Do Spatially Structured Soil Variables Influence the Plant Diversity in Tabuk Arid Region, Saudi Arabia?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Floristic Data
2.3. Soil Variables
2.4. Spatial Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel Khalik, K.; El-Sheikh, M.; El-Aidarous, A. Floristic diversity and vegetation analysis of wadi Al-Noman, Holy Mecca, Saudi Arabia. Turk. J. Bot. 2013, 37, 894–907. [Google Scholar] [CrossRef]
- Alsherif, E.A. Ecological studies of Commiphora genus (myrrha) in Makkah Region, Saudi Arabia. Heliyon 2019, 5, e01615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galal, T.M.; Al-Yasi, H.M.; Fadl, M.A. Vegetation zonation along the desert-wetland ecosystem of Taif Highland, Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 3374–3383. [Google Scholar] [CrossRef] [PubMed]
- Alfarhan, A.; Al-Turki, T.; Thomas, J.; Basahy, R.A. Annotated list to the flora of Farasan Archipelago, Southern Red Sea, Saudi Arabia. Taeckholmia 2002, 22, 1–33. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, R.; Gao, S.; Guo, J.; Sun, W. Responses of Plant Community Composition and Biomass Production to Warming and Nitrogen Deposition in a Temperate Meadow Ecosystem. PLoS ONE 2015, 10, e0123160. [Google Scholar] [CrossRef]
- Qin, Y.; Adamowski, J.F.; Deo, R.C.; Hu, Z.; Cao, J.; Zhu, M.; Feng, Q. Controlling factors of plant community composition with respect to the slope aspect gradient in the Qilian Mountains. Ecosphere 2019, 10, 02851. [Google Scholar] [CrossRef]
- Kumordzi, B.B.; Wardle, D.A.; Freschet, G.T. Plant assemblages do not respond homogenously to local variation in environmental conditions: Functional responses differ with species identity and abundance. J. Veg. Sci. 2014, 26, 32–45. [Google Scholar] [CrossRef]
- Lu, X.; Freschet, G.T.; Kazakou, E.; Wang, Z.-W.; Zhou, L.-S.; Han, X. Contrasting responses in leaf nutrient-use strategies of two dominant grass species along a 30-yr temperate steppe grazing exclusion chronosequence. Plant Soil 2015, 387, 69–79. