The Utilization of Modified Zeolite for the Removal of Cs Ions in an Aqueous Solution: Adsorption Capacity, Isotherms, Kinetics and Microscopic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Solutions and Sorbents
2.2. Experiments
2.3. Adsorption Kinetics and Isotherms
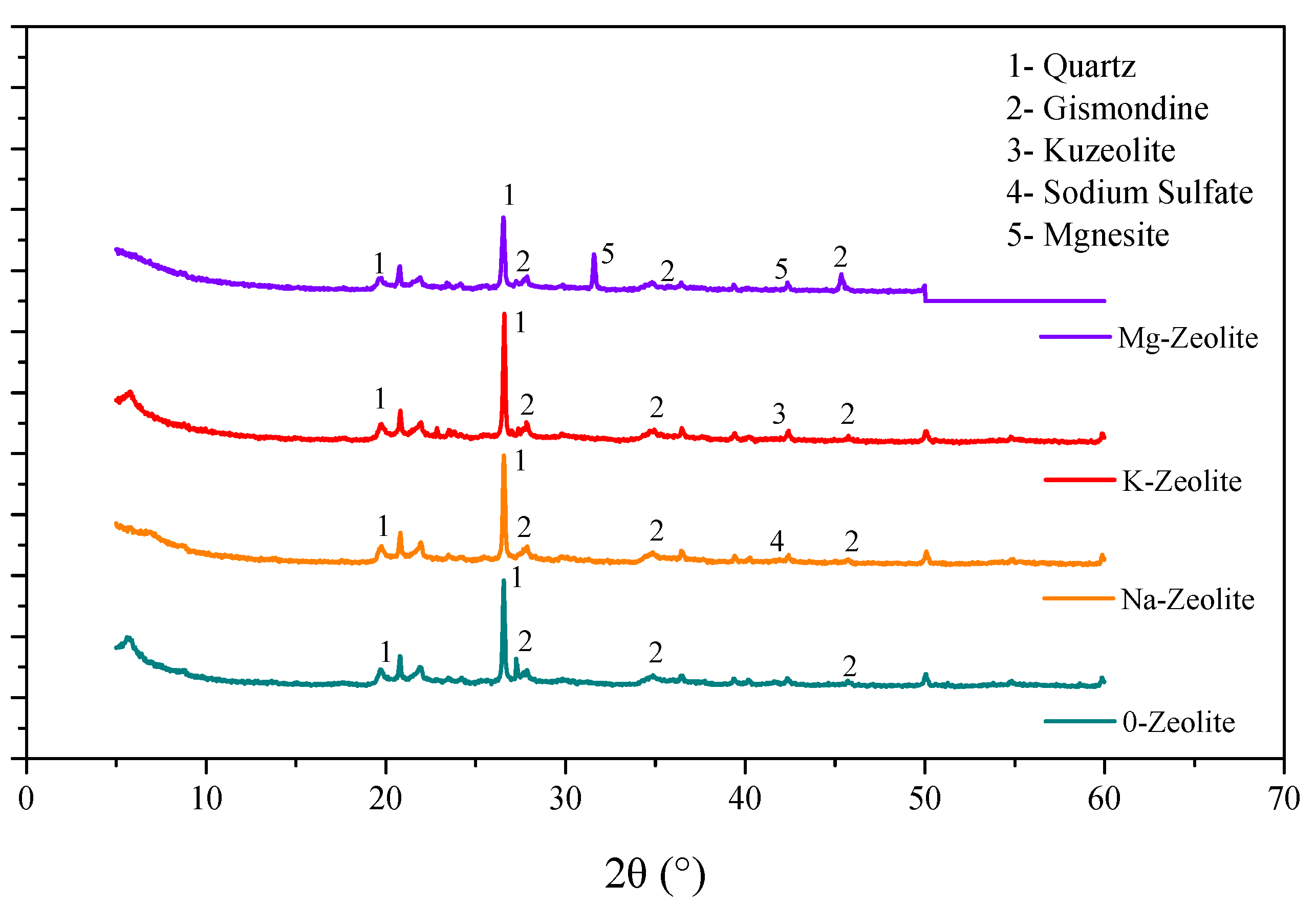
2.4. Sorbents Characterization
3. Results and Discussion
3.1. Experiment Results
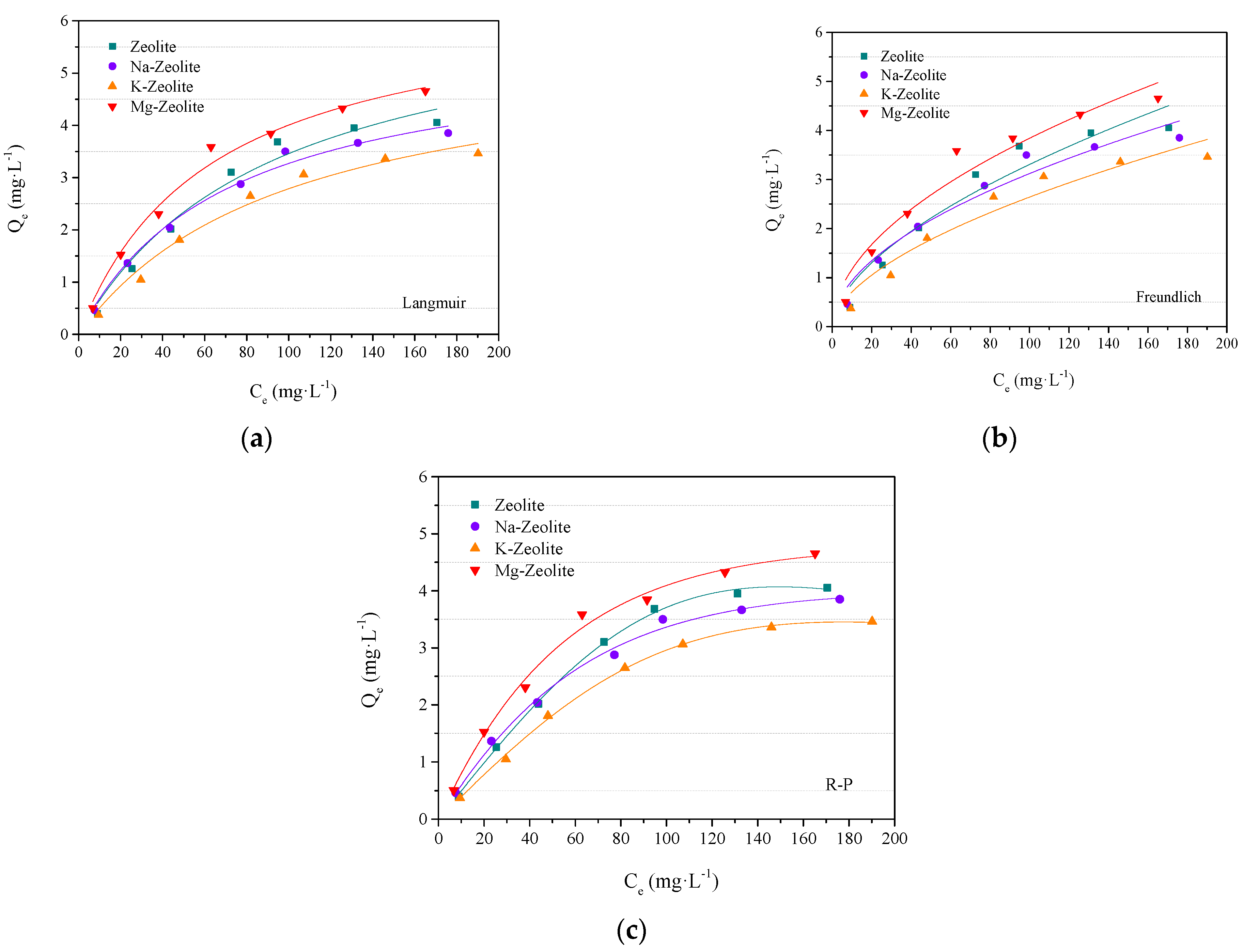
3.2. Adsorption Isotherms
