Biogas Production from Concentrated Municipal Sewage by Forward Osmosis, Micro and Ultrafiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sewage
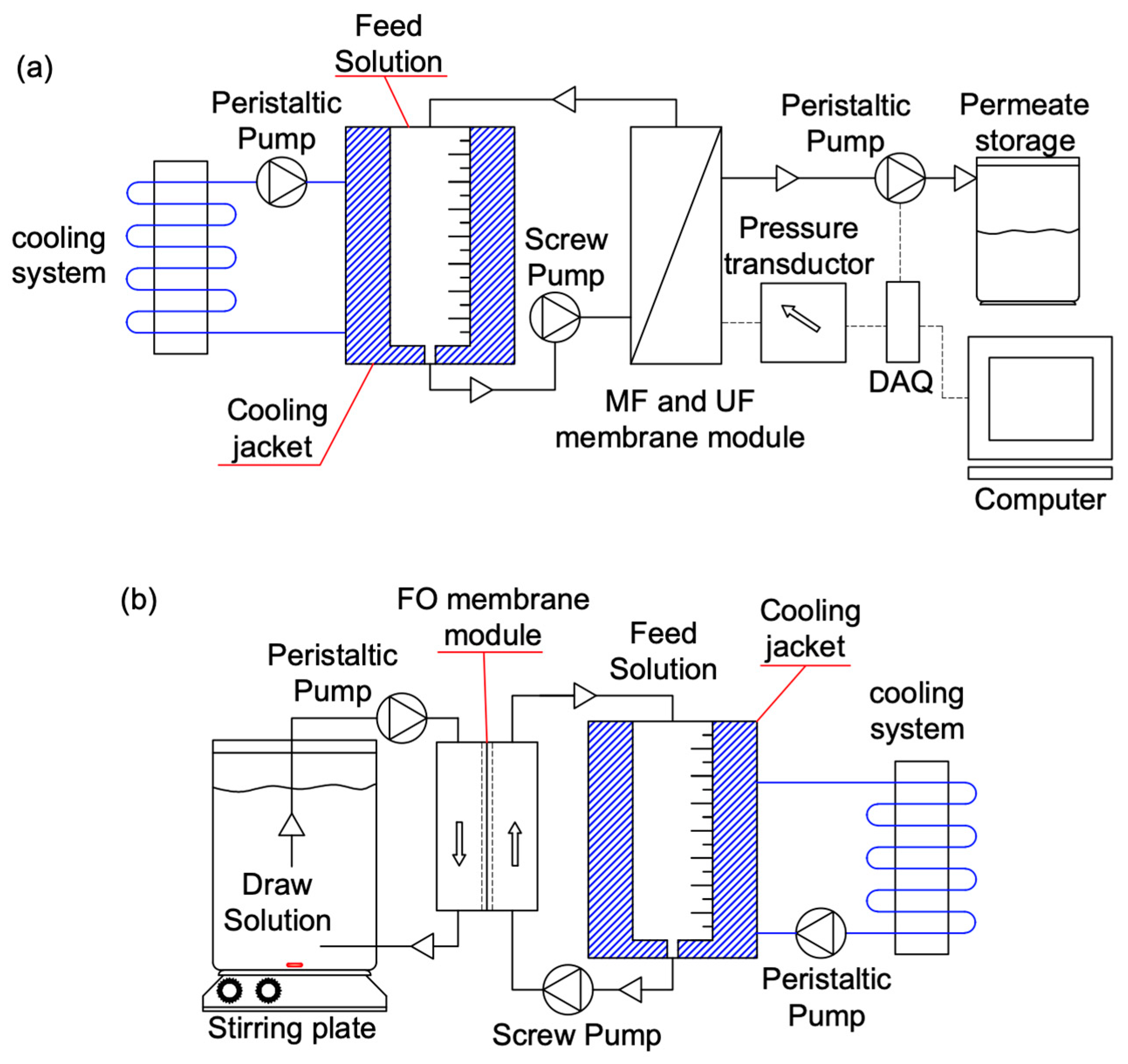
