Archeological Sites and Relict Landscapes as Refuge for Biodiversity: Case Study of Alexandria City, Egypt
Abstract
:1. Introduction
2. Materials and Methods
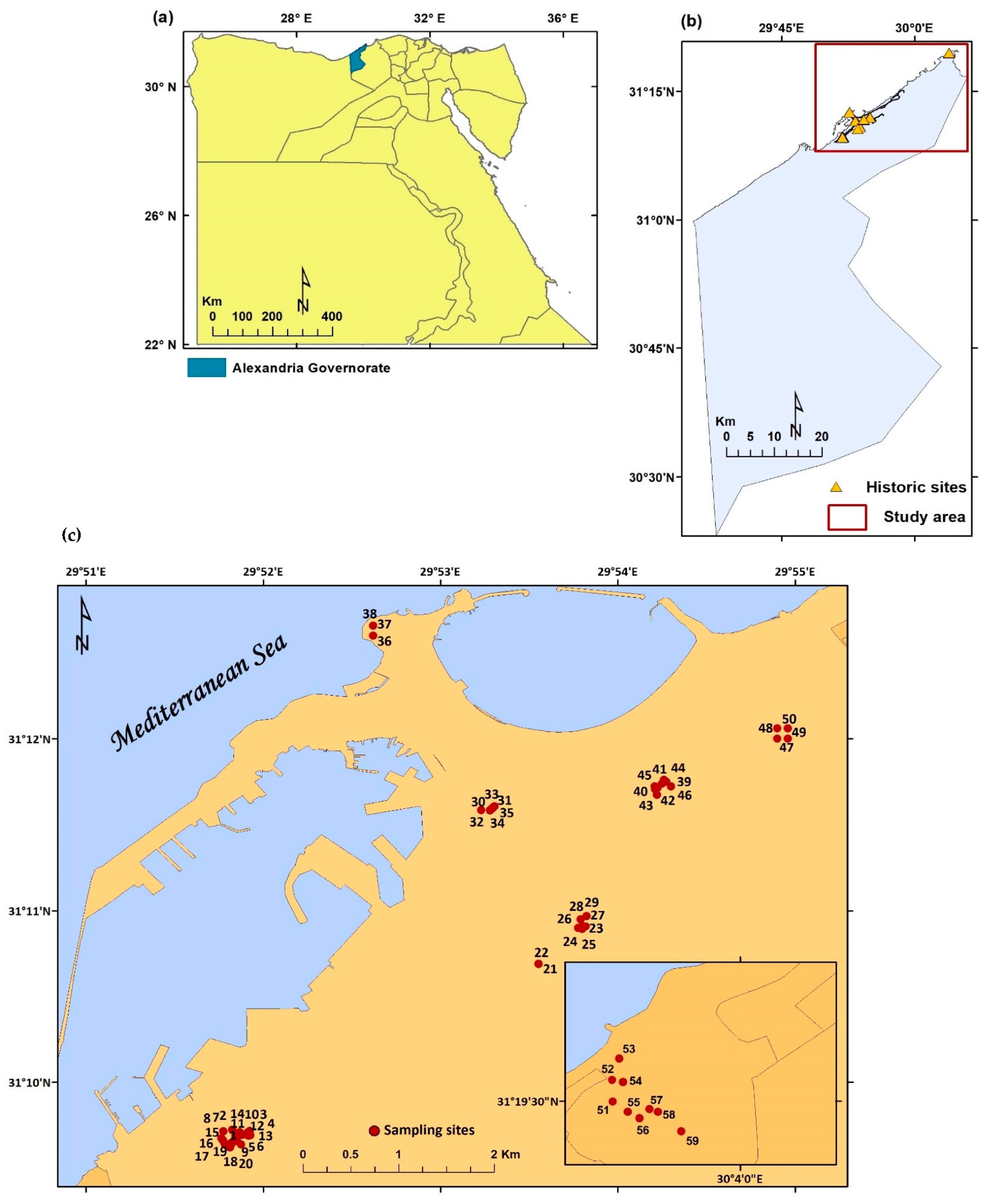
2.1. Study Area
2.2. Field Survey and Data Collection
2.3. Vegetation Measurements
2.4. Soil Analysis
2.5. Diversity Indices
2.6. Data Analysis
3. Results
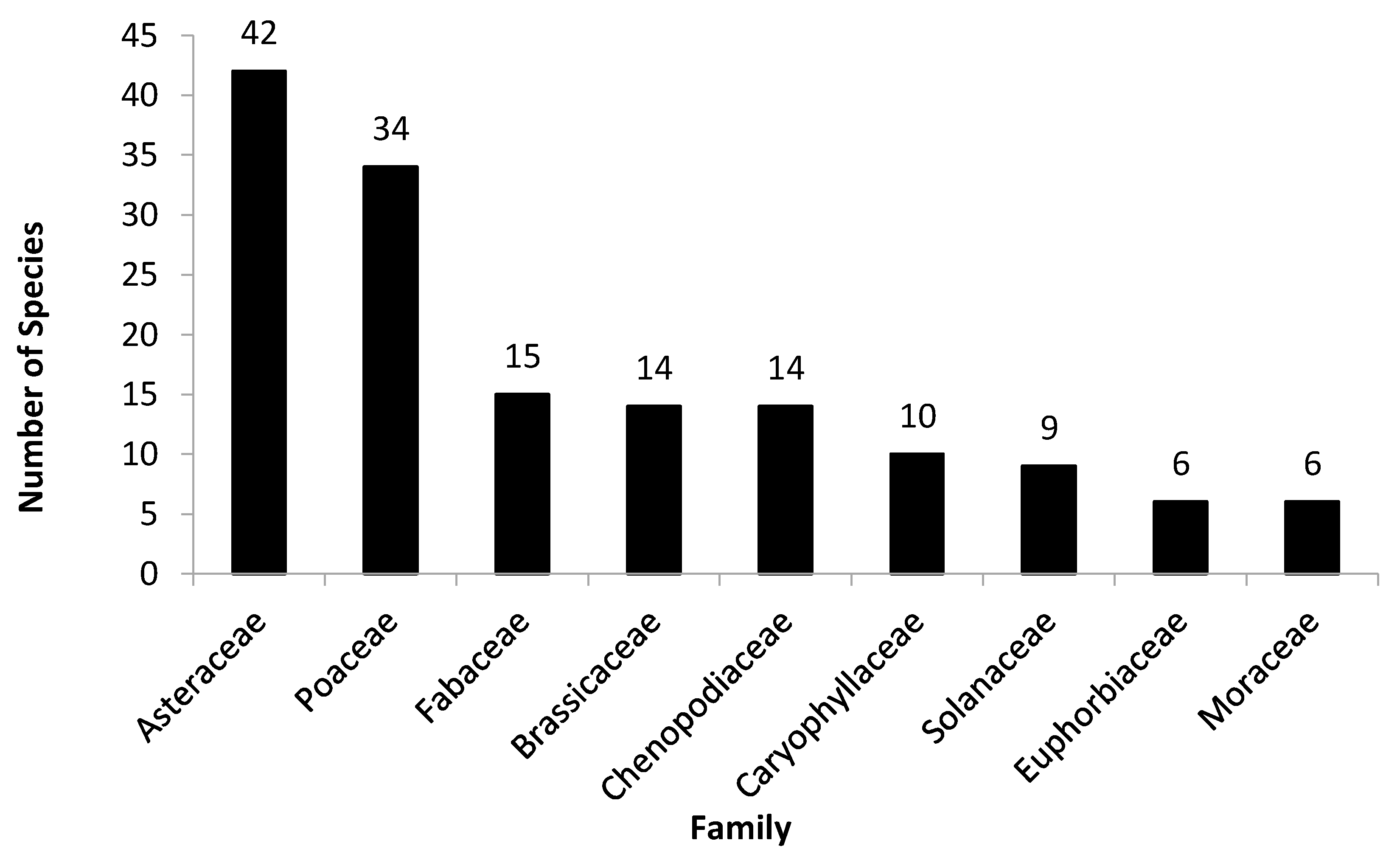
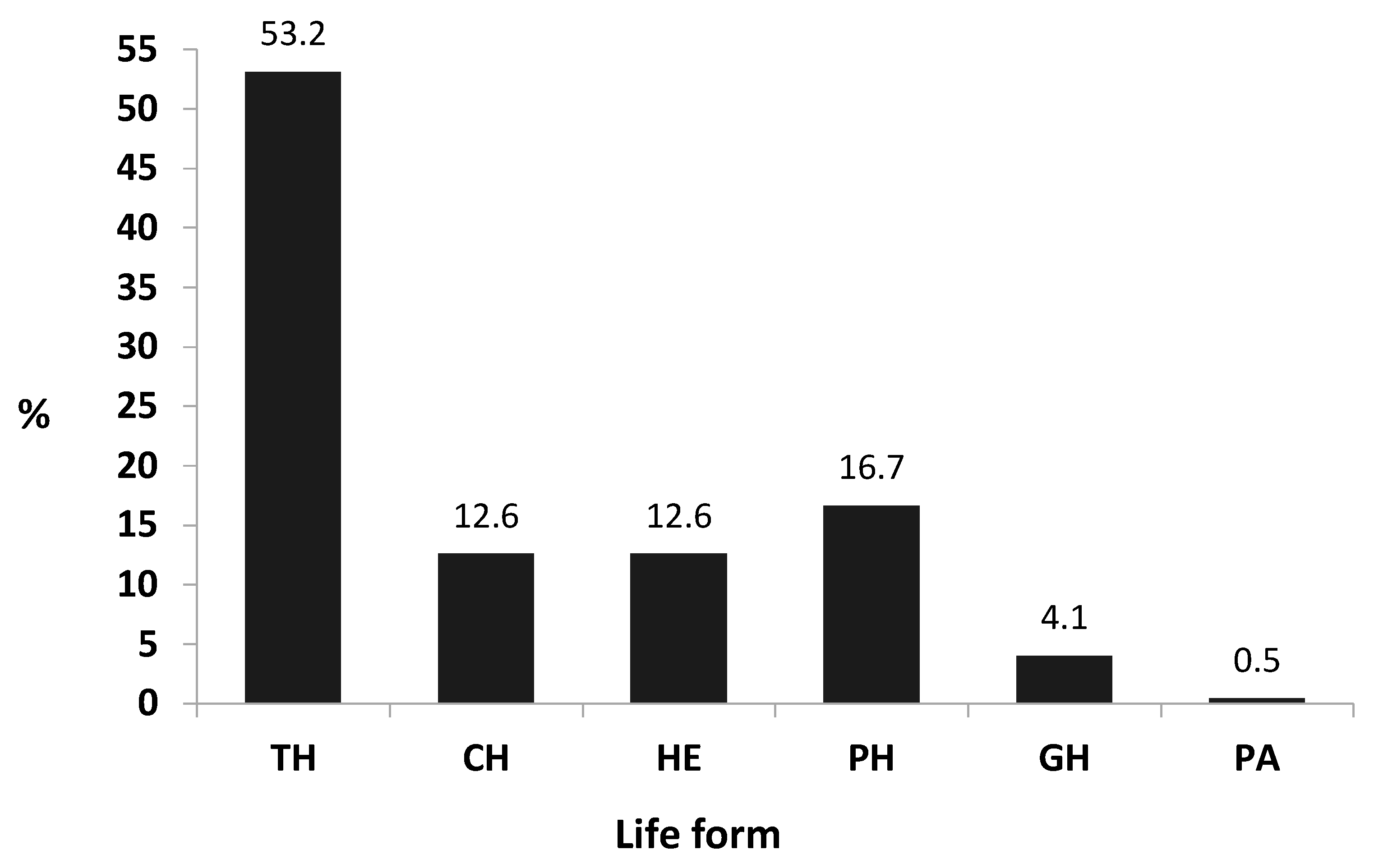
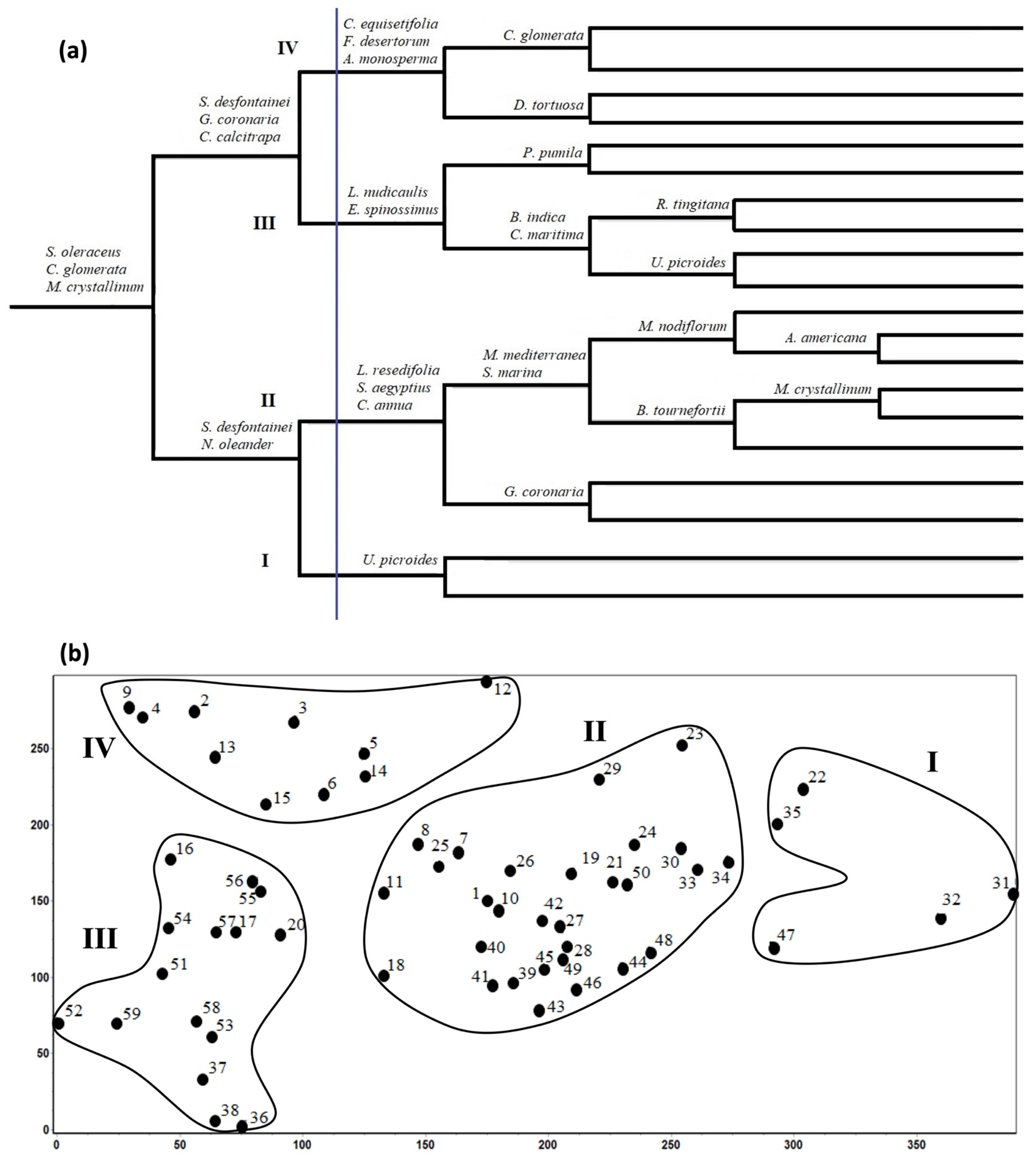
3.1. Floristic Analyses
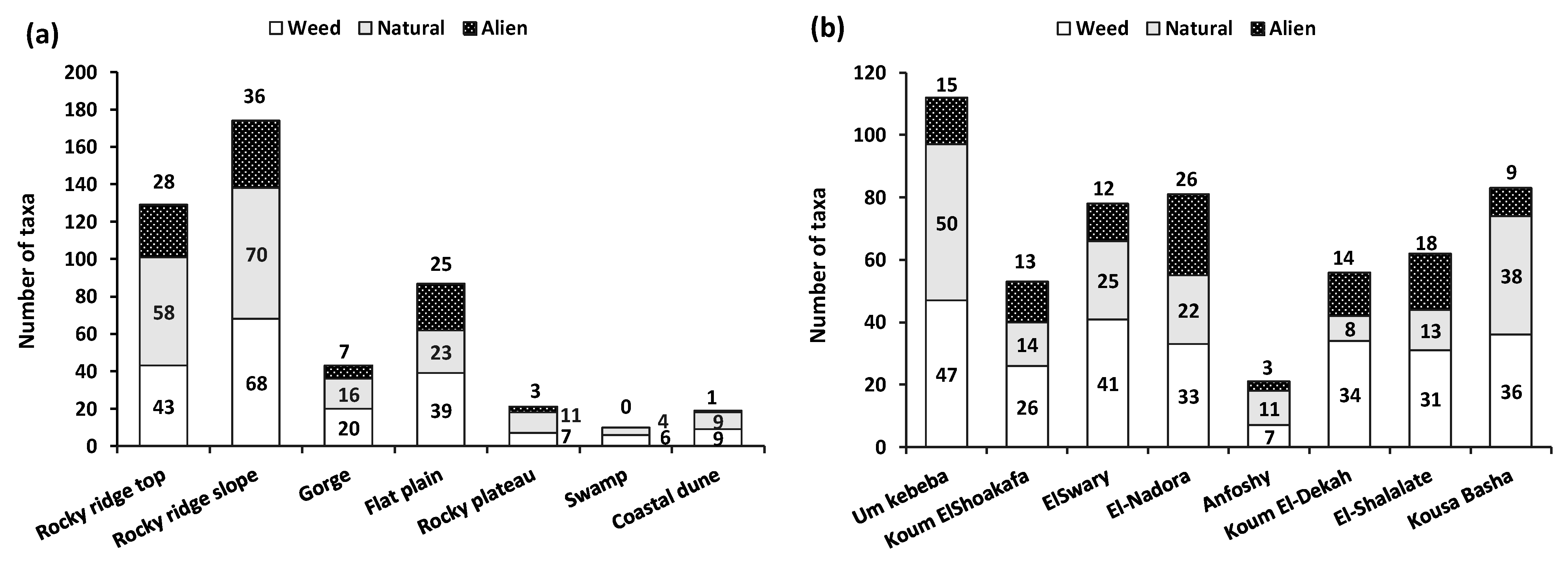
3.2. Plant Communities and Diversity Indices
3.3. Edaphic Factors and Vegetation Communities
4. Discussion
4.1. Floristic Composition
4.2. Plant Communities and Diversity Indices
4.3. Relationship between Edaphic Factors and Vegetation Communities
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caneva, G.; Benelli, F.; Bartoli, F.; Cicinelli, E. Safeguarding natural and cultural heritage on Etruscan tombs (La Banditaccia, Cerveteri, Italy). Rend. Lincei 2018, 29, 891–907. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Ricotta, C. Plant invasion as an emerging challenge for the conservation of heritage sites: The spread of ornamental trees on ancient monuments in Rome, Italy. Biol. Invasions 2021, 23, 1191–1206. [Google Scholar] [CrossRef]
- Cicinelli, E.; Salerno, G.; Caneva, G. An assessment methodology to combine the preservation of biodiversity and cultural heritage: The San Vincenzo al Volturno historical site (Molise, Italy). Biodivers. Conserv. 2018, 27, 1073–1093. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Sadori, L. Mediterranean Culture and Climatic Change: Past Patterns and Future Trends. In The Mediterranean Sea Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: London, UK, 2014; pp. 507–527. ISBN 978-94-007-6703-4. [Google Scholar]
- Baiamonte, G.; Domina, G.; Raimondo, F.M.; Bazan, G. Agricultural landscapes and biodiversity conservation: A case study in Sicily (Italy). Biodivers. Conserv. 2015, 24, 3201–3216. [Google Scholar] [CrossRef]
- Antrop, M. Why landscapes of the past are important for the future. Landsc. Urban Plan. 2005, 70, 21–34. [Google Scholar] [CrossRef]
- Bartoli, F.; Romiti, F.; Caneva, G. Aggressiveness of Hedera helix L. growing on monuments: Evaluation in Roman archaeological sites and guidelines for a general methodological approach. Plant Biosyst. 2017, 151, 866–877. [Google Scholar] [CrossRef]
- Ceschin, S.; Bartoli, F.; Salerno, G.; Zuccarello, V.; Caneva, G. Natural habitats of typical plants growing on ruins of Roman archaeological sites (Rome, Italy). Plant Biosyst. 2016, 150, 866–875. [Google Scholar] [CrossRef]
- Gao, H.; Ouyang, Z.; Chen, S.; van Koppen, C.S.A. Role of culturally protected forests in biodiversity conservation in Southeast China. Biodivers. Conserv. 2013, 22, 531–544. [Google Scholar] [CrossRef]
- Woods, C.L.; Cardelús, C.L.; Scull, P.; Wassie, A.; Baez, M.; Klepeis, P. Stone walls and sacred forest conservation in Ethiopia. Biodivers. Conserv. 2017, 26, 209–221. [Google Scholar] [CrossRef]
- Frosch, B.; Deil, U. Forest vegetation on sacred sites of the Tangier Peninsula (NW Morocco)–discussed in a SW-Mediterranean context. Phytocoenologia 2011, 41, 153–181. [Google Scholar] [CrossRef]
- Nielsen, A.B.; van den Bosch, M.; Maruthaveeran, S.; van den Bosch, C.K. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosyst. 2014, 17, 305–327. [Google Scholar] [CrossRef]
