industrial scale.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Elgallal, M.; Fletcher, L.; Evans, B. Assessment of potential risks associated with chemicals in wastewater used for irrigation in arid and semiarid zones: A review. Agric. Water Manag. 2016, 177, 419–431. [Google Scholar] [CrossRef]
- Al-Saleh, S.; Khan, A. Evaluation of Belgard EV 2000 as antiscalant control additive in MSF plants. Desalination 1994, 97, 87–96. [Google Scholar] [CrossRef]
- Bello, A.S.; Zouari, N.; Da’Ana, D.A.; Hahladakis, J.N.; Al-Ghouti, M.A. An overview of brine management: Emerging desalination technologies, life cycle assessment, and metal recovery methodologies. J. Environ. Manag. 2021, 288, 112358. [Google Scholar] [CrossRef]
- Zaichenko, V.M.; Shterenberg, V.Y. Capture of CO2 at Thermal Power Stations as One of the Main Trends towards Reducing Anthropogenic Carbon Dioxide Emissions. Therm. Eng. 2019, 66, 440–449. [Google Scholar] [CrossRef]
- Ye, Q. Hou’s Process for Soda Manufacture in China. J. Eng. Stud. 2009, 1, 368–379. [Google Scholar]
- Hertog, S.; Luciani, G. Energy and Sustainability Policies in the GCC; London School of Economics: London, UK, 2009. [Google Scholar]
- Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar]
- Anwar, M.; Fayyaz, A.; Sohail, N.; Khokhar, M.; Baqar, M.; Yasar, A.; Rasool, K.; Nazir, A.; Raja, M.; Rehan, M.; et al. CO2 utilization: Turning greenhouse gas into fuels and valuable products. J. Environ. Manag. 2020, 260, 110059. [Google Scholar] [CrossRef]
- Sema, T.; Naami, A.; Fu, K.; Edali, M.; Liu, H.; Shi, H.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Comprehensive mass transfer and reaction kinetics studies of CO2 absorption into aqueous solutions of blended MDEA–MEA. Chem. Eng. J. 2012, 209, 501–512. [Google Scholar] [CrossRef]