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.M.; Tuomisto, H.; Borcard, D.; Legendre, P.; Clark, D.B.; Olivas, P.C. Explaining variation in tropical plant community composition: Influence of environmental and spatial data quality. Oecologia 2007, 155, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, X.; Soininen, J.; Chu, C.-J.; Zhang, J.-Q.; Yu, K.-L.; Wang, G. Relative importance of spatial processes and environmental factors in shaping alpine meadow communities. J. Plant Ecol. 2011, 4, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Hautier, Y.; Isbell, F.; Borer, E.T.; Seabloom, E.W.; Harpole, W.S.; Lind, E.M.; MacDougall, A.S.; Stevens, C.; Adler, P.B.; Alberti, J.; et al. Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, A.; Ebeling, A.; Kunz, M.; Staab, M.; Guimarães-Steinicke, C.; Bachmann, D.; Buchmann, N.; Durka, W.; Fichtner, A.; Fornoff, F.; et al. Multiple plant diversity components drive consumer communities across ecosystems. Nat. Commun. 2019, 10, 1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, M.J.; Dale, M.R.; Ver Hoef, J.M. Spatial Analysis in Ecology. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–13. [Google Scholar]
- Yang, H.; Flower, R.J.; Battarbee, R.W. Influence of environmental and spatial variables on the distribution of surface sediment diatoms in an upland loch, Scotland. Acta Bot. Croat. 2009, 68, 367–380. [Google Scholar]
- Vormisto, J.; Svenning, J.-C.; Hall, P.; Balslev, H. Diversity and dominance in palm (Arecaceae) communities in terra firme forests in the western Amazon basin. J. Ecol. 2004, 92, 577–588. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, T.; Govaert, S.; De Kesel, W.; Berge, S.V.D.; Vangansbeke, P.; Meeussen, C.; Brunet, J.; Cousins, S.A.O.; Decocq, G.; Diekmann, M.; et al. Plant diversity in hedgerows and road verges across Europe. J. Appl. Ecol. 2020, 57, 1244–1257. [Google Scholar] [CrossRef]
- Hillebrand, H. On the Generality of the Latitudinal Diversity Gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [Green Version]