- Säumel, I.; Weber, F.; Kowarik, I. Toward livable and healthy urban streets: Roadside vegetation provides ecosystem services where people live and move. Environ. Sci. Policy 2016, 62, 24–33. [Google Scholar] [CrossRef]
- Jackson, W.; Ormsby, A. Urban sacred natural sites–a call for research. Urban Ecosyst. 2017, 20, 675–681. [Google Scholar] [CrossRef]
- Planchuelo, G.; von Der Lippe, M.; Kowarik, I. Untangling the role of urban ecosystems as habitats for endangered plant species. Landsc. Urban Plan. 2019, 189, 320–334. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G. Vascular plant colonisation of four castles in southern Italy: Effects of substrate bioreceptivity, local environment factors and current management. Int. Biodeterior. Biodegrad. 2018, 133, 26–33. [Google Scholar] [CrossRef]
- Caneva, G. A botanical approach to the planning of archaeological, parks in Italy. Conserv. Manag. Archaeol. Sites 1997, 3, 127–134. [Google Scholar] [CrossRef]
- Stanley, J.D.; Carslon, R.W.; Van Beek, G.; Jorstad, T.F.; Landau, E.A. Alexandria, Egypt, before Alexander the Great: A multidisciplinary approach yields rich discoveries. GSA Today 2007, 17, 4–10. [Google Scholar] [CrossRef]
- Véron, A.; Goiran, J.P.; Morhange, C.; Marriner, N.; Empereur, J.Y. Pollutant lead reveals the pre-Hellenistic occupation and ancient growth of Alexandria, Egypt. Geophys. Res. Lett. 2006, 33, L06409. [Google Scholar] [CrossRef] [Green Version]
- Mark, J.J. Alexandria, Egypt-World History Encyclopedia. Available online: https://www.worldhistory.org/alexandria/ (accessed on 10 November 2021).
- Boulos, L. Flora of Egypt, Volume 1; Al Hadara Publishing: Cairo, Egypt, 1999; ISBN 9789775429148. [Google Scholar]
- Boulos, L. Flora of Egypt, Volume 2; Al Hadara Publishing: Cairo, Egypt, 2000; ISBN 9789775429223. [Google Scholar]
- Boulos, L. Flora of Egypt, Volume 3; Al Hadara Publishing: Cairo, Egypt, 2002; ISBN 9789775429254. [Google Scholar]
- Boulos, L. Flora of Egypt, Volume 4; Al Hadara Publishing: Cairo, Egypt, 2005; ISBN 9789775429414. [Google Scholar]
- Raunkiær, C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Zohary, M. Flora Palestina. Part 2: Text Platanaceae to Umbelliferae; Israel Academy of Sciences and Humanities: Jerusalem, Palestine, 1972. [Google Scholar]
- Zohary, M. Flora Palaestina. Part 1: Text Equisetaceae to Moringaceae, 2nd ed.; Israel Academy of Sciences and Humanities: Jerusalem, Palestine, 1966; ISBN 9789652080004. [Google Scholar]
- Feinbrun-Dothan, N. Flora Palaestina Part 4: Alismataceae to Orchidaceae; Israel Academy of Sciences and Humanities: Jerusalem, Palestine, 1978. [Google Scholar]
- Feinbrun-Dothan, N. Flora Palaestina, Part 3: Ericaceae to Compositae; Israel Academy of Sciences and Humanities: Jerusalem, Palestine, 1977. [Google Scholar]
- Täckholm, V. Students’ Flora of Egypt, 2nd ed.; Cairo University: Cairo, Egypt, 1974. [Google Scholar]
- Boulos, L. Flora of Egypt Checklist, Revised Annotated Edition, 2nd ed.; Al Hadara Publishing: Cairo, Egypt, 2009; ISBN 9789774760020. [Google Scholar]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C.; Roberts, J.D. Chemical analysis. In Methods in Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell Scientific Publications: Oxford, UK, 1986; pp. 285–344. [Google Scholar]
- Stirling, G.; Wilsey, B. Empirical Relationships between Species Richness, Evenness, and Proportional Diversity. Am. Nat. 2001, 158, 286–299. [Google Scholar] [CrossRef]