3.3. Adsorption Kinetics
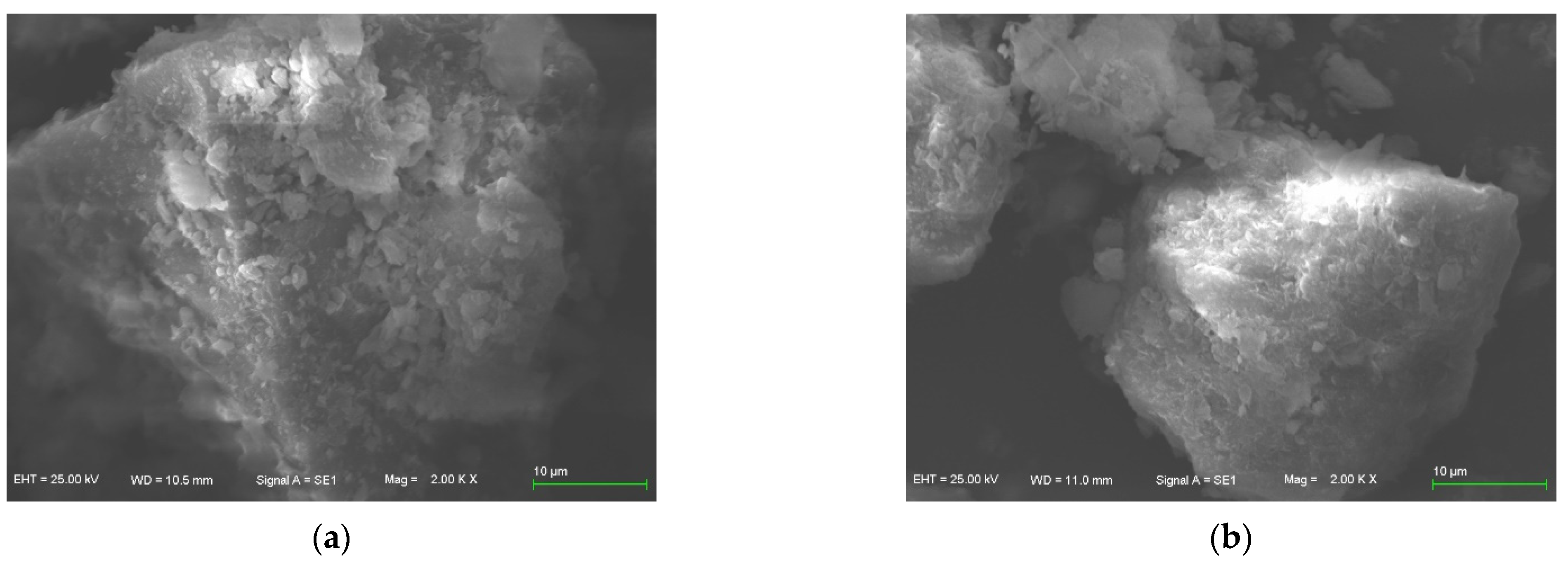
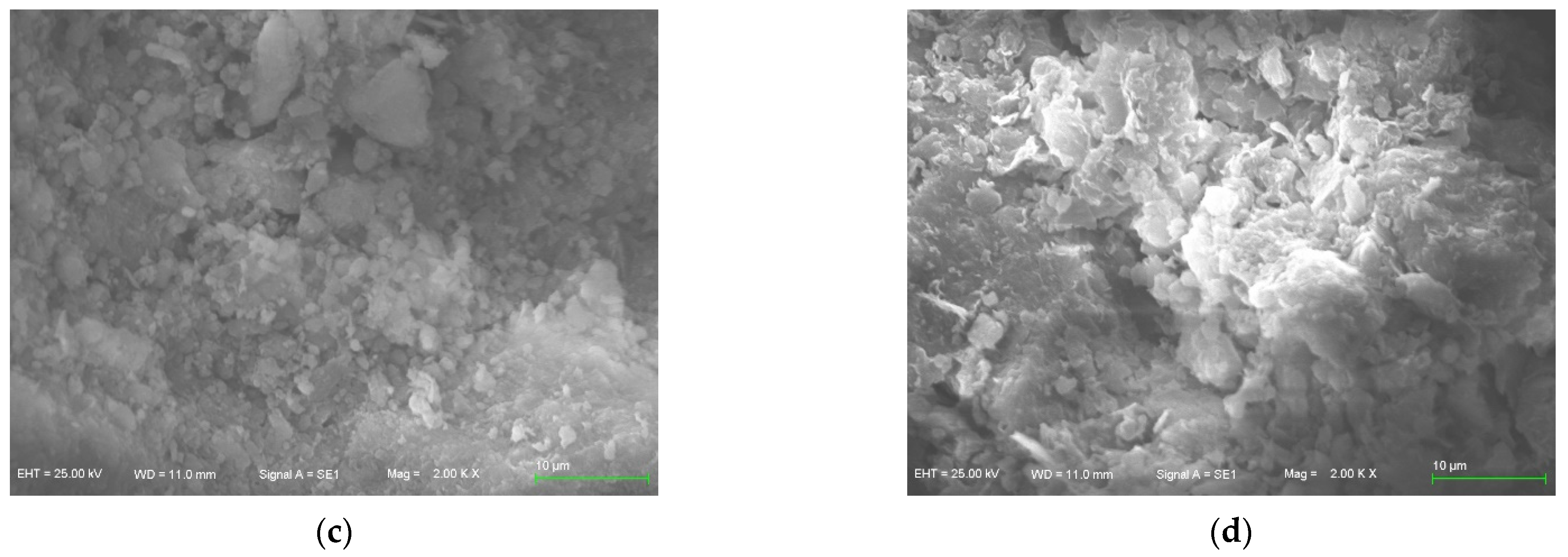
3.4. Sorbent’s Micro-Scope Characterization
4. Conclusions
- (1)
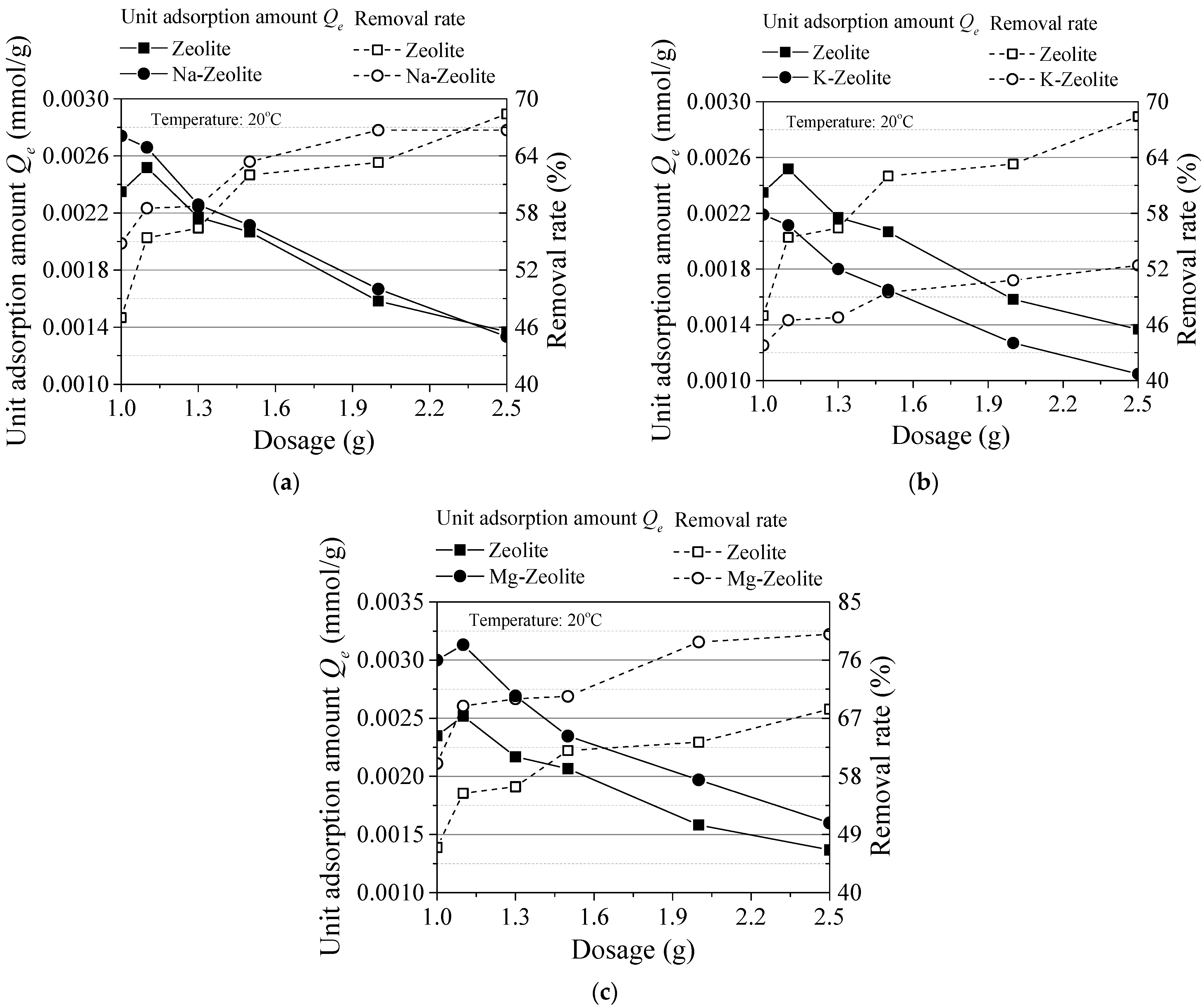
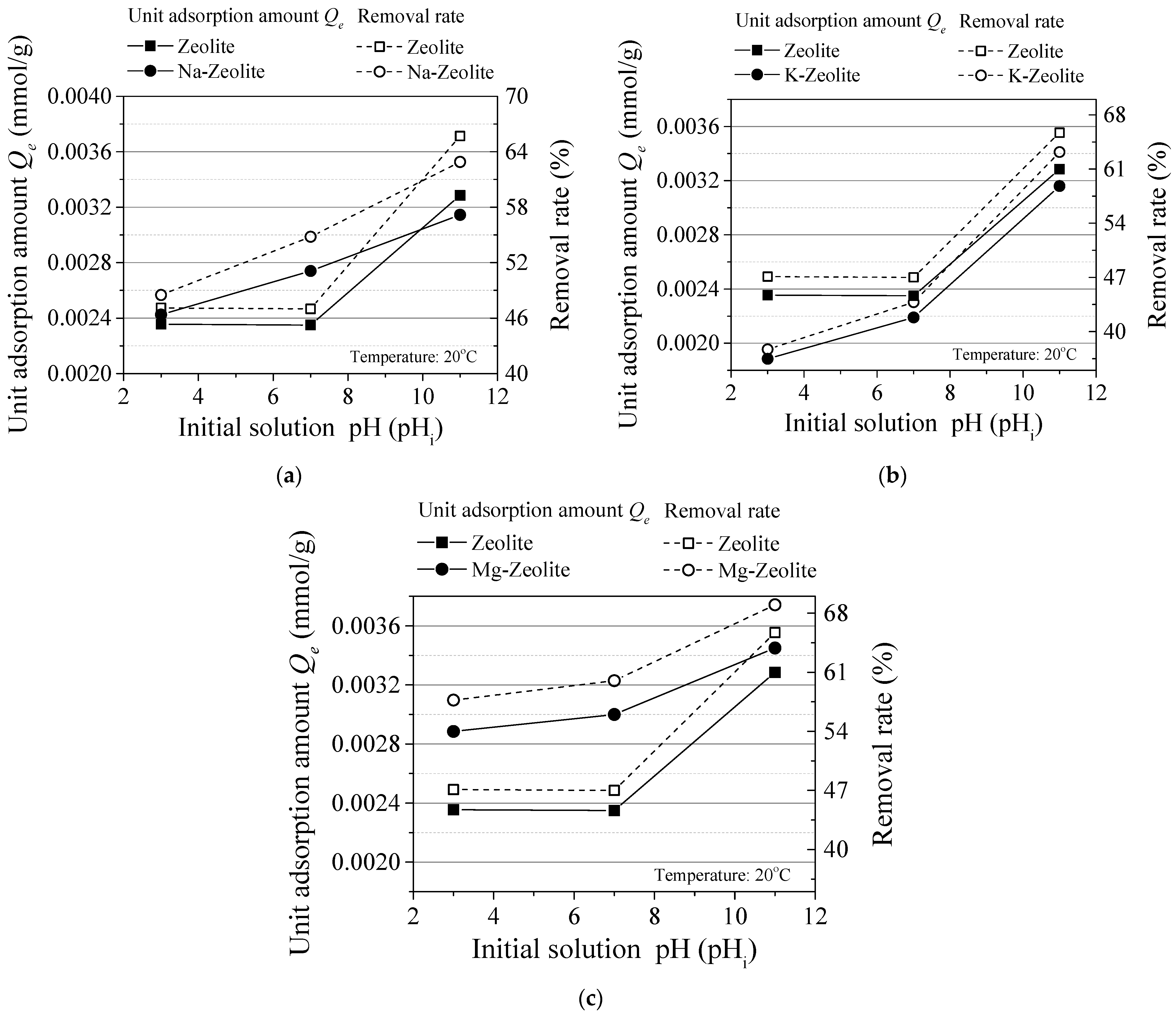
