Optimization of Carboniferous Egyptian Kaolin Treatment for Pharmaceutical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Sample Characterizations
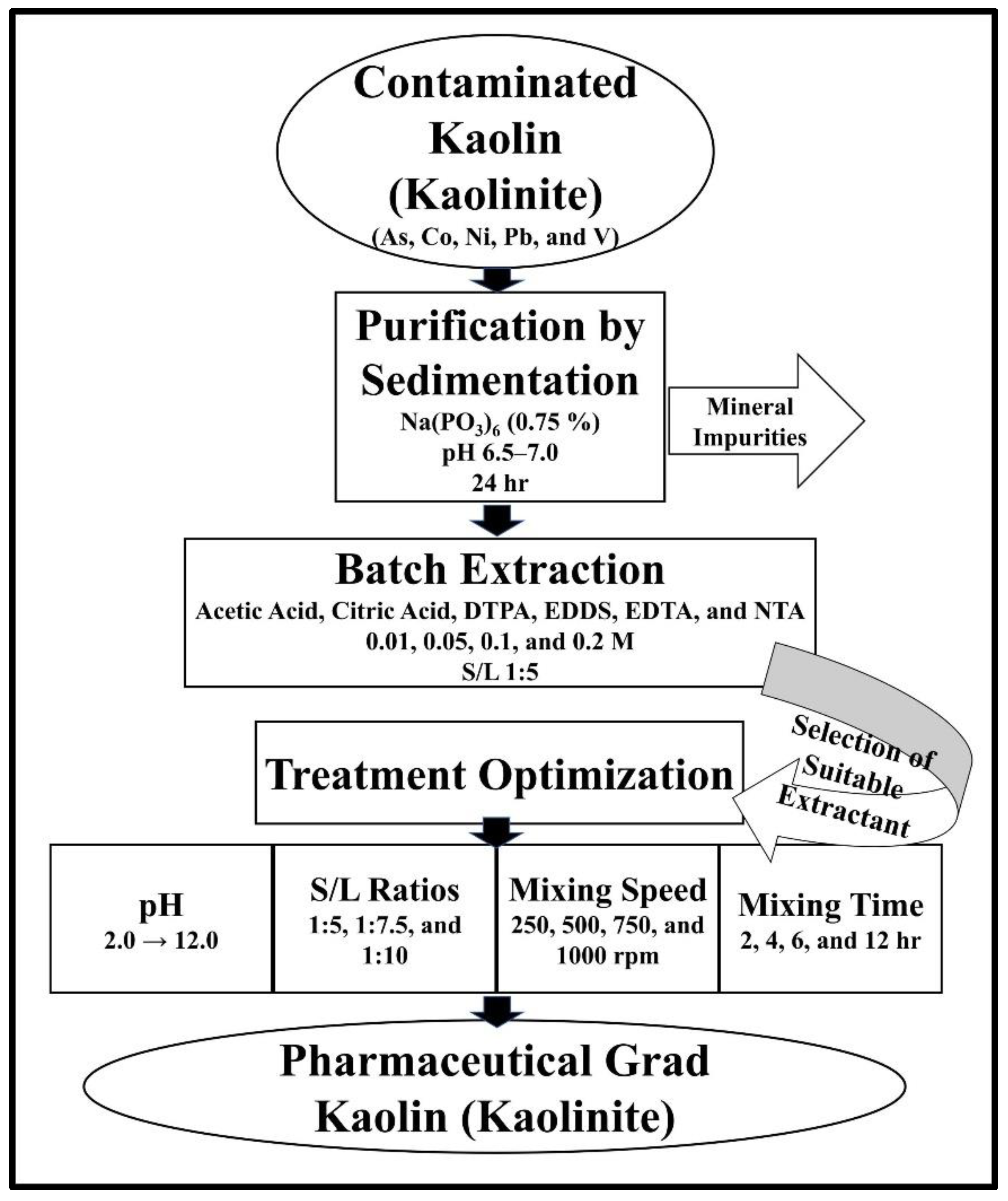
2.3. Purification of Kaolin
2.4. Batch Extraction Experiment
2.5. Treatment Optimization
2.6. PTE Determinations
2.7. Quality Control
3. Results
3.1. Sample Characterizations
3.2. PTE Concentrations
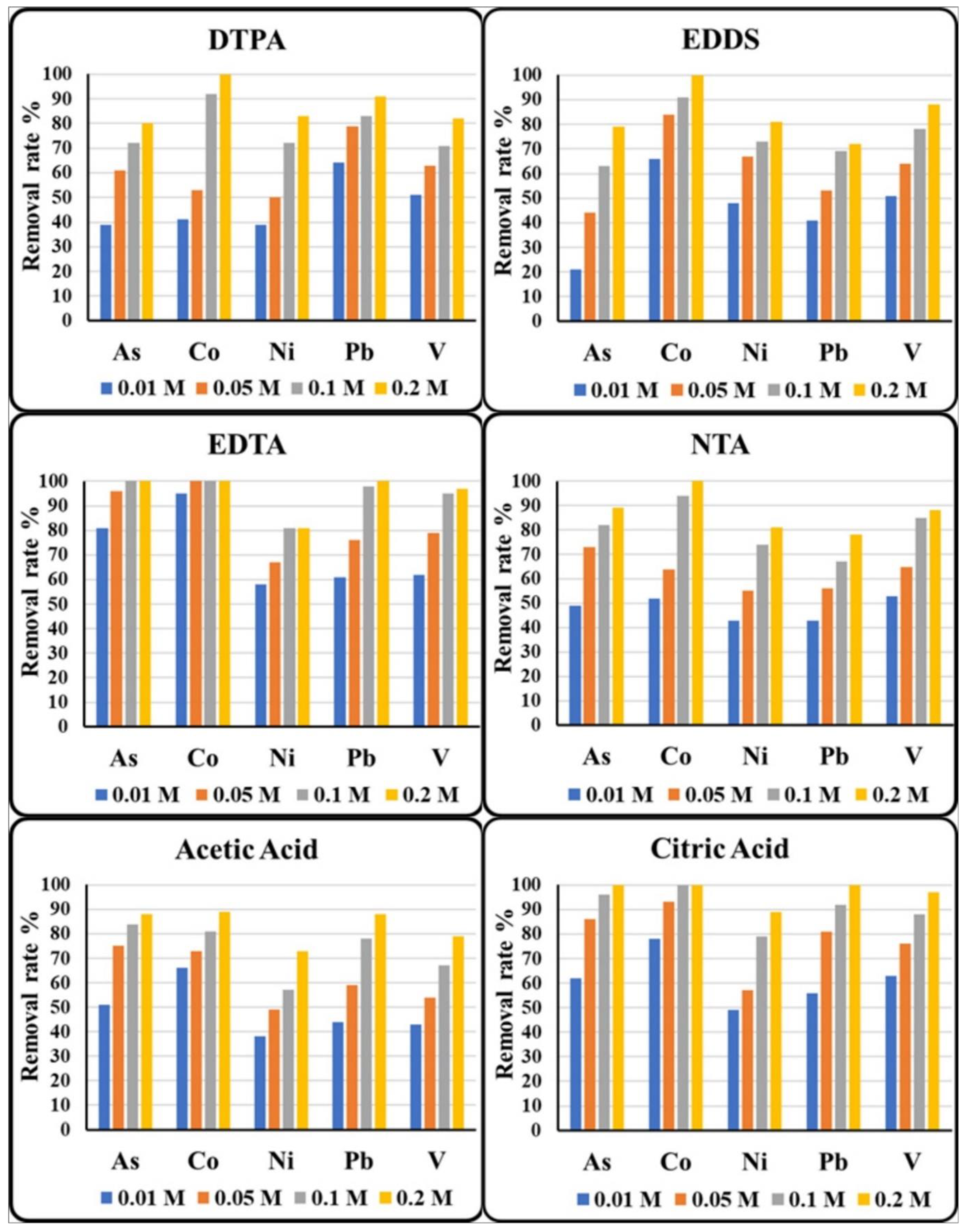
3.3. Selection of Extractant
3.4. Optimization of the Treatment Parameters Using Citric Acid and EDTA
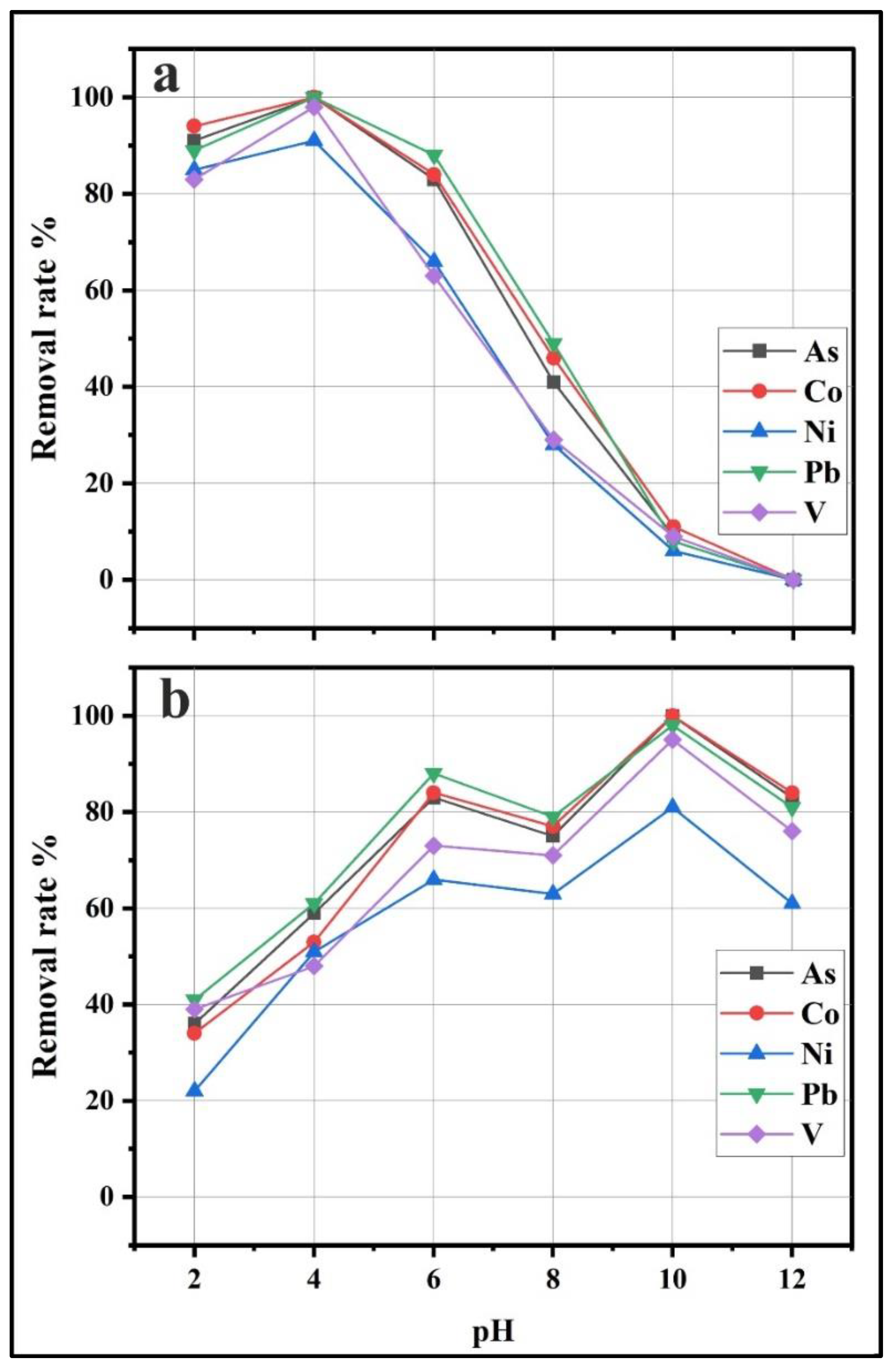
3.4.1. Effect of pH
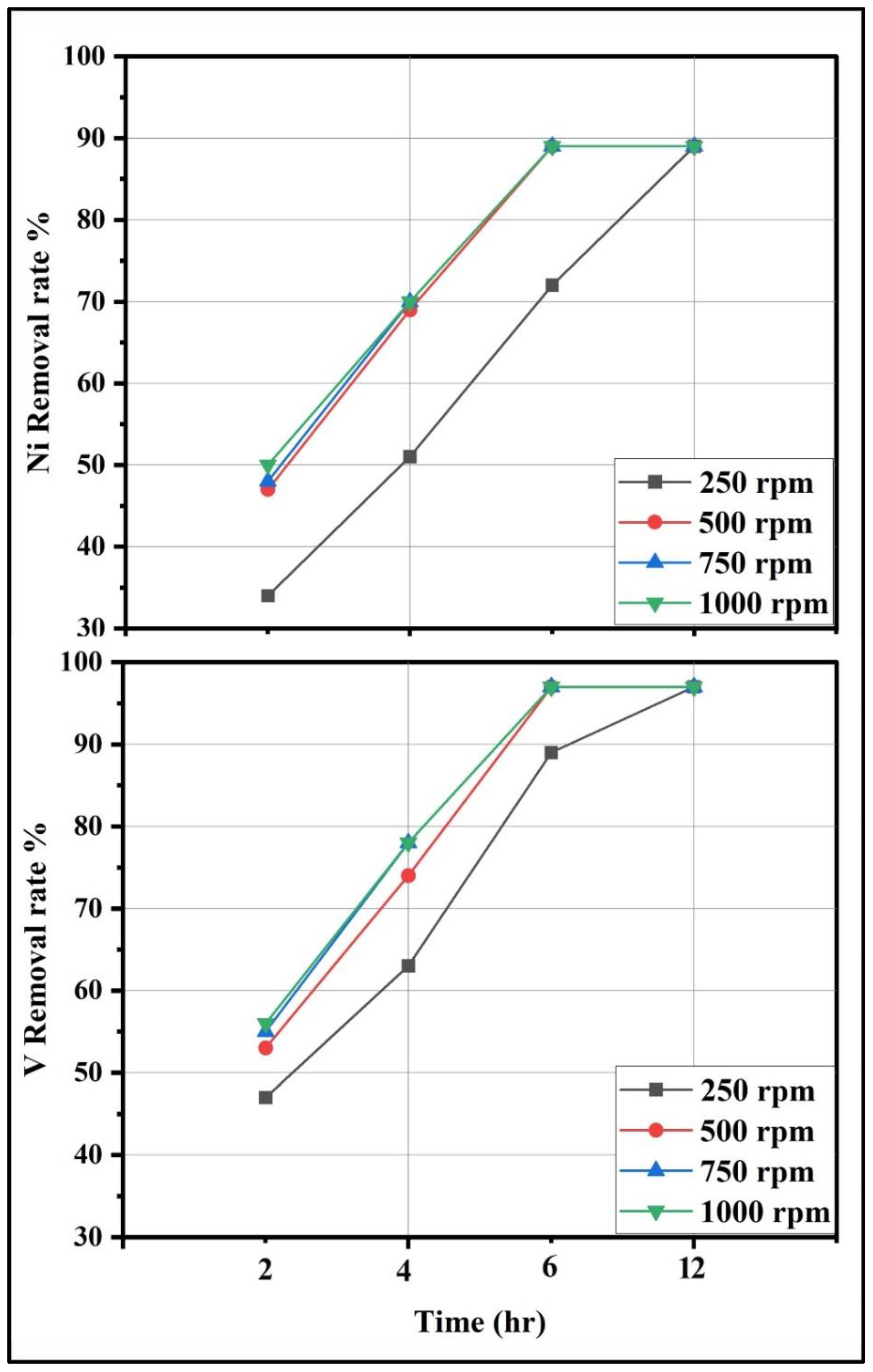
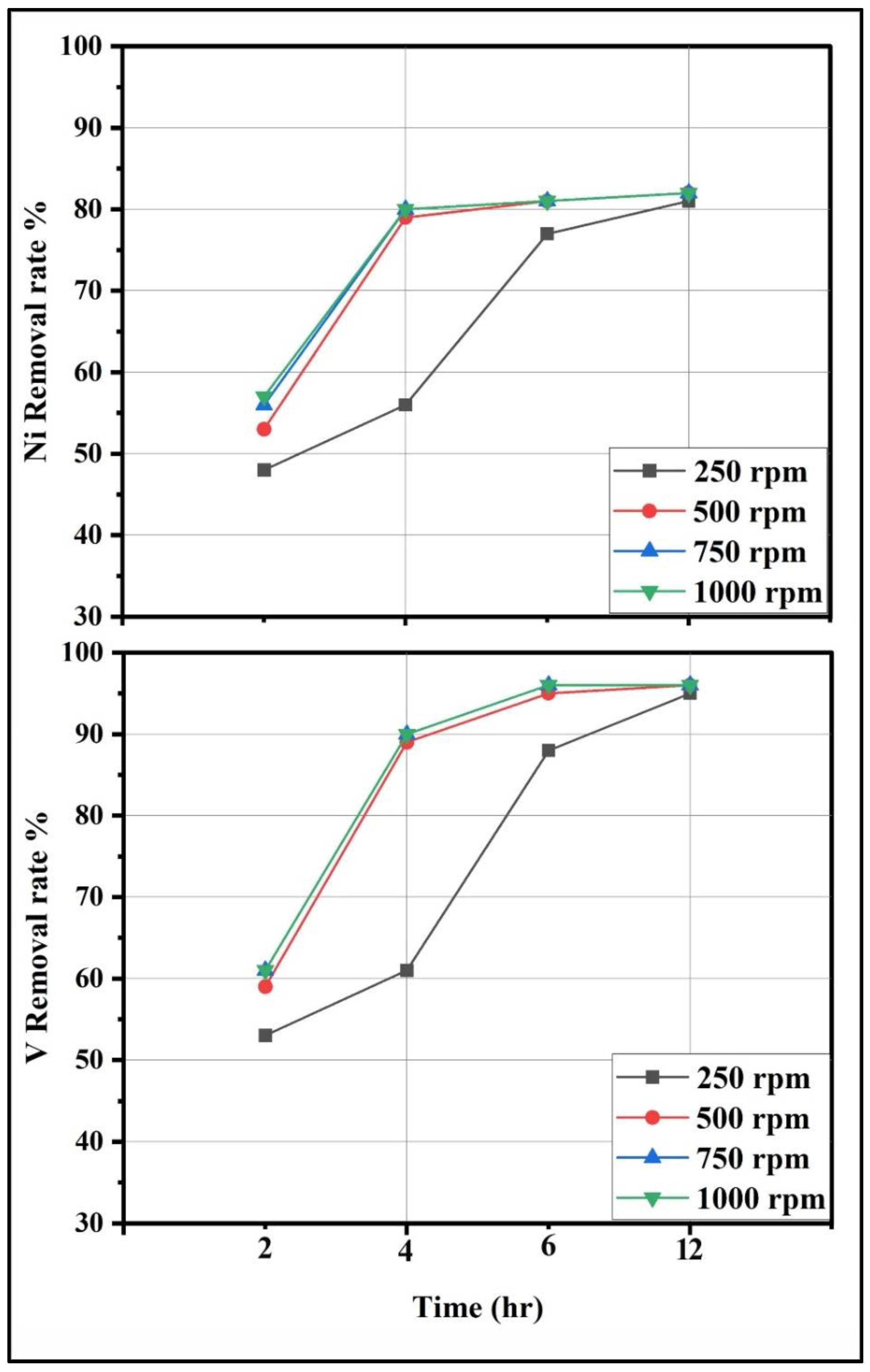
3.4.2. Effect of Mixing Speed and Time
3.4.3. Effect of S/L Ratio
3.5. Potential Applications
3.5.1. Healthcare
3.5.2. Geophagy
3.5.3. Soil Remediation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, C.; Rautureau, M.; Gomes, J.; Silva, E. Minerals in pharmacy and cosmetics. In Minerals Latu Sensu and Human Health Benefits, Toxicity and Pathologies; Gomes, C., Rautureau, M., Eds.; Springe: Wettingen, Switzerland, 2021; pp. 405–441. [Google Scholar] [CrossRef]
- Roselli, C.; Desideri, D.; Cantaluppi, C.; Mattioli, M.; Fasson, A.; Meli, M.A. Essential and toxic elements in clays for pharmaceutical and cosmetic use. J. Toxicol. Environ. Health A 2015, 78, 316–324. [Google Scholar] [CrossRef]