- Shatat, M.; Worall, M.; Riffat, S. Opportunities for solar water desalination worldwide. Sustain. Cities Soc. 2013, 9, 67–80. [Google Scholar] [CrossRef]
- Shatat, M.; Worall, M.; Riffat, S. A combined approach for the management of desalination reject brine and capture of CO2. Desalination 2010, 251, 70–74. [Google Scholar]
- Mohammad, A.F. Optimization of a Combined Approach for the Treatment of Desalination Reject Brine and Capture of CO2. Master’s Thesis, United Arab Emirates University, Abu Dhabi, United Arab Emirates, 2015. [Google Scholar]
- Børseth, K.E. Method for Desalination of Water and Removal of Carbon Dioxide from Exhaust Gases. Patent No. AU2003206275, 14 February 2003. [Google Scholar]
- Grauer, P.; Krass, F. Method and Device for Binding Gaseous CO2 to Sea Water for the Flue Gas Treatment with Sodium Carbonate Compounds. U.S. Patent 20100196244, 5 August 2010. [Google Scholar]
- Constantz, B.R.P.V.; Farsad, K.; Fernandez, M. Desalination Methods and Systems that Include Carbonate Compound Precipitation; Calera Corporation: Los Gatos, CA, USA, 2010. [Google Scholar]
- Jibril, B.E.-Y.; Ibrahim, A.A. Chemical conversions of salt concentrates from desalination plants. Desalination 2001, 139, 287–295. [Google Scholar] [CrossRef]
- Mohammad, A.F.; Al-Marzouqi, A.H.; El-Naas, M.H.; Van der Bruggen, B.; Al-Marzouqi, M.H. A New Process for the Recovery of Ammonia from Ammoniated High-Salinity Brine. Sustainability 2021, 13, 10014. [Google Scholar] [CrossRef]
- Mourad, A.A.-H.I.; Mohammad, A.F.; Al-Marzouqi, A.H.; El-Naas, M.H.; Al-Marzouqi, M.H.; Altarawneh, M. KOH-Based Modified Solvay Process for Removing Na Ions from High Salinity Reject Brine at High Temperatures. Sustainability 2021, 13, 10200. [Google Scholar] [CrossRef]