- Ricklefs, R.E. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 2004, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lan, G.; Getzin, S.; Wiegand, T.; Hu, Y.; Xie, G.; Zhu, H.; Cao, M. Spatial distribution and interspecific associations of tree species in a tropical seasonal rain forest of China. PLoS ONE 2012, 7, e46074. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, O.; Kunah, O.; Dubinina, Y.; Zhukova, Y.; Ganzha, D. The effect of soil on spatial variation of the herbaceous layer modulated by overstorey in an eastern European Poplar-willow forest. Ekologia 2019, 38, 253–272. [Google Scholar] [CrossRef] [Green Version]
- Pittarekki, M.; Lonati, M.; Enri, S.R.; Lombardi, G. Environmental factors and management intensity affect in different ways plant diversity and pastoral value of alpine pastures. Ecol. Indic. 2020, 115, 106429. [Google Scholar]
- Hegazy, A.; Demerdashb, M.-; Hosni, H. Vegetation, species diversity and floristic relations along an altitudinal gradient in south-west Saudi Arabia. J. Arid Environ. 1998, 38, 3–13. [Google Scholar] [CrossRef]
- Lan, G.; Hu, Y.-H.; Cao, M.; Zhu, H. Topography related spatial distribution of dominant tree species in a tropical seasonal rain forest in China. For. Ecol. Manag. 2011, 262, 1507–1513. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.-C.; Hernandez, C.; Romoleroux, K.; Losos, E.; Magard, E.; Balslev, H. Tree species distributions and local habitat variation in the Amazon: Large forest plot in eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Wyatt, J.L.; Silman, M.R. Distance-dependence in two Amazonian palms: Effects of spatial and temporal variation in seed predator communities. Oecologia 2004, 140, 26–35. [Google Scholar] [CrossRef]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia: Illustrated; Ministry of Agriculture and Water, National Herbarium, National Agriculture and Water Research Center: Riyadh, Saudi Arabia, 2000; Volume VI, 368p.
- Collenette, I.S. Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development: Riyadh, Saudi Arabia, 1999; Volume XXXII, 799p. [Google Scholar]
- Allen, S. Chemical Analysis for Ecological Materials; Blackwell Scientific Publications: Hoboken, NJ, USA, 1989. [Google Scholar]