- Magurran, A. Measuring Biologcial Diversity; Wiley-Blackwell: Hoboken, NJ, USA, 2004; ISBN 978-0-632-05633-0. [Google Scholar]
- Palmer, M.W. Putting Things in Even Better Order: The Advantages of Canonical Correspondence Analysis. Ecology 1993, 74, 2215–2230. [Google Scholar] [CrossRef]
- Hill, M.O. A Fortran Program for Detrended Correspondence Analysis and Reciprocal Averaging; Cornell University, Section of Ecology and Systematics: Ithaca, NY, USA, 1979. [Google Scholar]
- Hill, M.O. Twinspan: A Fortran Program for Arranging Multivariate Data in an Ordered Two-Way Table by Classification of the Individuals and Attributes; Cornell University Section of Ecology and Systematics: Ithaca NY, USA, 1979. [Google Scholar]
- Gauch, H.G.; Whittaker, R.H. Hierarchical Classification of Community Data. J. Ecol. 1981, 69, 537–557. [Google Scholar] [CrossRef]
- IBM. SPSS Statistics for Windows; IBM Crop: Armonk, NY, USA, 2012. [Google Scholar]
- Braak, C.J.F.T.; Looman, C.W.N. Regression. In Data Analysis in Community and Landscape Ecology; Jongman, R.H.G., Tongeren, O.F.R., TerBraak, C.J.F., van Tongeren, O.F.R., Eds.; Cambridge University Press: Wageningen, The Netherlands, 1987; pp. 29–77. [Google Scholar]
- Gopal, D.; von der Lippe, M.; Kowarik, I. Sacred sites, biodiversity and urbanization in an Indian megacity. Urban Ecosyst. 2019, 22, 161–172. [Google Scholar] [CrossRef]
- Kowarik, I.; Buchholz, S.; von der Lippe, M.; Seitz, B. Biodiversity functions of urban cemeteries: Evidence from one of the largest Jewish cemeteries in Europe. Urban For. Urban Green. 2016, 19, 68–78. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Soil Process. Curr. Trends Qual. Assess. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Shwartz, A.; Turbé, A.; Simon, L.; Julliard, R. Enhancing urban biodiversity and its influence on city-dwellers: An experiment. Biol. Conserv. 2014, 171, 82–90. [Google Scholar] [CrossRef]
- Shwartz, A.; Muratet, A.; Simon, L.; Julliard, R. Local and management variables outweigh landscape effects in enhancing the diversity of different taxa in a bigmetropolis. Biol. Conserv. 2013, 157, 285–292. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Getty Publications: Los Angeles, CA, USA, 2009. [Google Scholar]
- Heneidy, S.Z.; Halmy, M.W.A.; Toto, S.M.; Hamouda, S.K.; Fakhry, A.M.; Bidak, L.M.; Eid, E.M.; Al-Sodany, Y.M. Pattern of Urban Flora in Intra-City Railway Habitats (Alexandria, Egypt): A Conservation Perspective. Biology 2021, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Heneidy, S.Z.; Bidak, L.M. Multipurpose plant species in Bisha, Asir region, southwestern Saudi Arabia. J.-King Saud Univ. Sci. 2001, 13, 11–26. [Google Scholar]
- Le Houéou, H.N. The role of Browse in the management of natural grazing lands. In Browse in Africa; Le Houéou, H.N., Ed.; International Livestock Centre for Africa: Addis Ababa, Ethiopia, 1980; pp. 320–338. [Google Scholar]
- Heneidy, S.Z. Browsing and nutritive value of the most common range species in Matruh area, a coastal Mediterranean region, Egypt. Ecol. Mediterr. 2002, 28, 39–49. [Google Scholar] [CrossRef]
- Heneidy, S.Z. Diversity of Ecosystems. In Diversity of Ecosystems; Ali, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 127–166. ISBN 978-953-51-0572-5. [Google Scholar]
- Heneidy, S.Z.; Bidak, L.M. physical defenses and a version factor of some forage plant species in the western Mediterranean costal region of Egypt. J. Union Arab Biol. Cairo 1999, 9, 15–30. [Google Scholar]