- López-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Del Hoyo, C. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2007, 36, 103–121. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; El-Rahmany, M.M.; El-Desoky, H.M.; Viseras, C. Characterization of Egyptian kaolins for health-care uses. Appl. Clay Sci. 2017, 135, 176–189. [Google Scholar] [CrossRef]
- Eker, C.S.; Ari, U.V. Comparison of sandstone and mudstone with different methods for assessing toxic element contamination in the Early–Middle Jurassic sediments of Gümüşhane (NE Turkey). Environ. Earth Sci. 2020, 79, 444. [Google Scholar] [CrossRef]
- Oyebanjo, O.; Ekosse, G.; Odiyo, J. Health risk evaluation of trace elements in geophagic kaolinitic clays within Eastern Dahomey and Niger Delta Basins, Nigeria. Int. J. Environ. Res. Public Health 2020, 17, 4813. [Google Scholar] [CrossRef]
- Ekosse, G.-I.; Nkeng, G.E.; Bukalo, N.; Oyebanjo, O. Geophagic clays from cameroon: Provenance, metal contamination and health risk assessment. Int. J. Environ. Res. Public Health 2021, 18, 8315. [Google Scholar] [CrossRef]
- Baghdady, A.; Awad, S.; Gad, A. Assessment of metal contamination and natural radiation hazards in different soil types near iron ore mines, Bahariya Oasis, Egypt. Arab. J. Geosci. 2018, 11, 506. [Google Scholar] [CrossRef]
- Gonzalez-Nahm, S.; Nihlani, K.; House, J.S.; Maguire, R.L.; Skinner, H.G.; Hoyo, C. Associations between maternal cadmium exposure with risk of preterm birth and low birth weight: Effect of Mediterranean diet adherence on affected prenatal outcomes. Toxics 2020, 8, 90. [Google Scholar] [CrossRef]