- APHA. Water Environment Federation 2005. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Diniz-Filho, J.A.F.; Bini, L.M. Modelling geographical patterns in species richness using eigenvector-based spatial filters. Glob. Ecol. Biogeogr. 2005, 14, 177–185. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002, 153, 51–68. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2008. Available online: http://www.R-project.org/ (accessed on 12 January 2022).
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometerics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Schloerke, B.; Crowley, J.; Cook, D.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Larmarange, J. GGally: Extension to ’ggplot2’, R package version 1.4.0; 2018, p. 361. Available online: https://CRAN.R-project.org/package=GGally (accessed on 12 January 2022).
- El-Ghani, M.M.A.; Amer, W.M. Soil-vegetation relationships in a coastal desert plain of southern Sinai, Egypt. J. Arid. Environ. 2003, 55, 607–628. [Google Scholar] [CrossRef]
- Al-Turki, T.; Al-Olayan, H. Contribution to the flora of Saudi Arabia: Hail region. Saudi J. Biol. Sci. 2003, 10, 190–222. [Google Scholar]
- Al-Mutairi, K.; Al-Shami, S.; Khorshid, Z.; Moawed, M. Floristic diversity of Tabuk Province, North Saudi Arabia Arabia. J. Anim. Plant Sci. 2016, 26, 1019–1025. [Google Scholar]
- El-Sheikh, M.A.; Thomas, J.; Arif, I.A.; El-Sheikh, H.M. Ecology of inland sand dunes ‘‘nafuds” as a hyper-arid habitat, Saudi Arabia: Floristic and plant associations diversity. J. Biol. Sci. 2021, 28, 1503–1513. [Google Scholar] [CrossRef]
- Al-Yasi, H.M.; Alotaibi, S.S.; Al-Sodany, Y.M.; Galal, T.M. Plant distribution and diversity along altitudinal gradient of Sarrawat Mountains at Taif Province, Saudi Arabia. Biosci. Res. 2019, 16, 1198–1213. [Google Scholar]
- Elaidarous, A.A.; Osman, H.E.; Galal, T.M.; El-Morsy, M.H. Vegetation–environment relationship and foristic diversity of Wadi Al-Sharaea, Makkah Province, Saudi Arabia. Rend. Lincei Sci. Fis. 2022, in press. [Google Scholar] [CrossRef]
- El-Sheikh, M.A.; Al-Shehri, M.A.; Alfarhan, A.H.; Alatar, A.A.; Rajakrishnan, R.; Al-Rowaily, S.L. Threatened Prunus arabica in an ancient volcanic protected area of Saudi Arabia: Floristic diversity and plant associations. Saudi J. Biol. Sci. 2019, 26, 325–333. [Google Scholar] [CrossRef]