- Mourad, A.A.-H.; Mohammad, A.F.; Al-Marzouqi, A.H.; El-Naas, M.H.; Al-Marzouqi, M.H.; Altarawneh, M. CO2 capture and ions removal through reaction with potassium hydroxide in desalination reject brine: Statistical optimization. Chem. Eng. Process.-Process Intensif. 2022, 170, 108722. [Google Scholar] [CrossRef]
- Mourad, A.A.I.; Mohammad, A.F.; Altarawneh, M.; Al-Marzouqi, A.H.; El-Naas, M.H.; Al-Marzouqi, M.H. Effects of potassium hydroxide and aluminum oxide on the performance of a modified solvay process for CO2 capture: A comparative study. Int. J. Energy Res. 2021, 45, 13952–13964. [Google Scholar] [CrossRef]
- Mustafa, J.; Mourad, A.; Al-Marzouqi, A.H.; El-Naas, M. Simultaneous treatment of reject brine and capture of carbon dioxide: A comprehensive review. Desalination 2020, 483, 114386. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; AlNashef, I.; Abu-Zahra, M.R. A process for combined CO2 utilization and treatment of desalination reject brine. Desalination 2018, 442, 62–74. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Mohammad, A.F.; Suleiman, M.I.; Al Musharfy, M.; Al-Marzouqi, A.H. A new process for the capture of CO2 and reduction of water salinity. Desalination 2017, 411, 69–75. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Abu Zahra, M. Simultaneous carbon dioxide capture and utilization using thermal desalination reject brine. Appl. Energy 2015, 154, 298–308. [Google Scholar] [CrossRef]
- Huang, H.P.; Shi, Y.; Li, A.W.; Chang, S.G. Dual alkali approaches for the capture and separation of CO2. Energy Fuels 2001, 15, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, L.M.; Balbo, A.L.; Mac Cormack, W.; Ruberto, L.A.M. Bioremediation of a petroleum hydrocarbon-contaminated Antarctic soil: Optimization of a biostimulation strategy using response-surface methodology (RSM). Cold Reg. Sci. Technol. 2015, 119, 61–67. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, J.; Liu, R.; Chen, L. Optimization of Fenton and electro-Fenton oxidation of biologically treated coking wastewater using response surface methodology. Sep. Purif. Technol. 2011, 81, 444–450. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central composite design for response surface methodology and its application in pharmacy. In Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021. [Google Scholar]
- Heidari, M.; Vosoughi, M.; Sadeghi, H.; Dargahi, A.; Mokhtari, S.A. Degradation of diazinon from aqueous solutions by electro-Fenton process: Effect of operating parameters, intermediate identification, degradation pathway, and optimization using response surface methodology (RSM). Sep. Sci. Technol. 2021, 56, 2287–2299. [Google Scholar] [CrossRef]
- Malekian, R.; Abedi-Koupai, J.; Eslamian, S.S.; Mousavi, S.F.; Abbaspour, K.C.; Afyuni, M. Ion-exchange process for ammonium removal and release using natural Iranian zeolite. Appl. Clay Sci. 2011, 51, 323–329. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R.A. Enhancing the adsorption performance of carbon nanotubes with a multistep functionalization method: Optimization of Reactive Blue 19 removal through response surface methodology. Process Saf. Environ. Prot. 2016, 99, 20–29. [Google Scholar] [CrossRef]
- Boubakri, A.; Hafiane, A.; Bouguecha, S.A.T. Application of response surface methodology for modeling and optimization of membrane distillation desalination process. J. Ind. Eng. Chem. 2014, 20, 3163–3169. [Google Scholar] [CrossRef]
- Mohammad, A.F.; El-Naas, M.H.; Suleiman, M.I.; Al Musharfy, M. Optimization of a solvay-based approach for CO2 capture. Int. J. Chem. Eng. Appl. 2016, 7, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Muftah, E.-N. Process for Capture of Carbon Dioxide and Desalination. Patent No. WO2017029509A1, 23 February 2017. [Google Scholar]