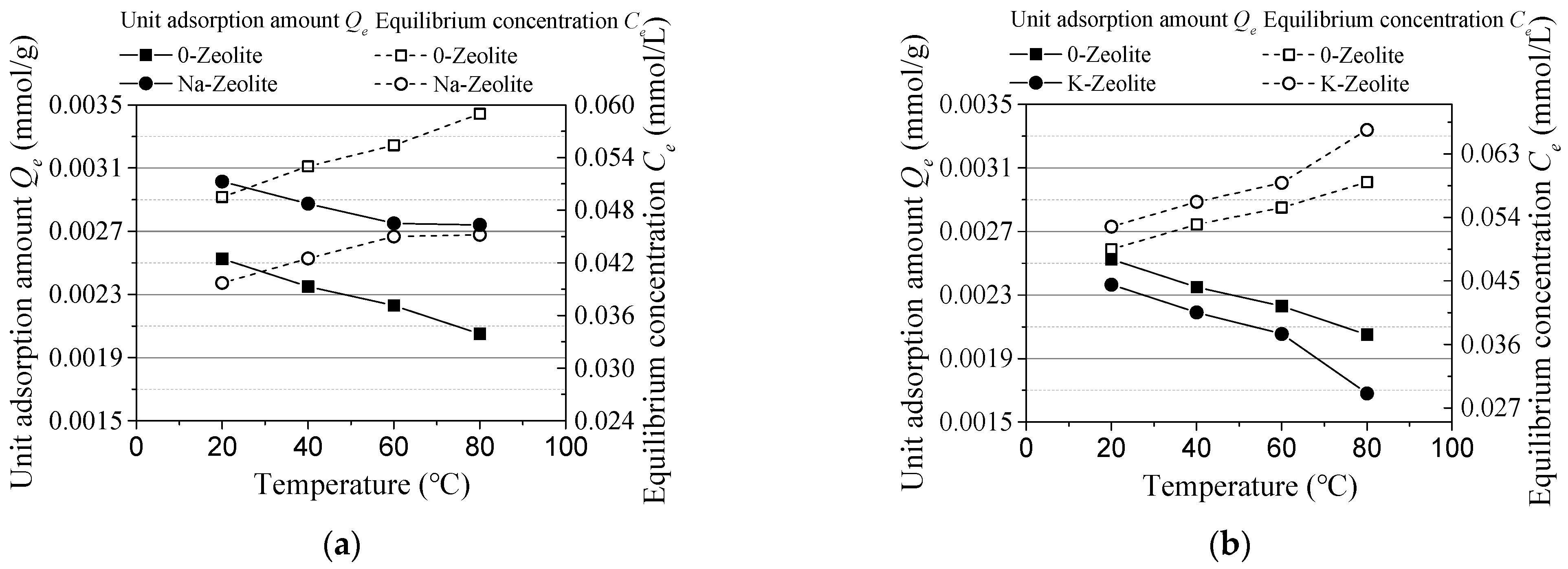
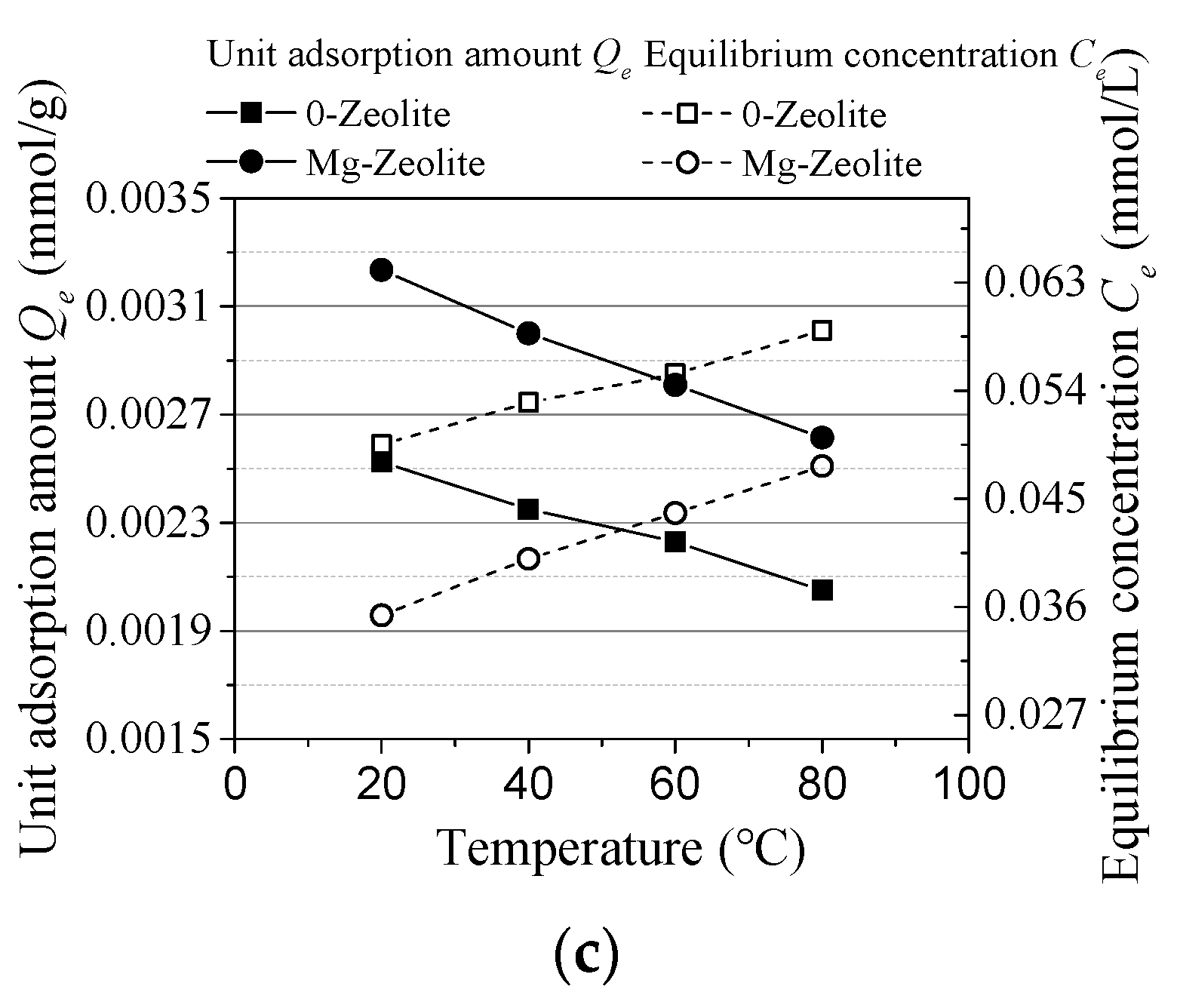
- The unit adsorption capacity changes in the same trend as an effect of the initial concentration and reaction time. It was also the same on the effect of the sorbent dosage and temperature. In these four groups, the removal rate changes in the reverse direction as the unit adsorption amount. Thus, an alkaline solution environment is better for cesium adsorption.