- Alharthi, A.; El-Sheikh, M.A.; Elhag, M.; Alatar, A.A.; Abbadi, G.A.; Abdel-Salam, E.M.; Arif, I.A.; Baeshen, A.A.; Eid, E.M. Remote sensing of 10 years changes in the vegetation cover of the northwestern coastal land of Red Sea, Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 3169–3179. [Google Scholar] [CrossRef]
- Al-Nafie, A. Phytogeography of Saudi Arabia. Saudi J. Biol. Sci. 2008, 15, 159–176. [Google Scholar]
- Al-Robai, S.A.; Mohamed, H.A.; Howladar, S.M.; Ahmed, A.A. Vegetation structure and species diversity of Wadi Turbah Zahran, Albaha area, southwestern Saudi Arabia. Ann. Agric. Sci. 2017, 62, 61–69. [Google Scholar] [CrossRef]
- Welter-Schultes, F.W.; Williams, M.R. History, island area and habitat availability determine land snail species richness of Aegean islands. J. Biogeogr. 1999, 26, 239–249. [Google Scholar] [CrossRef]
- Cody, M.L. Plants on Islands: Diversity and Dynamics on a Continental Archipelago; University of California Press: Oakland, CA, USA, 2006. [Google Scholar]
- Kreft, H.; Jetz, W.; Mutke, J.; Kier, G.; Barthlott, W. Global diversity of island floras from a macroecological perspective. Ecol. Lett. 2007, 11, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Lozano, F.D.; Price, J.; Otto, R.; Fernández-Palacios, J.M. Using taxonomic and phylogenetic evenness to compare diversification in two Island Floras. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 93–106. [Google Scholar] [CrossRef]
- Gwali, S.; Okullo, P.; Hafashimana, D.; Byabashaija, D.M. Taxonomic Diversity, Distinctness, and Abundance of Tree and Shrub Species in Kasagala Forest Reserve in Uganda: Implications for Management and Conservation Policy Decisions. Trop. Conserv. Sci. 2010, 3, 319–333. [Google Scholar] [CrossRef]
- Pineda, A.; Bortolini, J.C.; Rodrigues, L.C. Effects of space and environment on phytoplankton distribution in subtropical reservoirs depend on functional features of the species. Aquat. Sci. 2021, 84, 5. [Google Scholar] [CrossRef]