- Gad, A.; Abd El Bakey, S.M.; Sakr, S. Concentrations of heavy metals and associated human health risk in unrefined salts of inland hypersaline lakes, Egypt. Int. J. Environ. Anal. Chem. 2020, 1–15. [Google Scholar] [CrossRef]
- Jahromi, M.A.; Jamshidi-Zanjani, A.; Darban, A.K. Heavy metal pollution and human health risk assessment for exposure to surface soil of mining area: A comprehensive study. Environ. Earth Sci. 2020, 79, 365. [Google Scholar] [CrossRef]
- Medina-Estévez, F.; Zumbado, M.; Luzardo, O.P.; Rodríguez-Hernández, Á.; Boada, L.D.; Fernández-Fuertes, F.; Santandreu-Jimenez, M.E.; Henríquez-Hernández, L.A. Association between heavy metals and rare earth elements with acute ischemic stroke: A case-control study conducted in the Canary Islands (Spain). Toxics 2020, 8, 66. [Google Scholar] [CrossRef]
- Rauf, A.U.; Mallongi, A.; Lee, K.; Daud, A.; Hatta, M.; Al Madhoun, W.; Astuti, R.D.P. Potentially toxic element levels in atmospheric particulates and health risk estimation around industrial areas of Maros, Indonesia. Toxics 2021, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Khalek, N.A. The Egyptian kaolin: An outlook in the view of the new climate of investment. Appl. Clay Sci. 1999, 15, 325–336. [Google Scholar] [CrossRef]