- (2)
- The test data is in better fitting in the Redlich–Peterson isothermal model and intragranular diffusion model. The adsorption reaction is in the form of both physical and chemical adsorption, combined with intragranular diffusion. In this small-scale experiment, the highest adsorption capacity is in Mg-zeolite at 6.53 mg/g.
- (3)
- More reaction points may emerge on the surface of zeolite, and Mg-zeolite presents the best adsorption capacity in this study, where the surface crystal structure of Mg-zeolite is complex and diverse, and the amount of Al3+ that replaces Si2+ is comprehensive compared with other zeolites.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ishii, K.; Terakawa, A.; Matsuyama, S.; Kikuchi, Y.; Fujishiro, F.; Ishizaki, A.; Osada, N.; Arai, H.; Sugai, H.; Takahashi, H.; et al. Reducing logistical barriers to radioactive soil remediation after the Fukushima No. 1 nuclear power plant accident. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2014, 318, 70–75. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, D.; Patti, S. A bibliometric analysis of carbon labeling schemes in the Period 2007–2019. Energies 2020, 13, 4233. [Google Scholar] [CrossRef]
- Inui, T.; Yasutak, T.; Endo, K.; Katsumi, T. Geo-environmental issues induced by the 2011 off the Pacific Coast of Tohoku Earthquake and tsunami. Soils Found. 2012, 52, 856–871. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Wang, X.Q.; Chen, X.L.; Liu, Y.Y. Impacts of different aged landfill leachate on PVC corrosion. Environ. Sci. Pollut. Res. 2019, 26, 18256–18266. [Google Scholar] [CrossRef] [PubMed]
- Chino, M.; Nakayama, H.; Nagai, H.; Terada, H.; Katata, G.; Yamazawa, H. Preliminary estimation of release amounts of I-131 and Cs-137 accidentally discharged from the Fukushima Daiichi Nuclear Power Plant into the atmosphere. J. Nucl. Sci. Technol. 2011, 48, 1129–1134. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, T.W. Severe accident issues raised by the Fukushima accident and improvement suggested. Nucl. Eng. Technol. 2014, 46, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Lingyu, W.; Teng, L.; Xiuting, D.; Maobing, P.; Songtao, X.; Wen, Z. Thiophene-based MOFs for iodine capture: Effect of pore structures and interaction mechanism. Chem. Eng. J. 2021, 425, 130578. [Google Scholar]
- Lelieveld, J.; Kunkel, D.; Lawrence, M. Global risk of radioactive fallout after major nuclear reactor accidents. Atmos Chem. Phys. 2012, 12, 4245–4258. [Google Scholar] [CrossRef] [Green Version]
- Koo, Y.H.; Yang, Y.S.; Song, K.W. Radioactivity release from the Fukushima accident and its consequences: A review. Prog. Nucl. Energy 2014, 74, 61–70. [Google Scholar] [CrossRef]
- Sasaki, T.; Tanaka, S. Magnetic separation of cesium ion using Prussian blue modified magnetite. Chem. Lett. 2012, 41, 32–34. [Google Scholar] [CrossRef]
- Zhao, R.; Li, M.; Ma, S.D.; Yang, T.X.; Jing, L.Y. Material selection for landfill leachate piping by using a grey target decision-making approach. Environ. Sci. Pollut. Res. 2021, 28, 494–502. [Google Scholar] [CrossRef]
- Kajitani, Y.; Chang, S.E.; Tatano, H. Economic impacts of the 2011 Tohoku-Oki earthquake and tsunami. Earthq. Spectra 2013, 29, S457–S478. [Google Scholar] [CrossRef]
- Zhang, Q.; Mclellan Benjamin, C. Review of Japan’s power generation scenarios in light of the Fukushima nuclear accident. Int. J. Energy Res. 2014, 38, 539–550. [Google Scholar] [CrossRef]
- Vivoda, V. Japan’s energy security predicament post-Fukushima, energy policy. Energy Policy 2012, 46, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Yingxin, M.; Xihong, H.; Peng, C.; Song, Z.; Chengde, H.; Yuxin, W.; Jing, C. Robust porous polymers bearing phosphine oxide/chalcogenide ligands for volatile iodine capture. Chem. Eng. J. 2020, 379, 122365. [Google Scholar]
- Tsukada, T.; Uozumi, K.; Hijikata, T.; Koyama, T.; Ishikawa, K.; Ono, S.; Suzuki, S.; Denton, M.S.; Keenan, R.; Bonhomme, G. Early construction and operation of highly contaminated water treatment system in Fukushima Daiichi Nuclear Power Station (I)—Ion exchange properties of KURION herschelite in simulating contaminated water. J. Nucl. Sci. Technol. 2014, 51, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Saber-Samandari, S.; Saber-Samandari, S.; Nezafati, N.; Yahya, K. Efficient removal of lead (II) ions and methylene blue from aqueous solution using chitosan/Fe-hydroxyapatite nanocomposite beads. J. Environ. Manag. 2014, 146, 481–490. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, D.; Zhang, J. Policy Implications on Carbon Labeling Scheme Toward Carbon Neutrality in China. Front. Environment. Sci. 2021, 482. [Google Scholar] [CrossRef]