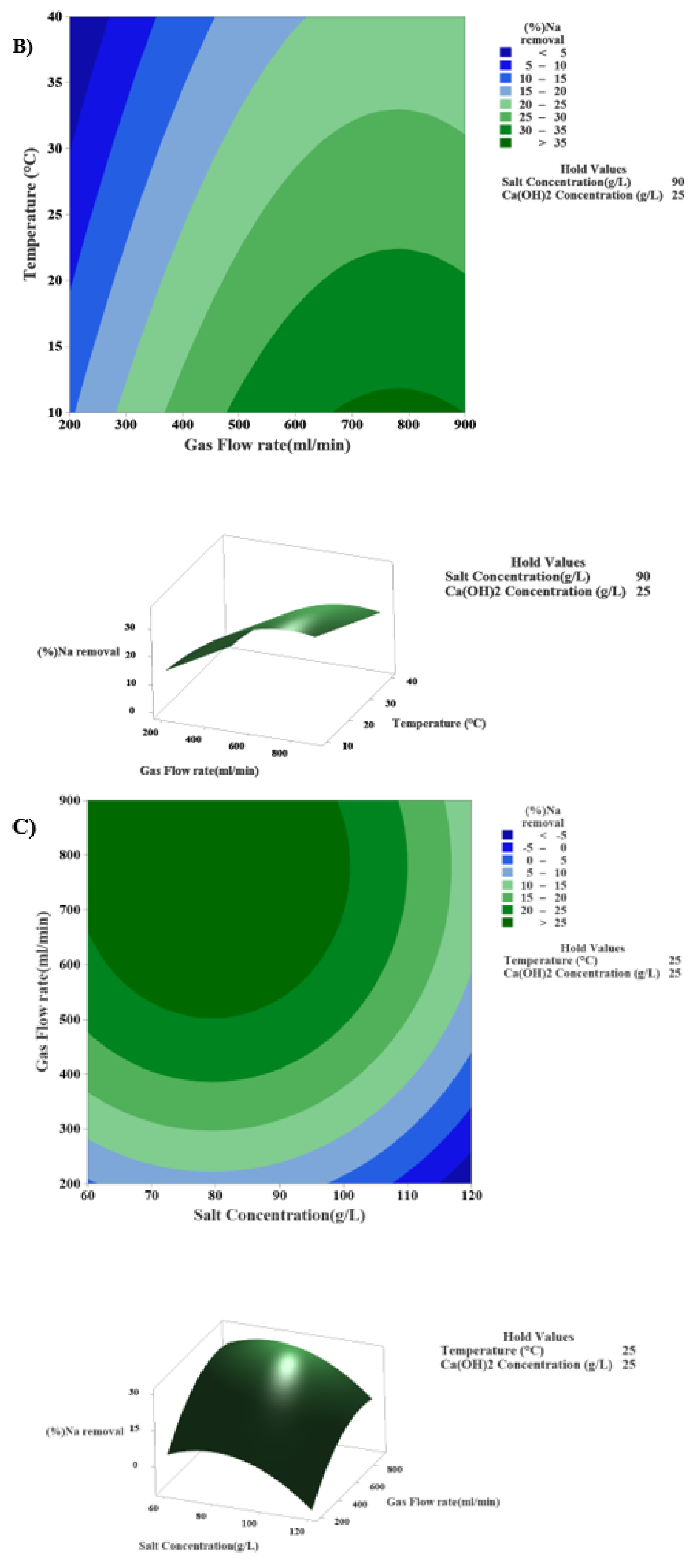
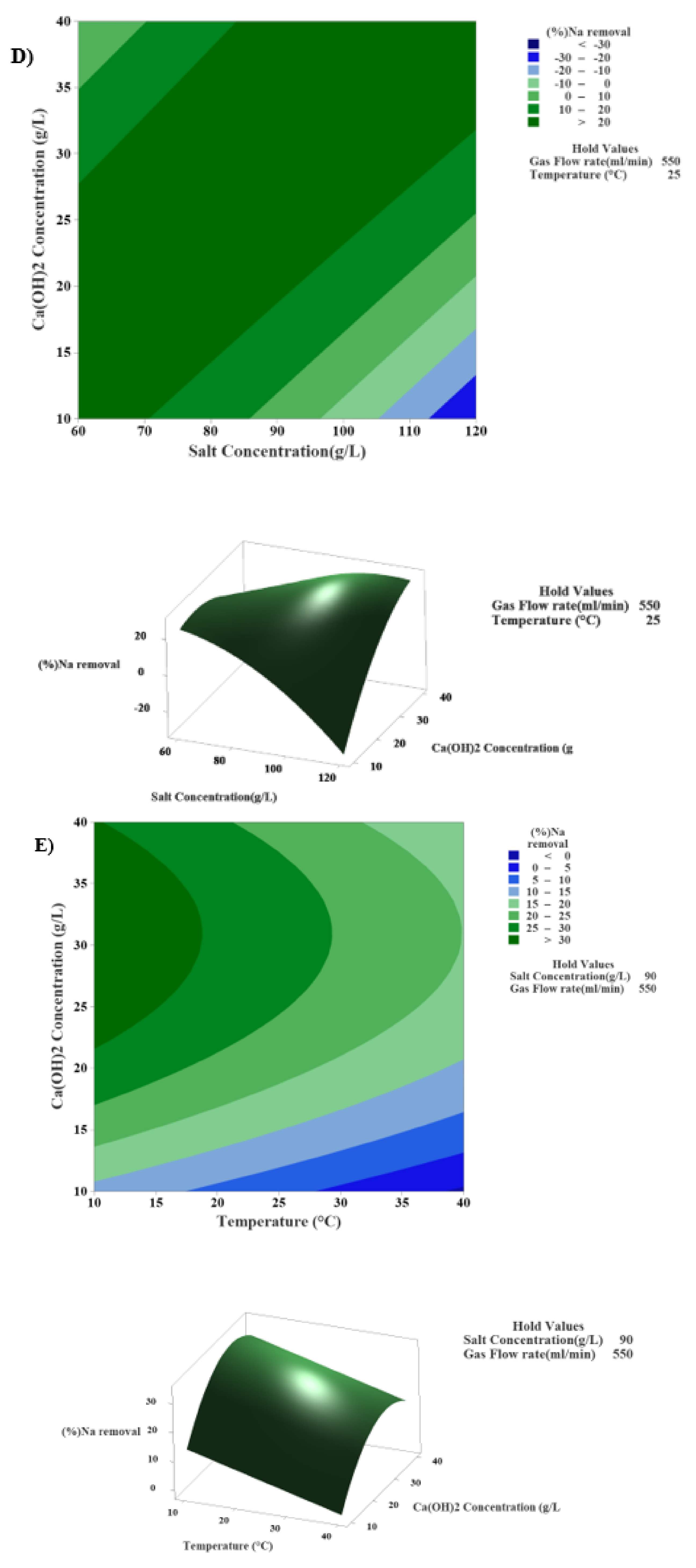
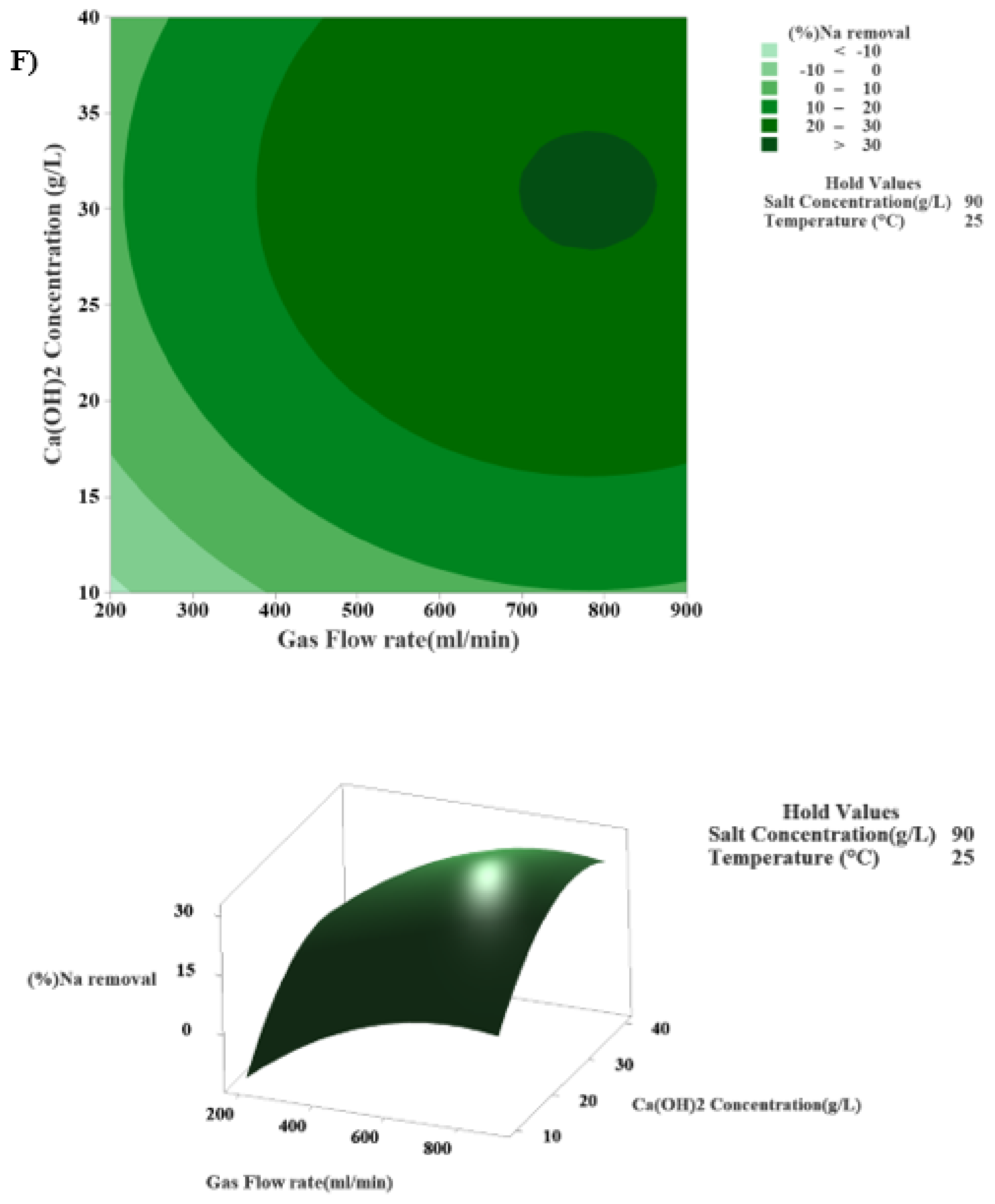
| Factor | Surface 1 | Surface 2 | Surface 3 | Surface 4 | Surface 5 |
|---|---|---|---|---|---|
| X1: Salt concentration (g/L) | 60 | 75 | 90 | 105 | 120 |
| X2: gas flow rate (mL/min) | 20 | 375 | 550 | 725 | 900 |
| X3: Temperature (°C) | 10 | 17.5 | 25 | 32.5 | 40 |
| X4: Calcium hydroxide concentration (g/L) | 10 | 17.5 | 25 | 32.5 | 40 |
| Run | X1: Concentrations of Sodium Chloride (g/L) | X2: Gas Flow Rate (mL/min) | X3: Temperature (°C) | X4: Concentrations of Calcium Hydroxide (g/L) | Actual Na Removal (%) | Predicted Na Removal (%) |
|---|---|---|---|---|---|---|
| 1 | 75 | 375 | 17.5 | 17.5 | 20.80 | 21.12 |
| 2 | 90 | 550 | 25.0 | 25.0 | 24.85 | 25.45 |
| 3 | 90 | 550 | 10.0 | 25.0 | 39.08 | 34.57 |
| 4 | 90 | 550 | 25.0 | 25.0 | 24.79 | 25.45 |
| 5 | 90 | 550 | 25.0 | 40.0 | 29.16 | 28.28 |
| 6 | 90 | 550 | 25.0 | 25.0 | 24.84 | 25.45 |
| 7 | 120 | 550 | 25.0 | 25.0 | 8.84 | 9.06 |
| 8 | 75 | 375 | 32.5 | 32.5 | 11.09 | 12.15 |
| 9 | 105 | 725 | 32.5 | 17.5 | 7.94 | 7.29 |
| 10 | 105 | 725 | 17.5 | 32.5 | 30.81 | 32.16 |
| 11 | 75 | 725 | 32.5 | 32.5 | 20.94 | 22.51 |
| 12 | 90 | 550 | 25.0 | 25.0 | 24.83 | 25.45 |
| 13 | 75 | 725 | 32.5 | 17.5 | 24.94 | 24.37 |
| 14 | 90 | 200 | 25.0 | 25.0 | 6.84 | 7.25 |
| 15 | 75 | 375 | 17.5 | 32.5 | 16.80 | 18.26 |
| 16 | 90 | 550 | 25.0 | 10.0 | 2.84 | 3.38 |
| 17 | 105 | 375 | 32.5 | 17.5 | 0.40 | 0.35 |
| 18 | 90 | 550 | 40.0 | 25.0 | 18.00 | 18.34 |
| 19 | 60 | 550 | 25.0 | 25.0 | 25.16 | 24.60 |
| 20 | 105 | 375 | 17.5 | 17.5 | 3.80 | 4.04 |
| 21 | 75 | 725 | 17.5 | 32.5 | 25.82 | 26.62 |
| 22 | 75 | 725 | 17.5 | 17.5 | 30.80 | 31.48 |