- Minissale, P.; Sciandrello, S. The wild vascular flora of the Archaeological Park of Neapolis in Syracuse and surrounding areas (Sicily, Italy). Biodivers. J. 2017, 8, 87–104. [Google Scholar]
- Lausi, D.; Nimis, P.L. Roadside vegetation in boreal South Yukon and adjacent Alaska. Phytocoenologia 1985, 13, 103–138. [Google Scholar] [CrossRef]
- Adams, K.D.; Goebel, T.; Graf, K.; Smith, G.M.; Camp, A.J.; Briggs, R.W.; Rhode, D. Late Pleistocene and Early Holocene lake-level fluctuations in the Lahontan Basin, Nevada: Implications for the distribution of archaeological sites. Geoarchaeology 2008, 23, 608–643. [Google Scholar] [CrossRef]
- Vernon, K.B.; Yaworsky, P.M.; Spangler, J.; Brewer, S.; Codding, B.F. Decomposing Habitat Suitability Across the Forager to Farmer Transition. Environ. Archeol. 2020, 1–14. [Google Scholar] [CrossRef]
- Anderson, M.K. Tending the Wild: Native American Knowledge and the Management of California’s Natural Resources, 1st ed.; University of California Press: Berkeley, CA, USA, 2013; ISBN 9780520280434. [Google Scholar]
- Trauernicht, C.; Brook, B.W.; Murphy, B.P.; Williamson, G.J.; Bowman, D.M.J.S. Local and global pyrogeographic evidence that indigenous fire management creates pyrodiversity. Ecol. Evol. 2015, 5, 1908–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, T.M. Swamps: Wooded wetlands. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2009; pp. 549–557. [Google Scholar]
- Nadeau, M.B.; Sullivan, T.P. Relationships between Plant Biodiversity and Soil Fertility in a Mature Tropical Forest, Costa Rica. Int. J. For. Res. 2015, 2015, 732946. [Google Scholar] [CrossRef]
- Ayyad, M.A.; Fakhry, A.M. Plant biodiversity in the western Mediterranean desert of Egypt. Verh. Ges. Für Okol. 1996, 25, 65–76. [Google Scholar]
- Huston, M. Soil nutrients and tree species richness in Costa Rican forests. J. Biogeogr. 1980, 7, 147–157. [Google Scholar] [CrossRef]
- Hofmeister, J.; Hošek, J.; Modrý, M.; Roleček, J. The influence of light and nutrient availability on herb layer species richness in oak-dominated forests in central Bohemia. Plant Ecol. 2009, 205, 57–75. [Google Scholar] [CrossRef]
- Hrivnák, R.; Slezák, M.; Jarcuška, B.; Jarolímek, I.; Kochjarová, J. Native and Alien Plant Species Richness Response to Soil Nitrogen and Phosphorus in Temperate Floodplain and Swamp Forests. Forests 2015, 6, 3501–3513. [Google Scholar] [CrossRef] [Green Version]
- Janssens, F.; Peeters, A.; Tallowin, J.R.B.; Bakker, J.P.; Bekker, R.M.; Fillat, F.; Oomes, M.J.M. Relationship between soil chemical factors and grassland diversity. Plant Soil 1998, 202, 69–78. [Google Scholar] [CrossRef]
- Lindgren, P.M.F.; Sullivan, T.P. Influence of alternative vegetation management treatments on conifer plantation attributes: Abundance, species diversity, and structural diversity. For. Ecol. Manag. 2001, 142, 163–182. [Google Scholar] [CrossRef]
- Guo, Y.; Gong, P.; Amundson, R. Pedodiversity in the United States of America. Geoderma 2003, 117, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Mendoza, C.; Reyes-Agüero, J.A.; Aguirre-Rivera, J.R.; Peña-Valdivia, C.B. Distribución Geográfica y Ecológica de Ephedra L. en el altipla-no potosino. Rev. Chapingo Ser. Hortic. 2000, VI, 131–138. [Google Scholar] [CrossRef]
- Proctor, J.; Woodell, S.R.J. The Ecology of Serpentine Soils. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1975; Volume 9, pp. 255–366. [Google Scholar]