- Al-Mutairi, K.A.; Al-Shami, S.A. Spatial and Environmental determinants of plant diversity in Farasan Archipelago, Saudi Arabia. Life Sci. J. 2014, 11, 61–69. [Google Scholar]
- Bartha, S.; Czárán, T.; Oborny, B. Spatial constraints masking community assembly rules: A simulation study. Folia Geobot. 1995, 30, 471–482. [Google Scholar] [CrossRef]
- Karst, J.; Gilbert, B.; Lechowicz, M.J. Fern Community Assembly: The Roles of Chance and the Environment at Local and Intermediate Scales. Ecology 2005, 86, 2473–2486. [Google Scholar] [CrossRef]
- Zhao, C.-M.; Chen, W.-L.; Tian, Z.-Q.; Xie, Z.-Q. Altitudinal Pattern of Plant Species Diversity in Shennongjia Mountains, Central China. J. Integr. Plant Biol. 2005, 47, 1431–1449. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; von Gadow, K. Partitioning temperate plant community structure at different scales. Acta Oecologica 2010, 36, 306–313. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, T.; Zhang, J.-L.; Sun, Q.-M. Spatial and environmental determinants of plant species diversity in a temperate desert. J. Plant Ecol. 2016, 9, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.T.; Long, R.J.; Wang, Q.J.; Ding, L.M.; Wang, M.P. Effects of altitude on plant-species diversity and productivity in an alpine meadow, Qinghai–Tibetan plateau. Aust. J. Bot. 2007, 55, 110–117. [Google Scholar] [CrossRef] [Green Version]

| Aldesah | Alzetah | Alawz | Harrah | Sharma | F-Value | p | |
|---|---|---|---|---|---|---|---|
| pH | 7.860 ± 0.212 | 7.983 ± 0.129 | 8.167 ± 0.025 | 7.963 ± 0.218 | 7.947 ± 0.270 | 1.040 | 0.434 |
| EC (mS cm−1) | 0.340 ± 0.312 | 0.193 ± 0.035 | 0.207 ± 0.067 | 0.260 ± 0.095 | 0.200 ± 0.050 | 0.499 | 0.737 |
| Sand | 74.253 ± 21.911 | 89.117 ± 6.885 | 86.267 ± 9.621 | 77.237 ± 5.690 | 86.830 ± 6.450 | 0.930 | 0.785 |
| Silt | 21.043 ± 19.661 | 8.230 ± 4.451 | 10.847 ± 9.358 | 18.970 ± 5.698 | 10.960 ± 5.152 | 0.860 | 0.520 |
| Clay | 4.703 ± 2.318 | 2.653 ± 2.686 | 2.887 ± 2.794 | 3.793 ± 0.854 | 2.210 ± 2.126 | 0.582 | 0.683 |
| Na (mg kg−1) | 0.948 ± 0.708 | 0.512 ± 0.331 | 0.564 ± 0.367 | 0.451 ± 0.278 | 0.568 ± 0.133 | 0.683 | 0.620 |
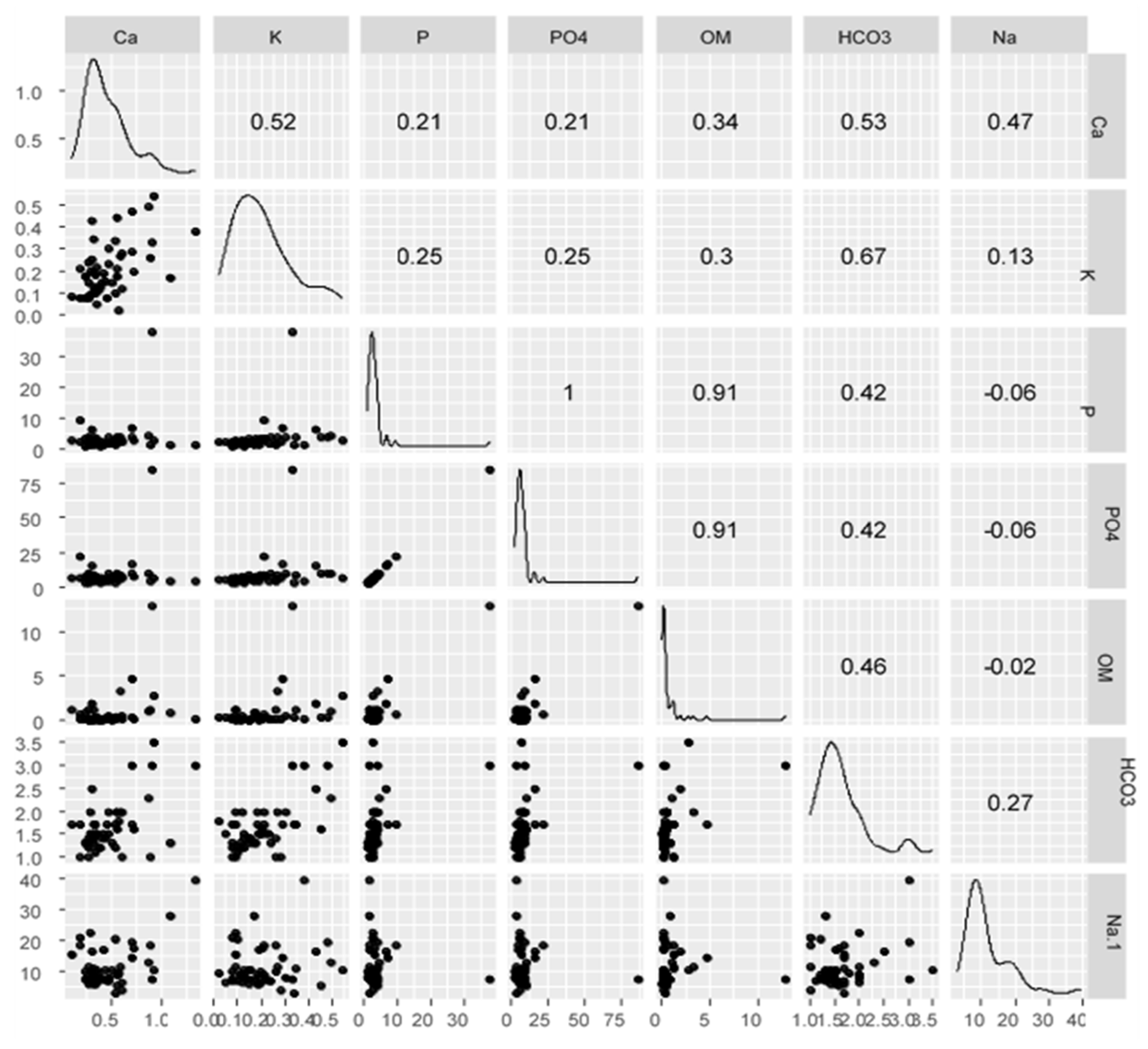