| 23 | 90 | 550 | 25.0 | 25.0 | 24.84 | 25.45 |
| 24 | 90 | 550 | 25.0 | 25.0 | 24.82 | 25.45 |
| 25 | 105 | 725 | 17.5 | 17.5 | 13.79 | 14.40 |
| 26 | 105 | 725 | 32.5 | 32.5 | 24.94 | 26.05 |
| 27 | 90 | 900 | 25.0 | 25.0 | 30.70 | 29.97 |
| 28 | 75 | 375 | 32.5 | 17.5 | 15.02 | 14.01 |
| 29 | 105 | 375 | 32.5 | 32.5 | 14.94 | 15.69 |
| 30 | 90 | 550 | 25.0 | 25.0 | 24.82 | 25.45 |
| 31 | 105 | 375 | 17.5 | 32.5 | 20.80 | 22.80 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14 | 2489.42 | 177.815 | 19.25 | 0.000 |
| Linear | 4 | 1648.62 | 412.156 | 44.61 | 0.000 |
| X1 | 1 | 276.29 | 276.285 | 29.91 | 0.000 |
| X2 | 1 | 641.18 | 641.183 | 69.40 | 0.000 |
| X3 | 1 | 303.67 | 303.668 | 32.87 | 0.000 |
| X4 | 1 | 427.49 | 427.486 | 46.27 | 0.000 |
| Square | 4 | 415.08 | 103.770 | 11.23 | 0.000 |
| X1 × X1 | 1 | 157.73 | 157.726 | 17.07 | 0.001 |
| X2 × X2 | 1 | 103.89 | 103.890 | 11.25 | 0.004 |
| X3 × X3 | 1 | 8.23 | 8.229 | 0.89 | 0.359 |
| X4 × X4 | 1 | 193.09 | 193.093 | 20.90 | 0.000 |
| 2-Way Interaction | 6 | 425.71 | 70.952 | 7.68 | 0.001 |
| X1 × X2 | 1 | 0.10 | 0.098 | 0.01 | 0.919 |
| X1 × X3 | 1 | 0.10 | 0.098 | 0.01 | 0.919 |
| X1 × X4 | 1 | 425.08 | 425.081 | 46.01 | 0.000 |
| X2 × X3 | 1 | 0.18 | 0.183 | 0.02 | 0.890 |
| X2 × X4 | 1 | 0.13 | 0.128 | 0.01 | 0.908 |
| X3 × X4 | 1 | 0.13 | 0.128 | 0.01 | 0.908 |
| Error | 16 | 147.81 | 9.238 | ||
| Lack-of-Fit | 10 | 147.81 | 14.781 | 37,854.32 | <0.00001 |
| Pure Error | 6 | 0.1865 | 0.099 | ||
| Total | 30 | 2637.23 |
| Source | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | PRESS | Remarks |
|---|---|---|---|---|---|---|
| Linear | 0.6720 | 0.5549 | 0.4837 | 0.3295 | 17.01 | |
| 2FI | 0.4966 | 0.8152 | 0.7180 | 0.4249 | 14.59 | |
| Quadratic | 0.3590 | 0.9444 | 0.9283 | 0.8850 | 11.14 | Suggested |