- Krasilnikov, P.; García-Calderón, N.E.; Elizabeth, F.-R. Pedogenesis and slope processes in subtropical mountain areas, Sierra Sur de Oaxaca, Mexico. Rev. Mex. Cienc. Geológicas 2007, 24, 469–486. [Google Scholar]
- Krasilnikov, P.; García-Calderón, N.E.; Galicia Palacios, M. del S. Soils Developed on different parent materials. TERRA Latinoam. 2007, 25, 335–344. [Google Scholar]
- Ström, L.; Olsson, T.; Tyler, G. Differences between calcifuge and acidifuge plants in root exudation of low-molecular organic acids. Plant Soil 1994, 167, 239–245. [Google Scholar] [CrossRef]
- Bárcenas-Argüello, M.L.; del Carmen Gutiérrez-Castorena, M.; Terrazas, T. The Role of Soil Properties in Plant Endemism—A Revision of Conservation Strategies. In Soil Processes and Current Trends in Quality Assessment; Hernandez Soriano, M.C., Ed.; InTech: Rijeka, Croatia, 2013; pp. 381–398. [Google Scholar]
- Veblen, K.E.; Young, T.P. A California Grasslands Alkali Specialist, Hemizonia pungens ssp. pungens, Prefers Non-Alkali Soil. J. Veg. Sci. 2009, 20, 170–176. [Google Scholar] [CrossRef]
- Ecker, A.J.E. Influence of Substrate Type and Microsite Availability on the Persistence of Foxtail Pine (Pinus balfouriana, Pinaceae) in the Klamath Mountains, California. Am. J. Bot. 2006, 93, 1615–1624. [Google Scholar] [CrossRef]
- Kirkpatrick, J.B.; Bridle, K.L. Environmental relationships of floristic variation in the alpine vegetation of southeast Australia. J. Veg. Sci. 1998, 9, 251–260. [Google Scholar] [CrossRef]


| Site | No. of Stands | Establishment Date [20] | Status | Area (m2) | Location | Description [20] |
|---|---|---|---|---|---|---|
| Um Kebeba Fortress/Tabieh Um Kebeba | 20 (Stand 1–20) | 19th century | Not protected, no constraint preventing common activities in the area such as grazing, fire, waste disposal | 62,156 | West of Alexandria | An archeological place, “Tabieh”, which the residents call a “cave”, and which was built to repel the attacks of invaders. It has two doors and four rooms, and is abandoned, which allowed the fishermen to take it as a place to gather and store some of their tools. |
| Catacombs of Koum ElShoakafa | 3 (Stand 21–23) | 2nd century | Protected, archeological sites restrictions | 15,144 | Short distance southwest of Pompey’s Pillar | The site comprises a multi-level labyrinth, with a big spiral staircase, in addition to lots of chambers ornamented with carved pillars, statues, and other religious symbols, burial niches, and sarcophagi of the syncretic Romano-Egyptian era, discovered in the early 20th century. |
| Pompey’s Pillar/ElSwary | 6 (Stand 24–29) | 298–302 A.D. | Protected, archeological sites restrictions | 22,391 | The middle of Alexandria | The 30 m high red Aswan granite pillar is a victory monument constructed about 300 A.D. for the Roman Emperor Diocletian, in an archeological site that previously included the Serapeum of Alexandria. |
| El-Nadora | 6 (Stand 30–35) | 14th century A.D. | Protected, archeological sites restrictions | 34,414 | In the customs district in Alexandria | Tel Kom Nadora appears as an elegant archeological tower, which was used by Napoleon’s armies to assess the numbers of ships coming to Alexandria; Muhammad Ali set up an observatory for the movement of stars and planets. On this hill, the companion Amr ibn al-Aas ascended when he opened the city (25 A.H.–645 A.D.) where he built his mosque, which has been known since that time as the first mosque in the city of Alexandria. The antique tower of Kom Nadora was built after the collapse of the ancient lighthouse of Alexandria. The tower is in the shape of an octagon, with a height of about 25 m, and it consists of 4 floors, connected by a spiral staircase of wood. The oldest plan for this tower was made by an Italian traveler (Hugo Comnelli), 1472 A.D. |