- Baioumy, H.M. Geochemistry and origin of the Cretaceous sedimentary kaolin deposits, Red Sea, Egypt. Geochemistry 2014, 74, 195–203. [Google Scholar] [CrossRef]
- El-Kammar, A.; Abu-Zied, H.T.; Galal, M.; Osman, D. Composition, radioactivity, and possible applications of kaolin deposits of Sinai, Egypt. Arab. J. Geosci. 2017, 10, 463. [Google Scholar] [CrossRef]
- Kamel, O.A.; Soliman, F.H.A.; Abdel Maaboud, M.H.M. Sinai Carboniferous kaolins: Their mineralogy, geochemical characteristics and suitability for ceramic industry. Egypt. J. Geol. 1997, 41, 219–238. [Google Scholar]
- Baioumy, H.M.; Gilg, H.A.; Taubald, H. Mineralogy and geochemistry of the sedimentary kaolin deposits from Sinai, Egypt: Implications for control by the source rocks. Clays Clay Min. 2012, 60, 633–654. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Sánchez-Espejo, R.; Sainz-Díaz, C.I.; El-Rahmany, M.M.; Viseras, C. Crystallite size as a function of kaolinite structural order-disorder and kaolin chemical variability: Sedimentological implication. Appl. Clay Sci. 2018, 162, 261–267. [Google Scholar] [CrossRef]
- Saber, E.; Ali, M.; El-Sheikh, A. Provenance studies of Kalabsha kaolin deposits, Egypt: A petrographical and geochemical approach. Arab. J. Geosci. 2018, 11, 339. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Medarević, D.; Đuriš, J.; El-Rahmany, M.M.; Ibrić, S.; Viseras, C. Flow and tableting behaviors of some Egyptian kaolin powders as potential pharmaceutical excipients. Minerals 2020, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Pintauro, P.N. Desorption-complexation-dissolution characteristics of adsorbed cadmium from kaolin by chelators. Water Air Soil Pollut. 1996, 86, 35–50. [Google Scholar] [CrossRef]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Removal of nickel and phenanthrene from kaolin soil using different extractants. Environ. Eng. Sci. 2004, 24, 691–704. [Google Scholar] [CrossRef]
- Maturi, K.; Reddy, K.R. Extractants for the removal of mixed contaminants from soils. Soil Sediment Contam. 2008, 17, 586–608. [Google Scholar] [CrossRef]
- Niinae, M.; Nishigaki, K.; Aoki, K. Removal of Lead from Contaminated Soils with Chelating Agents. Mater. Trans. 2008, 49, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Zhu, L.; Zhou, W. Simultaneous removal of phenanthrene and cadmium from contaminated soils by saponin, a plant-derived biosurfactant. Environ. Pollut. 2008, 156, 1368–1370. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.A.; Bozeya, A.; Abu-Irmaileh, B.; Abu-Mallouh, S.; Al Bawab, A.; Al-Dujaili, A.H. Assessment of some heavy metals in the Dead Sea Mud and treatment optimization. Soil Sediment Contam. 2017, 26, 364–376. [Google Scholar] [CrossRef]