| Ca (mg kg−1) | 0.610 ± 0.628 | 0.482 ± 0.097 | 0.292 ± 0.047 | 0.640 ± 0.257 | 0.378 ± 0.171 | 0.660 | 0.634 |
| K (mg kg−1) | 0.227 ± 0.142 | 0.218 ± 0.198 | 0.122 ± 0.029 | 0.337 ± 0.182 | 0.261 ± 0.172 | 0.739 | 0.587 |
| P (mg kg−1) | 4.567 ± 4.291 | 3.067 ± 1.097 | 3.100 ± 0.608 | 2.470 ± 0.590 | 4.100 ± 2.651 | 0.397 | 0.806 |
| PO4 (mg kg−1) | 10.458 ± 9.827 | 7.023 ± 2.512 | 7.099 ± 1.393 | 5.656 ± 1.350 | 9.389 ± 6.072 | 0.379 | 0.806 |
| OM (%) | 0.341 ± 0.260 | 0.211 ± 0.173 | 0.079 ± 0.036 | 1.022 ± 1.504 | 2.117 ± 1.051 | 3.087 | 0.048 |
| HCO3 (mg kg−1) | 1.933 ± 0.971 | 1.333 ± 0.231 | 1.633 ± 0.321 | 2.000 ± 1.323 | 1.833 ± 0.764 | 0.317 | 0.860 |
| Aldesah | Alzetah | Alawz | Harah | Sharma | ANOVA | |
|---|---|---|---|---|---|---|
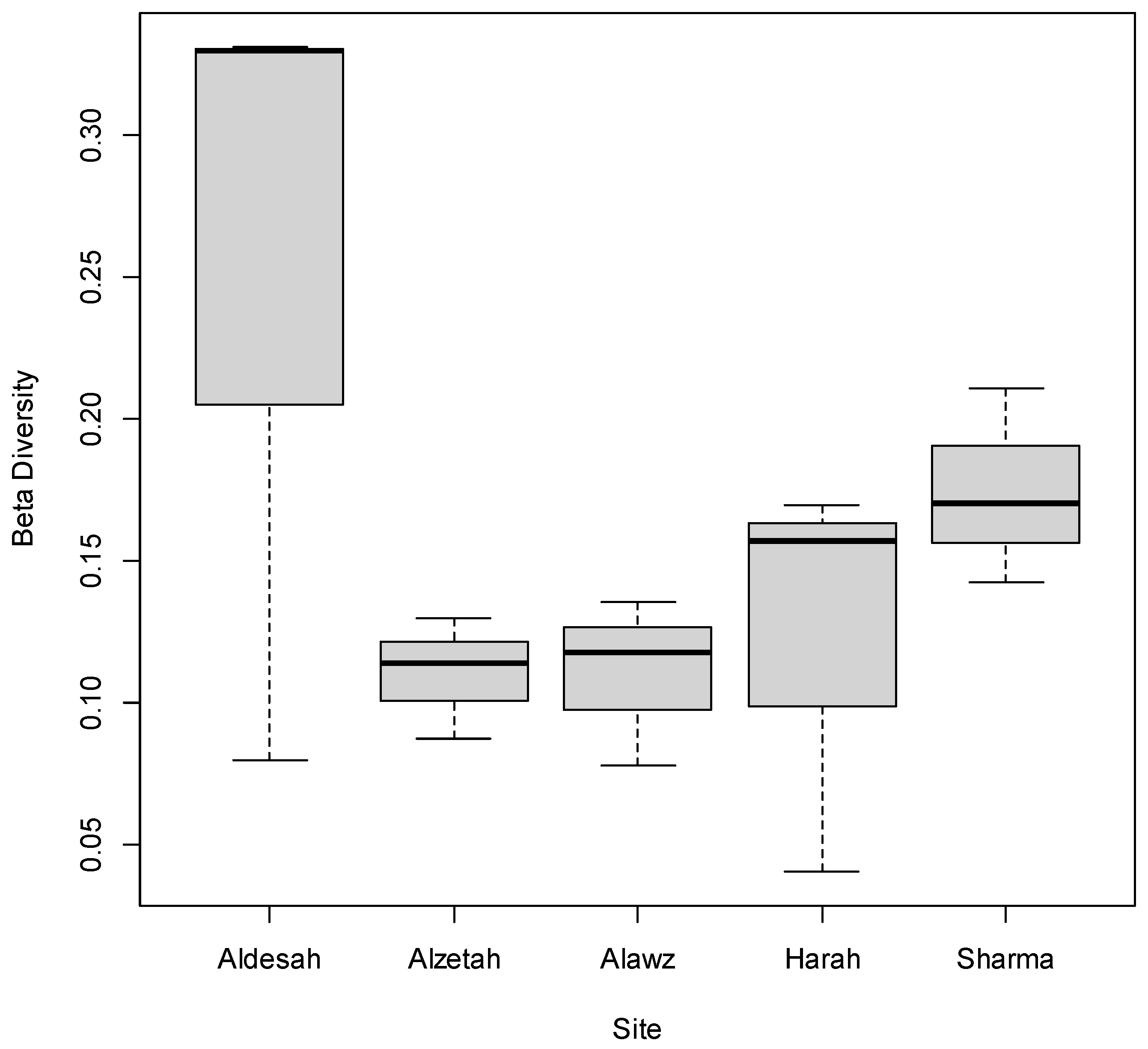
| Alpha diversity | 8.333 ± 3.512 | 26.333 ± 4.509 | 29.667 ± 4.163 | 24.000 ± 1.000 | 43.000 ± 4.583 | 32.204 * |
| Shannon | 2.490 ± 0.468 | 3.742 ± 0.175 | 3.866 ± 0.148 | 3.657 ± 0.043 | 4.246 ± 0.111 | 22.927 * |
| Evenness | 1.541 ± 0.045 | 1.617 ± 0.005 | 1.621 ± 0.004 | 1.615 ± 0.002 | 1.629 ± 0.002 | 9.391 * |
| Brillouin | 1.317 ± 0.354 | 2.359 ± 0.158 | 2.472 ± 0.135 | 2.282 ± 0.038 | 2.823 ± 0.103 | 26.188 * |
| Menhinick | 2.843 ± 0.614 | 5.119 ± 0.439 | 5.438 ± 0.389 | 4.898 ± 0.102 | 6.551 ± 0.353 | 31.823 * |
| Margalef | 3.426 ± 0.972 | 7.734 ± 0.972 | 8.448 ± 0.884 | 7.236 ± 0.220 | 11.160 ± 0.907 | 32.774 * |
| Equitability_J | 1.214 ± 0.033 | 1.148 ± 0.007 | 1.143 ± 0.005 | 1.151 ± 0.002 | 1.130 ± 0.004 | 14.042 * |
| Chao-1 | 39.333 ± 30.333 | 354.000 ± 120.003 | 446.333 ± 119.822 | 288.833 ± 24.002 | 932.000 ± 193.845 | 23.717 * |
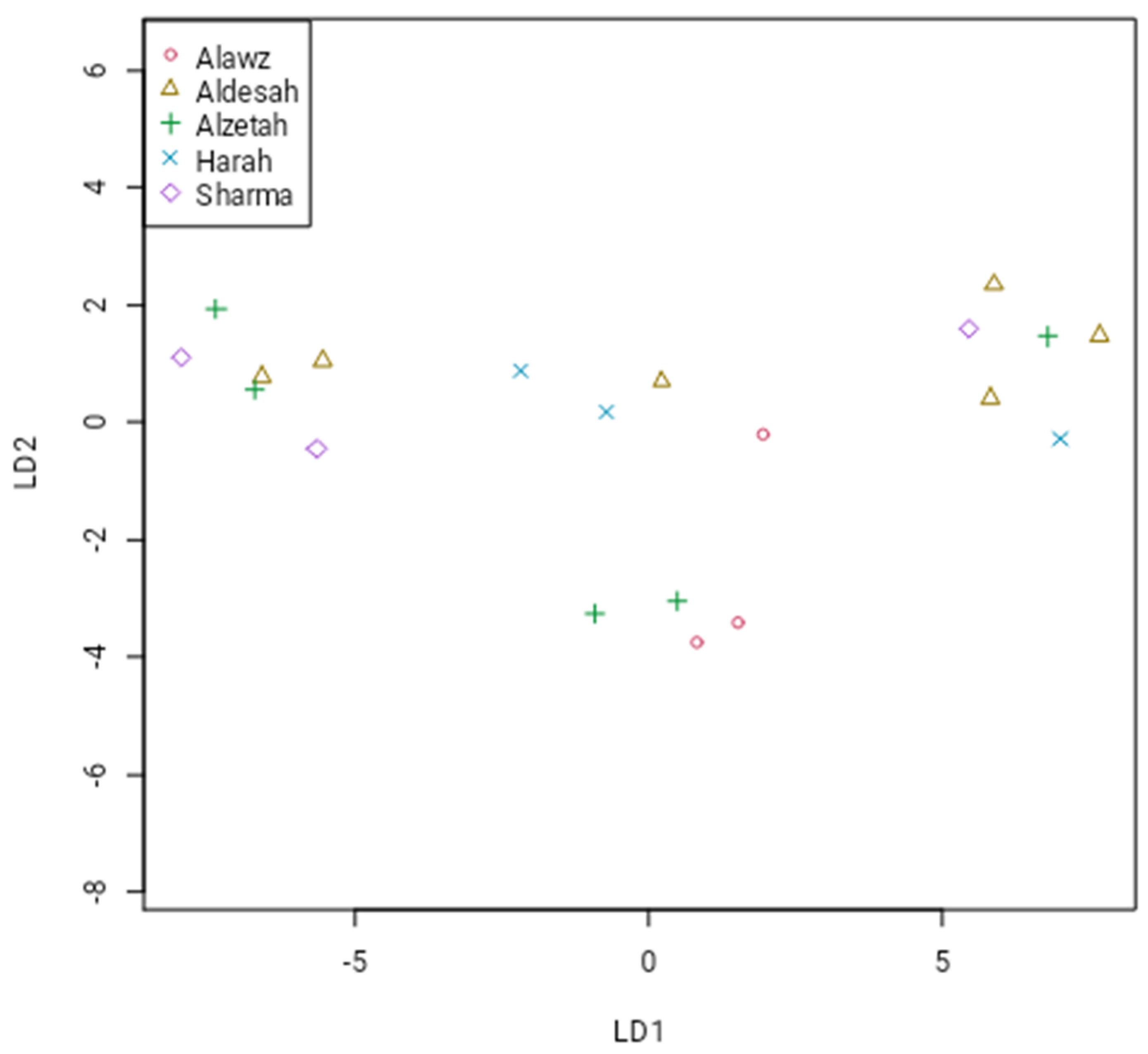
| PCoA1 | PCoA2 | PCoA3 | PCoA4 | |
|---|---|---|---|---|
| Aldesah | −0.492 | −0.039 | −0.165 | −0.129 |
| Alzetah | −0.357 | −0.0984 | 0.172 | 0.163 |
| Alawz | 0.115 | 0.504 | 0.043 | −0.035 |
| Harah | 0.445 | −0.232 | −0.243 | 0.054 |
| Sharma | 0.239 | −0.082 | 0.335 | −0.131 |
| ANOVA | F = 3.876 p = 0.0491 | |||
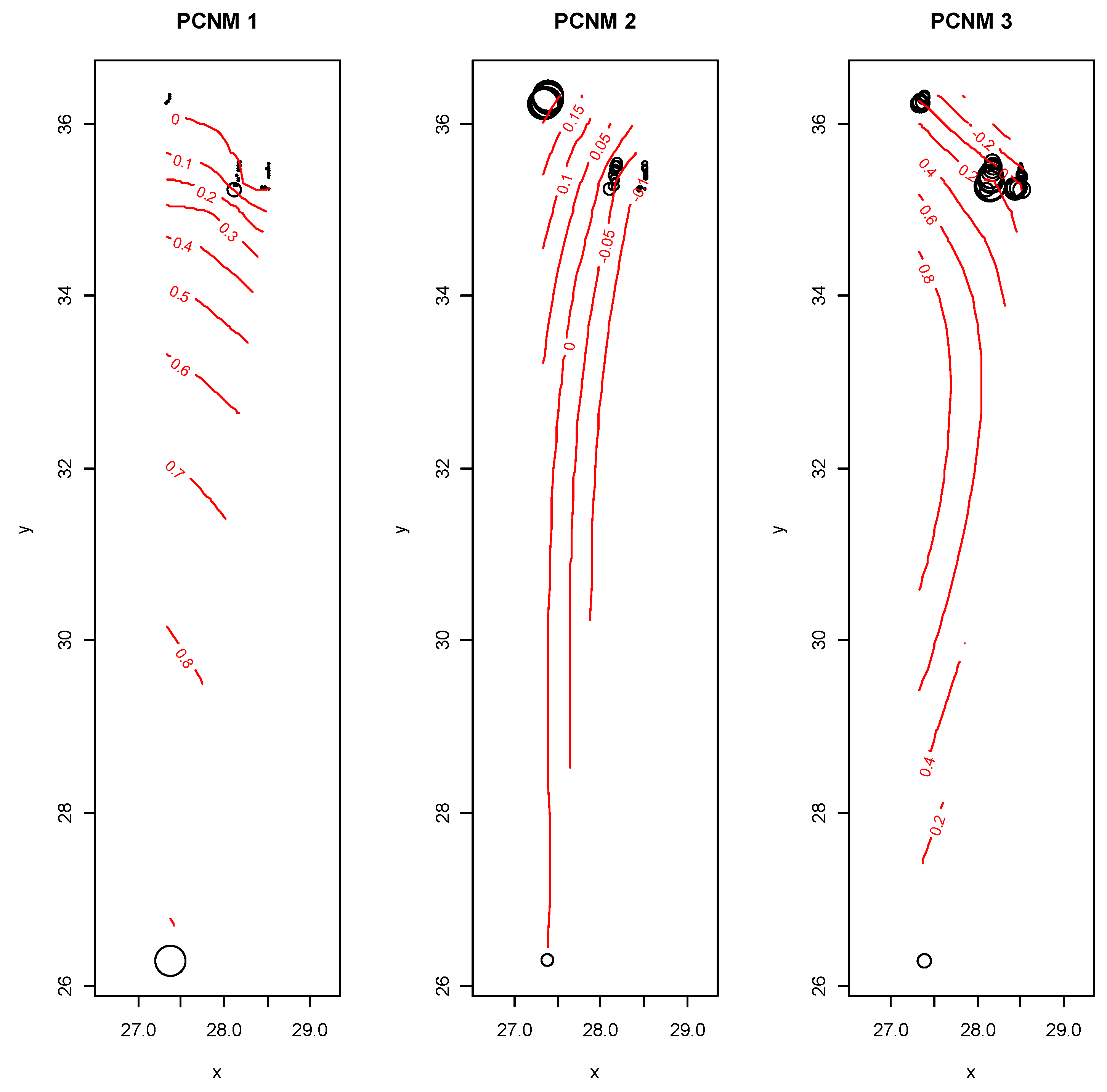