| Atta Fortress/Tabieh Atta/Anfoshy | 3 (Stand 36–38) | Not protected, no constraint preventing common activities in the area such as grazing, fire, waste disposal | 27,360 | Ras El-Tin Palace, the Naval Base and Qaitbay Citadel | Neighborhood used to hear the Ramadan cannon from the Atta site twice a day, firing at fast breaking at the time of the Maghrib call to prayer with another shot at Suhoor before the call to dawn prayer, until the silencing of the cannon in the late eighties. | |
| Koum El-Dekah | 8 (Stand 39–46) | 3rd century Roman Era | Protected, archeological site restrictions | 39,213 | In the heart of modern Alexandria | Represents part an ancient Roman cityscape complete with theater, public baths, houses, and palatial villas. What was once a Roman town was neglected, later rediscovered and excavated in 1960. Over the past half-century, discoveries have continued to be made on this site, including a series of lecture halls. The remains of a Roman Theater represent a noteworthy monument on the site. It contains 13 rows of seats arranged in a simple U-shaped formation. Most of the complex at Koum El-Dekah is residential, which includes opulent villas and homes for the wealthiest citizens of Alexandria throughout the 1st and 2nd centuries BCE. |
| El-Nahassen Fortress/Tabieh El-Nahassen/El-Shalalate Gardens | 4 (Stand 47–50) | The Greco-Roman era | Not fully protected | 37,474 | Al-Shatby district in middle of Alexandria | The site contained the royal palace gardens and old Alexandria library of the Greco-Roman era, and the third major public park in Alexandria. The parks were established following the ideas of the American landscaper and park-maker Frederick Law Olmsted, and include towers that were once a part of the ancient Roman Alexandrian wall and Copper Fortress, ‘Tabieh El-Nahassen’, where copper tools were manufactured in the era of Mohammed Ali. |
| Kusa Basha Fortress/Tabieh Kousa Basha | 9 (Stand 51–59) | 1807 AD | Not protected, no constraint preventing common activities in the area such as grazing, fire, waste disposal, completely degraded due to overexploitation by local inhabitants | 36,720 | Abu Qir east of Alexandria | One of the oldest taboos, it was established by order of Muhammad Ali Basha, who directed one of the senior commanders called “Muhammad Kousa Basha” to fortify Alexandria from French aggression. It was intended to be an impenetrable fortress to repel the attacks of invaders from eastern Alexandria, especially after the departure of the French campaign, and the failure of the English Fraser campaign. Tabieh is surrounded by a high external wall on all sides. This wall is separated from the sandy hill surrounding it by a trench 20 m wide and 8 m deep. |
| Vegetation Group | VG1 | VG2 | VG3 | VG4 |
|---|---|---|---|---|
| First dominant | Chenopodium murale | Glebionis coronaria | Reichardia tingitana | Emex spinosa |
| Presence (%) | 83 | 96 | 100 | 92 |
| Second dominant | Emex spinosa | Malva parviflora | Mesembryanthemum crystallinum | Glebionis coronaria |
| Presence (%) | 83 | 79 | 88 | 92 |
| Habitats | ||||
| Rocky ridge top | 16.7 | 12.5 | 25.0 | 30.8 |
| Rocky ridge slope | 33.3 | 62.5 | 43.8 | 46.2 |