- Amir, M.; Mohsen, S.; Afsaneh, M. Simultaneous desorption and desorption kinetics of phenanthrene, anthracene, and heavy metals from kaolinite with different organic matter content. Soil Sediment Contam. 2018, 27, 200–220. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, S.; Chen, S.; Ma, Y. Change of the Extractability of Cadmium Added to Different Soils: Aging Effect and Modeling. Sustainability 2018, 10, 885. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Luo, D.; Yao, G.; Huang, X.; Wei, L.; Liu, Y.; Wu, Q.; Mai, X.; Liu, G.; Xiao, T. Comparative Activation Process of Pb, Cd and Tl Using Chelating Agents from Contaminated Red Soils. Int. J. Environ. Res. Public Health 2020, 17, 497. [Google Scholar] [CrossRef] [Green Version]
- Lumia, L.; Giustra, M.G.; Viviani, G.; Di Bella, G. Washing batch test of contaminated sediment: The case of Augusta Bay (SR, Italy). Appl. Sci. 2020, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Koo, N.; Kim, J.-G.; Lee, S.-H. Effects of washing solution, washing time, and solid-solution rate on the maximum heavy metals removal efficiency. Appl. Sci. 2021, 11, 6398. [Google Scholar] [CrossRef]
- Kora, M.; El Shahat, A.; Abu Shabana, M. Lithostratigraphy of the manganese-bearing Urn Bogma Formation, west-central Sinai, Egypt. J. Afr. Earth Sci. 1994, 18, 151–162. [Google Scholar] [CrossRef]
- Ramadan, S.R.; Gad, A.; Yehia, M.M.; Dawood, Y.H.; Kawady, N.A. Grain size and mineralogical characteristics of the stream sediments, east of Abu Zeneima area, southwestern Sinai, Egypt. Arab. J. Geosci. 2019, 12, 192. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD). ICDD Powder Diffraction File Data Sets 01 to 19; ICDD: Livermore, CA, USA, 2000. [Google Scholar]
- Maynard, R.N. A method for removing titanium dioxide impurities from kaolin. Clays Clay Min. 1969, 17, 59–62. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Leckie, J.O. Multiple site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J. Colloid Interface Sci. 1981, 79, 209–221. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Borkovec, M.; Grolimund, D.; Elimelech, M. Mobile subsurface colloids and their role in contaminant transport. Adv. Agron. 1999, 66, 121–193. [Google Scholar] [CrossRef]
- Han, F.X.; Singer, A. Binding and distribution of trace elements among solid-phase components in arid zone soils. In Biogeochemistry of Trace Elements in Arid Environments; Han, F.X., Singer, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 13, pp. 131–167. [Google Scholar] [CrossRef]
- USP-NF. United States Pharmacopeia 42 and National Formulary 37 (USP 42-NF37); US Pharmacopeial Convention: Rockville, MD, USA, 2018. [Google Scholar]
- ICH. The International Conference on Harmonization of Technical Requirements and Registration of Pharmaceuticals for Human Use (ICH), Guideline for Elemental Impurities Q3D. 2019. Available online: https://database.ich.org/sites/default/files/Q3D-R1EWG_Document_Step4_Guideline_2019_0322.pdf (accessed on 15 June 2021).