| Cubic | 0.1696 | 0.9921 | 0.9671 | −0.1428 | 28.98 |
| Kinetic Model | Equation | Linear Form of Equation |
|---|---|---|
| Zero order | ||
| first order | ||
| second order |
| Kinetic Model | Zero Order | First Order | Second Order |
|---|---|---|---|
| R2 | 0.9101 | 0.915 | 0.9141 |
| K0 (mg·L−1·min−1) | 97.811 | - | - |
| K1 (min−1) | - | 0.0016 | - |
| K2 (L·mg−1·min−1) | - | - | 3 × 10−8 |
| Kinetic Model | Zero Order | First Order | Second Order |
|---|---|---|---|
| R2 | 0.9115 | 0.9324 | 0.9532 |
| K0 (mg·L−1·min−1) | 70.783 | - | - |
| K1 (min−1) | - | 0.0017 | - |
| K2 (L·mg−1·min−1) | - | - | 4 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setayeshmanesh, T.; Parivazh, M.M.; Abbasi, M.; Osfouri, S.; Dianat, M.J.; Akrami, M. Reducing the Environmental Impacts of Desalination Reject Brine Using Modified Solvay Process Based on Calcium Oxide. Sustainability 2022, 14, 2298. https://doi.org/10.3390/su14042298
Setayeshmanesh T, Parivazh MM, Abbasi M, Osfouri S, Dianat MJ, Akrami M. Reducing the Environmental Impacts of Desalination Reject Brine Using Modified Solvay Process Based on Calcium Oxide. Sustainability. 2022; 14(4):2298. https://doi.org/10.3390/su14042298
Chicago/Turabian StyleSetayeshmanesh, Tahereh, Mohammad Mehdi Parivazh, Mohsen Abbasi, Shahriar Osfouri, Mohammad Javad Dianat, and Mohammad Akrami. 2022. "Reducing the Environmental Impacts of Desalination Reject Brine Using Modified Solvay Process Based on Calcium Oxide" Sustainability 14, no. 4: 2298. https://doi.org/10.3390/su14042298
APA StyleSetayeshmanesh, T., Parivazh, M. M., Abbasi, M., Osfouri, S., Dianat, M. J., & Akrami, M. (2022). Reducing the Environmental Impacts of Desalination Reject Brine Using Modified Solvay Process Based on Calcium Oxide. Sustainability, 14(4), 2298. https://doi.org/10.3390/su14042298