| Gorge | 12.5 | 6.3 | 15.4 | |
| Flat plain | 50.0 | 8.3 | 7.7 | |
| Rocky plateau | 18.8 | |||
| Swamp | 4.2 | |||
| Coastal dune | 6.3 | |||
| Sites | ||||
| Tabieh Um Kebeba | 12.5 | 25.0 | 100.0 | |
| Koum ElShoakafa | 33.3 | 4.2 | ||
| ElSwary | 25.0 | |||
| El-Nadora | 50.0 | 12.5 | ||
| Anfoshy | 18.8 | |||
| Koum El-Dekah | 33.3 | |||
| Tabieh El-Nahassen | 16.7 | 12.5 | ||
| Tabieh Kousa Basha | 56.3 |
| Habitat | Richness | Simpson’s D | Shannon’s H′ | Hill’s N1 | Hill’s N2 | Evenness E1 | Evenness E5 |
|---|---|---|---|---|---|---|---|
| Rocky ridge top | 23.67 ± 6.08 | 0.06 ± 0.03 | 2.97 ± 0.36 | 20.49 ± 5.65 | 18.07 ± 5.16 | 0.95 ± 0.02 | 0.87 ± 0.03 |
| Rocky ridge slope | 25.60 ± 7.29 | 0.06 ± 0.02 | 3.05 ± 0.28 | 21.99 ± 6.25 | 19.42 ± 5.45 | 0.95 ± 0.01 | 0.88 ± 0.03 |
| Gorge | 18.83 ± 2.40 | 0.07 ± 0.01 | 2.77 ± 0.15 | 16.16 ± 2.38 | 14.38 ± 2.52 | 0.95 ± 0.02 | 0.88 ± 0.04 |
| Flat plain | 28.00 ± 7.13 | 0.05 ± 0.02 | 3.14 ± 0.29 | 24.00 ± 6.54 | 20.66 ± 5.76 | 0.95 ± 0.01 | 0.85 ± 0.02 |
| Rocky plateau | 12.33 ± 6.66 | 0.12 ± 0.04 | 2.31 ± 0.47 | 10.83 ± 5.51 | 9.78 ± 4.70 | 0.95 ± 0.02 | 0.90 ± 0.03 |
| Swamp | 10.00 | 0.15 | 2.12 | 8.29 | 6.74 | 0.92 | 0.79 |
| Coastal dune | 19.00 | 0.07 | 2.82 | 16.82 | 15.13 | 0.96 | 0.89 |
| F-value | 3.59 ** | 6.04 *** | 4.89 *** | 3.37 ** | 3.22 ** | 0.76 | 2.13 |
| Site | |||||||
| Tabieh Um Kebeba | 21.80 ± 6.56 | 0.07 ± 0.03 | 2.88 ± 0.35 | 18.82 ± 5.89 | 16.77 ± 5.37 | 0.95 ± 0.02 | 0.88 ± 0.03 |
| Koum ElShoakafa | 26.33 ± 7.37 | 0.06 ± 0.02 | 3.06 ± 0.33 | 22.10 ± 6.57 | 18.91 ± 5.91 | 0.94 ± 0.01 | 0.84 ± 0.02 |
| ElSwary | 28.50 ± 11.00 | 0.05 ± 0.02 | 3.16 ± 0.34 | 24.83 ± 9.06 | 22.18 ± 7.44 | 0.96 ± 0.01 | 0.89 ± 0.04 |
| El-Nadora | 28.33 ± 4.23 | 0.05 ± 0.01 | 3.17 ± 0.16 | 23.97 ± 3.78 | 20.73 ± 3.27 | 0.95 ± 0.01 | 0.86 ± 0.02 |
| Anfoshy | 12.33 ± 6.66 | 0.12 ± 0.04 | 2.31 ± 0.47 | 10.83 ± 5.51 | 9.78 ± 4.70 | 0.95 ± 0.02 | 0.90 ± 0.03 |
| Koum El-Dekah | 23.63 ± 4.66 | 0.06 ± 0.01 | 2.98 ± 0.19 | 20.10 ± 4.14 | 17.58 ± 3.91 | 0.95 ± 0.01 | 0.87 ± 0.03 |
| Tabieh El-Nahassen | 26.00 ± 11.69 | 0.07 ± 0.05 | 3.00 ± 0.61 | 22.61 ± 10.47 | 19.51 ± 9.21 | 0.95 ± 0.02 | 0.84 ± 0.04 |
| Tabieh Kousa Basha | 23.67 ± 5.43 | 0.06 ± 0.02 | 3.00 ± 0.24 | 20.53 ± 4.98 | 18.15 ± 4.58 | 0.95 ± 0.01 | 0.88 ± 0.03 |
| F-value | 2.23 * | 2.58 * | 2.60 * | 2.06 | 1.90 | 0.48 | 1.82 |
| Vegetation group | |||||||
| I | 27.83 ± 7.11 | 0.05 ± 0.02 | 3.13 ± 0.28 | 23.59 ± 6.41 | 20.33 ± 5.51 | 0.95 ± 0.01 | 0.85 ± 0.02 |
| II | 25.04 ± 7.75 | 0.06 ± 0.02 | 3.02 ± 0.32 | 21.49 ± 6.67 | 18.84 ± 5.83 | 0.95 ± 0.01 | 0.87 ± 0.04 |
| III | 20.75 ± 6.74 | 0.07 ± 0.03 | 2.84 ± 0.38 | 18.07 ± 5.90 | 16.11 ± 5.25 | 0.95 ± 0.01 | 0.89 ± 0.03 |
| IV | 23.00 ± 7.47 | 0.07 ± 0.04 | 2.92 ± 0.41 | 19.81 ± 6.78 | 17.61 ± 6.19 | 0.95 ± 0.02 | 0.88 ± 0.03 |
| Total | 23.71 ± 7.52 | 0.06 ± 0.03 | 2.96 ± 0.36 | 20.41 ± 6.54 | 17.98 ± 5.75 | 0.95 ± 0.02 | 0.87 ± 0.03 |
| F-value | 1.79 | 0.91 | 1.40 | 1.44 | 1.10 | 0.50 | 1.53 |
| Soil Variable | Richness | Simpson’s D | Shannon’s H′ | Hill’s N1 | Hill’s N2 | Evenness E1 | Evenness E5 |
|---|---|---|---|---|---|---|---|
| pH | 0.089 | −0.070 | 0.079 | 0.078 | 0.058 | 0.008 | −0.146 |
| EC | 0.127 | −0.105 | 0.128 | 0.145 | 0.161 | 0.165 | 0.119 |
| Ca2+ | 0.161 | −0.132 | 0.159 | 0.174 | 0.184 | 0.140 | 0.062 |
| Mg2+ | 0.095 | −0.082 | 0.098 | 0.112 | 0.130 | 0.145 | 0.126 |
| Na+ | 0.096 | −0.096 | 0.107 | 0.120 | 0.143 | 0.194 | 0.181 |