- Wen, Z.; Xiuting, D.; Yingxin, M.; Yuxin, W.; Jing, C. Constructing adjacent phosphine oxide ligands confined in mesoporous Zr-MOFs for uranium capture from acidic medium. J. Mater. Chem. A 2021, 9, 16685–16691. [Google Scholar]
- Lasheen, M.R.; Ammar, N.S. Speciation and stabilization of some heavy metals in the sediments from drains, Egypt. Desalination Water Treat. 2014, 52, 3271–3279. [Google Scholar] [CrossRef]
- Wen, Z.; An, B.; Qingyuan, J.; Luofu, M.; Song, Z.; Yuxin, W.; Jing, C. pK(a)-Directed Incorporation of Phosphonates into MOF-808 via Ligand Exchange: Stability and Adsorption Properties for Uranium. ACS Appl. Mater. Interfaces 2019, 11, 33931–33940. [Google Scholar]
- Tang, Q.; Tang, X.W.; Li, Z.Z.; Chen, Y.M.; Kou, N.Y.; Sun, Z.F. Adsorption and desorption behaviour of Pb(II) on a natural kaolin: Equilibrium, kinetic and thermodynamic studies. J. Chem. Technol. Biotechnol. 2009, 84, 1371–1380. [Google Scholar] [CrossRef]
- Peng, C.; Xihong, H.; Maobin, P.; Xiuting, D.; Song, Z.; Wen, Z. Iodine Capture Using Zr-Based Metal-Organic Frameworks (Zr-MOFs): Adsorption Performance and Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 20429–20439. [Google Scholar]
- Tang, Q.; Tang, X.W.; Li, Z.Z.; Wang, Y.; Hu, M.M.; Zhang, X.J.; Chen, Y.M. Zn(II) Removal with Activated Firmiana Simplex Leaf: Kinetics and Equilibrium Studies. J. Environ. Eng. (ASCE) 2012, 138, 19199. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, W.; Li, Z.Z.; Wang, Y.; Tang, X.W. Removal of Aqueous Cu(II) with Natural Kaolin: Kinetics and Equilibrium Studies. Environ. Eng. Manag. J. 2018, 17, 467–476. [Google Scholar] [CrossRef]
- Tang, Q.; Katsumi, T.; Inui, T.; Li, Z.Z. Membrane behavior of bentonite-amended compacted clay towards Zn(II) and Pb(II). Membr. Water Treat. 2015, 6, 393–409. [Google Scholar] [CrossRef]
- Tang, Q.; Chu, J.M.; Wang, Y.; Zhou, T.; Liu, Y. Characteristics and factors influencing Pb(II) desorption from a Chinese clay by citric acid. Sep. Sci. Technol. 2016, 51, 2734–2743. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, T.; Gu, F.; Wang, Y.; Chu, J.M. Removal of Cd(II) and Pb(II) from soil through desorption using citric acid: Kinetic and equilibrium studies. J. Cent. South Univ. 2017, 24, 1941–1952. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, W.; Wang, H.Y.; Cheng, R.; Qian, Y.F. Membrane behavior of bentonite-amended Fukakusa clay in K, Na and Ca solutions. J. Cent. South Univ. 2016, 23, 3122–3131. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, H.Y.; Tang, X.W.; Wang, Y. Removal of aqueous Ni(II) with carbonized leaf powder: Kinetic and equilibrium studies. J. Cent. South Univ. 2016, 27, 778–786. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, P.X.; Yuan, Z.; Shi, S.J.; Xu, X.J.; Katsumi, T. Potential of zero-valent iron in remediation of Cd(II) contaminated soil: From laboratory experiment, mechanism study to field application. Soils Found. (JGS) 2019, 59, 2099–2109. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ning, Y.; Zhang, T.; Wu, J.J. Measuring Carbon Emissions of Pavement Construction in China. Sustainability 2016, 8, 723. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Zhou, X.; Han, J.J.; Liu, C.L. For the sustainable performance of the carbon reduction labeling policies under an evolutionary game simulation. Technol. Forecast. Soc. Chang. 2016, 112, 262–274. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Pholosi, A.; Naidoo, E.B. Kinetics and competitive modeling of cesium biosortion onto chemically modified pine cone powder. J. Taiwan Inst. Chem. Eng. 2013, 44, 943–951. [Google Scholar] [CrossRef]
- Ozeroglu, C.; Dogan, E.; Keçeli, G. Investigation of Cs(I) adsorption on densely crosslinked poly(sodium methacrylate) from aqueous solutions. J. Radioanal. Nucl. Chem. 2011, 289, 577–586. [Google Scholar] [CrossRef]
- Lingyu, W.; Peng, C.; Xiuting, D.; Wen, Z.; Song, Z.; Songtao, X.; Yinggen, O. Porous MOF-808@PVDF beads for removal of iodine from gas streams. RSC Adv. 2020, 10, 44679–44687. [Google Scholar]
- Kumagai, S.; Hayashi, K.; Kameda, T.; Yoshioka, T. Removal and condensation of cesium ion from water phase into ionic associate phase using tetraphenylborate. J. Soc. Remediat. Radioact. Contam. Environ. 2016, 4, 239–245. [Google Scholar]
- Thilagavathy, P.; Santhi, T. Kinetics, isotherms and equilibrium study of Co(II) adsorption from single and binary qqueous solutions by Acacia nilotica leaf carbon. Chin. J. Chem. Eng. 2014, 22, 1193–1198. [Google Scholar] [CrossRef]
- Liu, Z.; Ciais, P.; Deng, Z.; Davis, S.J.; Zheng, B.; Wang, Y.; Cui, D.; Zhu, B.; Dou, X.; Ke, P.; et al. Carbon Monitor, a near-real-time daily dataset of global CO emission from fossil fuel and cement production. Sci. Data 2020, 7, 392. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Chen, W.; Zhu, S.; Liu, N.; Zhu, L. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ. Pollut. 2010, 158, 514–519. [Google Scholar] [CrossRef]