| PCNM1 | PCNM2 | PCNM3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate ± SE | t | p | Estimate ± SE | t | p | Estimate ± SE | t | p | |
| Intercept | −3.224 ± 1.984 | −1.624 | 0.113 | 0.509 ± 1.281 | 0.398 | 0.693 | 0.053 ± 1.726 | 0.032 | 0.975 |
| pH | 0.375 ± 0.052 | 2.473 | 0.018 | −0.192 ± 0.978 | −1.964 | 0.057 | −0.124 ± 0.1.2 | −0.300 | 0.355 |
| EC | −0.522 ± 0.578 | −0.902 | 0.373 | −0.073 ± 0.038 | −0.195 | 0.847 | 0.511 ± 0.503 | 1.016 | 0.316 |
| Sand | 0.007 ± 0.013 | 0.055 | 0.956 | 0.011 ± 0.008 | 1.301 | 0.201 | 0.008 ± 0.015 | 0.698 | 0.489 |
| Silt | 0.004 ± 0.016 | 0.224 | 0.824 | 0.013 ± 0.010 | 1.320 | 0.195 | 0.014 ± 0.014 | 1.041 | 0.304 |
| Ca | −0.008 ± 0.019 | −0.399 | 0.692 | −0.169 ± 0.126 | −1.348 | 0.186 | 0.006 ± 0.169 | 0.038 | 0.970 |
| K | 0.693 ± 0.231 | 2.997 | 0.005 | 0.213 ± 0.179 | 1.428 | 0.161 | 0.026 ± 0.201 | 0.131 | 0.896 |
| P | 0.005 ± 0.004 | 1.236 | 0.224 | 0.004 ± 0.027 | 1.580 | 0.122 | 0.003 ± 0.004 | 0.068 | 0.394 |
| PO4 | −0.002 ± 0.002 | −1.263 | 0.261 | −0.002 ± 0.001 | −1.570 | 0.124 | −0.001 ± 0.002 | −0.063 | 0.393 |
| OM | 0.002 ± 0.029 | 0.679 | 0.501 | 0.116 ± 0.019 | 5.660 | 0.001 | −0.008 ± 0.025 | −0.297 | 0.768 |
| HCO3 | 0.046 ± 0.051 | 0.791 | 0.434 | −0.070 ± 0.038 | −1.858 | 0.071 | 0.095 ± 0.050 | 1.885 | 0.067 |
| Na | 0.138 ± 0.114 | 1.237 | 0.223 | 0.172 ± 0.072 | 2.392 | 0.022 | −0.354 ± 0.097 | −3.658 | 0.001 |
| A dj-R2 | 0.175 | 0.561 | 0.365 | ||||||
| F-value | 1.944 | 6.703 | 3.565 | ||||||
| p | 0.046 | 0.000 | 0.002 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mutairi, K.A. Do Spatially Structured Soil Variables Influence the Plant Diversity in Tabuk Arid Region, Saudi Arabia? Sustainability 2022, 14, 2611. https://doi.org/10.3390/su14052611
Al-Mutairi KA. Do Spatially Structured Soil Variables Influence the Plant Diversity in Tabuk Arid Region, Saudi Arabia? Sustainability. 2022; 14(5):2611. https://doi.org/10.3390/su14052611
Chicago/Turabian StyleAl-Mutairi, Khalid Awadh. 2022. "Do Spatially Structured Soil Variables Influence the Plant Diversity in Tabuk Arid Region, Saudi Arabia?" Sustainability 14, no. 5: 2611. https://doi.org/10.3390/su14052611