| K+ | −0.026 | −0.052 | 0.015 | −0.028 | −0.025 | −0.006 | −0.027 |
| HCO3− | 0.079 | −0.123 | 0.104 | 0.078 | 0.081 | 0.041 | 0.003 |
| Cl− | 0.104 | −0.073 | 0.099 | 0.122 | 0.138 | 0.155 | 0.121 |
| SO42− | 0.159 | −0.160 | 0.174 | 0.177 | 0.193 | 0.169 | 0.107 |
| SAR | −0.078 | 0.001 | −0.036 | −0.044 | −0.007 | 0.237 | 0.331 * |
| TDS | 0.127 | −0.105 | 0.128 | 0.145 | 0.161 | 0.165 | 0.119 |
| N | −0.030 | −0.039 | 0.001 | −0.046 | −0.058 | −0.124 | −0.126 |
| P | 0.290 * | −0.263 * | 0.281 * | 0.274 * | 0.242 | 0.099 | −0.224 |
| Fe | −0.170 | 0.159 | −0.180 | −0.184 | −0.190 | −0.238 | −0.090 |
| Zn | −0.170 | 0.092 | −0.133 | −0.156 | −0.142 | −0.013 | 0.074 |
| Mn | −0.327 * | 0.345 ** | −0.360 ** | −0.319 * | −0.308 * | 0.052 | 0.188 |
| Cu | −0.112 | 0.000 | −0.064 | −0.101 | −0.092 | 0.140 | 0.141 |
| OM | 0.104 | −0.019 | 0.065 | 0.081 | 0.046 | −0.149 | −0.278 * |
| CaCO3 | −0.044 | −0.003 | −0.018 | −0.041 | −0.052 | −0.013 | −0.114 |
| Gravel | 0.225 | −0.230 | 0.249 | 0.245 | 0.238 | 0.184 | −0.070 |
| Sand | −0.069 | 0.083 | −0.079 | −0.055 | −0.029 | 0.024 | 0.185 |
| Silt | −0.020 | 0.001 | −0.014 | −0.033 | −0.052 | −0.070 | −0.138 |
| Clay | 0.110 | −0.120 | 0.120 | 0.097 | 0.071 | 0.006 | −0.186 |
| SP | 0.057 | −0.122 | 0.094 | 0.060 | 0.053 | 0.057 | −0.035 |
| Alt | 0.126 | −0.291 * | 0.221 | 0.112 | 0.111 | 0.021 | −0.014 |
| Soil Variable | F-Value | ||||
|---|---|---|---|---|---|
| Location | Habitat | VG | |||
| Physical properties | pH | 2.19 * | 1.34 | 0.78 | |
| EC | dS/m | 0.96 | 0.22 | 0.47 | |
| Gravel | % | 7.75 *** | 1.97 | 0.81 | |
| Sand | 12.73 *** | 0.28 | 5.03 ** | ||
| Silt | 6.15 *** | 0.12 | 2.35 | ||
| Clay | 11.77 *** | 0.56 | 5.95 *** | ||
| SP | 6.87 *** | 0.50 | 3.10* | ||
| SAR | 0.92 | 1.46 | 2.09 | ||
| OM | 13.63 *** | 0.68 | 5.37 *** | ||
| CaCO3 | 1.09 | 0.45 | 1.04 | ||
| Available nutrients | TDS | ppm | 0.96 | 0.22 | 0.85 |
| N | 6.38 *** | 0.92 | 7.37 *** | ||
| P | 16.34 *** | 2.26 | 13.28 *** | ||
| Cations | Ca2+ | meq/L | 1.03 | 0.15 | 1.11 |
| Mg2+ | 1.58 | 0.39 | 1.67 | ||
| Na+ | 0.83 | 0.31 | 0.86 | ||
| K+ | 2.90 ** | 0.90 | 3.25 * | ||
| Anions | HCO3− | 2.30 * | 0.35 | 2.09 | |
| Cl− | 1.20 | 0.51 | 0.82 | ||
| SO42− | 0.81 | 0.01 | 0.87 | ||
| Heavy metals | Fe | ppm | 5.35 *** | 1.72 | 4.65 ** |
| Zn | 3.98 ** | 1.94 | 1.30 | ||
| Mn | 9.24 *** | 13.82 *** | 2.34 | ||
| Cu | 4.82 *** | 2.07 | 0.60 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heneidy, S.Z.; Al-Sodany, Y.M.; Bidak, L.M.; Fakhry, A.M.; Hamouda, S.K.; Halmy, M.W.A.; Alrumman, S.A.; Al-Bakre, D.A.; Eid, E.M.; Toto, S.M. Archeological Sites and Relict Landscapes as Refuge for Biodiversity: Case Study of Alexandria City, Egypt. Sustainability 2022, 14, 2416. https://doi.org/10.3390/su14042416
Heneidy SZ, Al-Sodany YM, Bidak LM, Fakhry AM, Hamouda SK, Halmy MWA, Alrumman SA, Al-Bakre DA, Eid EM, Toto SM. Archeological Sites and Relict Landscapes as Refuge for Biodiversity: Case Study of Alexandria City, Egypt. Sustainability. 2022; 14(4):2416. https://doi.org/10.3390/su14042416
Chicago/Turabian StyleHeneidy, Selim Z., Yassin M. Al-Sodany, Laila M. Bidak, Amal M. Fakhry, Sania K. Hamouda, Marwa W. A. Halmy, Sulaiman A. Alrumman, Dhafer A. Al-Bakre, Ebrahem M. Eid, and Soliman M. Toto. 2022. "Archeological Sites and Relict Landscapes as Refuge for Biodiversity: Case Study of Alexandria City, Egypt" Sustainability 14, no. 4: 2416. https://doi.org/10.3390/su14042416