- Mohammad, A.M.; Eldin, T.A.S.; Hassan, M.A.; El-Anadouli, B.E. Efficient treatment of lead-containing wastewater by hydroxyapatite/chitosan nanostructures. Arab. J. Chem. 2017, 10, 683–690. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sheikh, T.A.; Asiri, A.M.; Awual, M.R. Development of 3-methoxyaniline sensor probe based on thin Ag2O@La2O3nanosheets for environmental safety. N. J. Chem. 2019, 43, 4624632. [Google Scholar] [CrossRef]
- Erzsébet, S.B.; Melinda, C.; Réka, B.; Csavdari, A.A. Influence of synthesis method of nanohydroxyapatite-based materials on cadmium sorption processes. J. Iran. Chem. Soc. 2014, 11, 53–68. [Google Scholar]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite micro-fibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Long, Y.; Jiang, J.; Hu, J.; Hu, X.; Yang, Q.; Zhou, S. Removal of Pb (II) from aqueous solution by hydroxyapatite/carbon composite: Preparation and adsorption behavior. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 471–479. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, L.; Yang, W.; Shi, W.; Tong, Y.; Chai, L.; Gao, S.; Liao, Q. Simultaneous immobilization of cadmium and lead in contaminated soils by hybrid bio-nanocomposites of fungal hyphae and nano-hydroxyapatites. Environ. Sci. Pollut. Res. 2018, 25, 119711980. [Google Scholar] [CrossRef]
- Eljamal, O.; Shubair, T.; Tahara, A.; Sugihara, Y.; Matsunaga, N. Iron based nanoparticles-zeolite composites for the removal of cesium from aqueous solutions. J. Mol. Liq. 2019, 277, 613–623. [Google Scholar] [CrossRef]
- El-Khaiary, M.I.; Malash, G.F. Common data analysis errors in batch adsorption studies. Hydrometallurgy 2011, 105, 314. [Google Scholar] [CrossRef]
- Scheckel, K.G.; Sparks, D.L. Temperature Effects on Nickel Sorption Kinetics at the Mineral–Water Interface. Soil Sci. Soc. Am. J. 2011, 65, 719. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: Reactivity, characterization and mechanism. Water Res. 2011, 25, 3481. [Google Scholar] [CrossRef]



| Factors | Parameters |
|---|---|
| Initial Concentration | 0.3 mmol/L, 0.5 mmol/L, 0.8 mmol/L, and 1.0 mmol/L |
| Adsorbent Dosage | 0.1, 0.3, 0.5, 1, and 1.5 g |
| Solution pH | 3, 7, 11 |
| Reaction Time | 4, 6, 10, and 12 h |
| Temperature | 40, 60, and 80 °C |
| Model | Langmuir Isothermal Model | Freundlich Isothermal Model | Redlich–Peterson Isothermal Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Qm (mg/g) | KL | R2 | K (mg/g) | 1/n | R2 | KRP (L/g) | aRP (L/mg) | β | R2 |
| Zeolite | 5.60 | 0.0140 | 0.989 | 0.230 | 0.579 | 0.923 | 0.049 | 1.16 × 10−5 | 2.23 | 0.998 |
| Na-zeolite | 5.69 | 0.0130 | 0.987 | 0.282 | 0.522 | 0.947 | 0.061 | 0.001 | 1.37 | 0.992 |
| K-zeolite | 5.57 | 0.0099 | 0.979 | 0.189 | 0.573 | 0.932 | 0.039 | 3.49 × 10−6 | 1.98 | 0.998 |
| Mg-zeolite | 6.53 | 0.0159 | 0.987 | 0.357 | 0.516 | 0.944 | 0.083 | 0.003 | 1.30 | 0.989 |
| Model | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Intragranular Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | k1 (mg/g) | Qe (mg/g) | R2 | k2 (g/mg·min) | Qe (mg/g) | R2 | kint (g/mg·min1/2) | C (mg/g) | R2 |
| Zeolite | 0.0086 | 9.12 | 0.977 | 0.0018 | 9.92 | 0.894 | 0.097 | 6.66 | 0.728 |
| Na-zeolite | 0.0122 | 9.66 | 0.382 | 0.0039 | 10.10 | 0.736 | 0.059 | 8.26 | 0.907 |
| K-zeolite | 0.0111 | 7.77 | 0.376 | 0.0037 | 8.22 | 0.721 | 0.060 | 6.33 | 0.888 |
| Mg-zeolite | 0.0084 | 11.35 | 0.665 | 0.0013 | 12.47 | 0.840 | 0.142 | 7.82 | 0.906 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Chen, J.; Peng, X.; Zhang, Y.; Mo, J.; Liao, X.; Tang, Q. The Utilization of Modified Zeolite for the Removal of Cs Ions in an Aqueous Solution: Adsorption Capacity, Isotherms, Kinetics and Microscopic Studies. Sustainability 2022, 14, 2615. https://doi.org/10.3390/su14052615
Sun J, Chen J, Peng X, Zhang Y, Mo J, Liao X, Tang Q. The Utilization of Modified Zeolite for the Removal of Cs Ions in an Aqueous Solution: Adsorption Capacity, Isotherms, Kinetics and Microscopic Studies. Sustainability. 2022; 14(5):2615. https://doi.org/10.3390/su14052615
Chicago/Turabian StyleSun, Junfang, Ji Chen, Xiang Peng, Yu Zhang, Jialin Mo, Xin Liao, and Qiang Tang. 2022. "The Utilization of Modified Zeolite for the Removal of Cs Ions in an Aqueous Solution: Adsorption Capacity, Isotherms, Kinetics and Microscopic Studies" Sustainability 14, no. 5: 2615. https://doi.org/10.3390/su14052615
APA StyleSun, J., Chen, J., Peng, X., Zhang, Y., Mo, J., Liao, X., & Tang, Q. (2022). The Utilization of Modified Zeolite for the Removal of Cs Ions in an Aqueous Solution: Adsorption Capacity, Isotherms, Kinetics and Microscopic Studies. Sustainability, 14(5), 2615. https://doi.org/10.3390/su14052615