- Elliott, H.A.; Brown, G.A. Comparative evaluation of NTA and EDTA for extractive decontamination of pb-polluted soils. Water Air Soil Pollut. 1989, 45, 361–369. [Google Scholar] [CrossRef]
- Pichtel, J.; Pichtel, T.M. Comparison of solvents for Ex situ removal of chromium and lead from contaminated soil. Environ. Eng. Sci. 1997, 14, 97–104. [Google Scholar] [CrossRef]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Effect of different extraction agents on metal and organic contaminant removal from a field soil. J. Hazard. Mater. 2005, 117, 15–24. [Google Scholar] [CrossRef]
- Di Palma, L.; Mecozzi, R. Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater. 2007, 147, 768–775. [Google Scholar] [CrossRef]
- Abollino, O.; Aceto, M.; Malandrino, M.; Sarzanini, C.; Mentasti, E. Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res. 2003, 37, 1619–1627. [Google Scholar] [CrossRef]
- Tuin, B.J.W.; Tels, M. Removing heavy metals from contaminated clay soils by extraction with hydrochloric acid, edta or hypochlorite solutions. Environ. Technol. 1990, 11, 1039–1052. [Google Scholar] [CrossRef]
- Quaghebeur, M.; Rate, A.; Rengel, Z.; Hinz, C. Desorption kinetics of arsenate from kaolinite as influenced by pH. J. Environ. Qual. 2005, 34, 479–486. [Google Scholar] [CrossRef]
- White, G.N.; Dixon, J.B. Kaolin–serpentine minerals. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; SSSA Book Series; Wiley: Hoboken, NJ, USA, 2002; Volume 7, pp. 389–414. [Google Scholar] [CrossRef]
- Li, L.Y. Removal of multiple-metals from contaminated clay minerals. Environ. Technol. 2006, 27, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, Y.; Ong, S.K. Factors affecting EDTA extraction of lead from lead-contaminated soils. Chemosphere 2003, 51, 845–853. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Zhang, S.; Zhong, Q.; Zhou, W.; Zhang, X.; Wu, J.; Vijver, M.G.; Peijnenburg, W.J. Remediation of heavy metal contaminated soil by biodegradable chelator–induced washing: Efficiencies and mechanisms. Environ. Res. 2020, 186, 109554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yang, M.; Gao, Y.; Wang, J.; Li, D.; Li, T. Removal of toxic metals from vanadium-contaminated soils using a washing method: Reagent selection and parameter optimization. Chemosphere 2017, 180, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, J.; Huang, X.; Zia, B.; Su, C.; Luo, G.; Xu, Y.; Wu, Y.; Mao, Z.; Qiu, R. Chelant extraction of heavy metals from contaminated soils using new selective EDTA derivatives. J. Hazard. Mater. 2013, 262, 464–471. [Google Scholar] [CrossRef]
- Wargala, E.; Sławska, M.; Zalewska, A.; Toporowska, M. Health effects of dyes, minerals, and vitamins used in cosmetics. Women 2021, 1, 223–237. [Google Scholar] [CrossRef]
- Kutalek, R.; Wewalka, G.; Gundacker, C.; Auer, H.; Wilson, J.; Haluza, D.; Huhulescu, S.; Hillier, S.; Sager, M.; Prinz, A. Geophagy and potential health implications: Geohelminths, microbes and heavy metals. Trans. Roy. Soc. Trop. Med. Hyg. 2010, 104, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lar, U.A.; Agene, J.I.; Umar, A.I. Geophagic clay materials from Nigeria: A potential source of heavy metals and human health implications in mostly women and children who practice it. Environ. Geochem. Health 2015, 37, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Asowata, I.T. Geophagic clay around Uteh-Uzalla near Benin: Mineral and trace elements compositions and possible health implications. SN Appl. Sci. 2021, 3, 569. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Luo, H.-Q.; Zhu, Y.-Y.; Yu, Y.-Q.; He, W.-Y.; Wu, Z.-Z.; Wang, B. Adsorption-desorption and co-migration of vanadium on colloidal kaolinite. Environ. Sci. Pollut. Res. 2020, 27, 17910–17922. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad, A.; Al-Mur, B.A.; Alsiary, W.A.; Abd El Bakey, S.M. Optimization of Carboniferous Egyptian Kaolin Treatment for Pharmaceutical Applications. Sustainability 2022, 14, 2388. https://doi.org/10.3390/su14042388
Gad A, Al-Mur BA, Alsiary WA, Abd El Bakey SM. Optimization of Carboniferous Egyptian Kaolin Treatment for Pharmaceutical Applications. Sustainability. 2022; 14(4):2388. https://doi.org/10.3390/su14042388
Chicago/Turabian StyleGad, Ahmed, Bandar A. Al-Mur, Waleed A. Alsiary, and Sahar M. Abd El Bakey. 2022. "Optimization of Carboniferous Egyptian Kaolin Treatment for Pharmaceutical Applications" Sustainability 14, no. 4: 2388. https://doi.org/10.3390/su14042388
APA StyleGad, A., Al-Mur, B. A., Alsiary, W. A., & Abd El Bakey, S. M. (2022). Optimization of Carboniferous Egyptian Kaolin Treatment for Pharmaceutical Applications. Sustainability, 14(4), 2388. https://doi.org/10.3390/su14042388