2.2. Sewage Concentration Setup
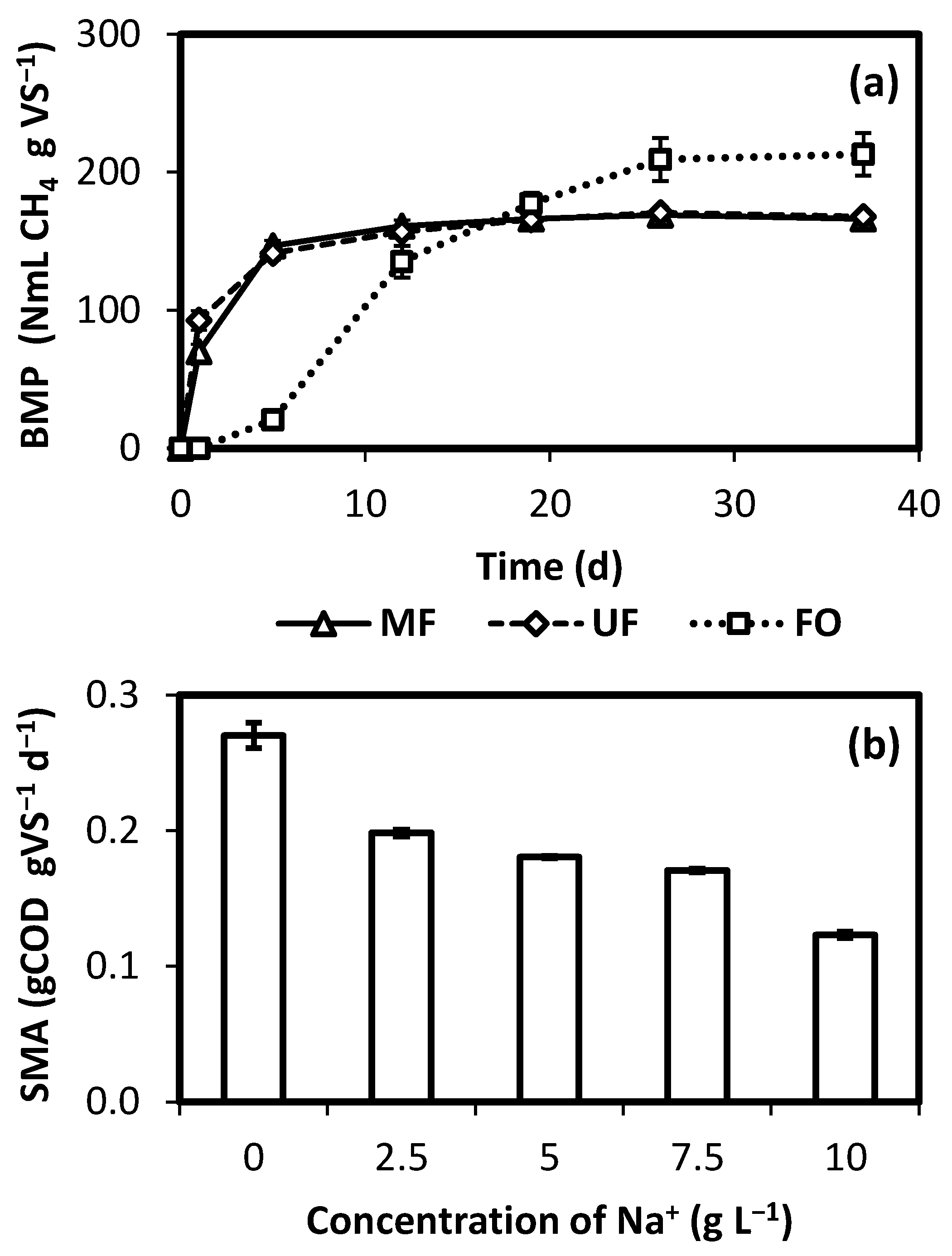
2.3. Biogenic Methane Potential
2.4. Specific Methanogen Activity (SMA)
2.5. Analyses
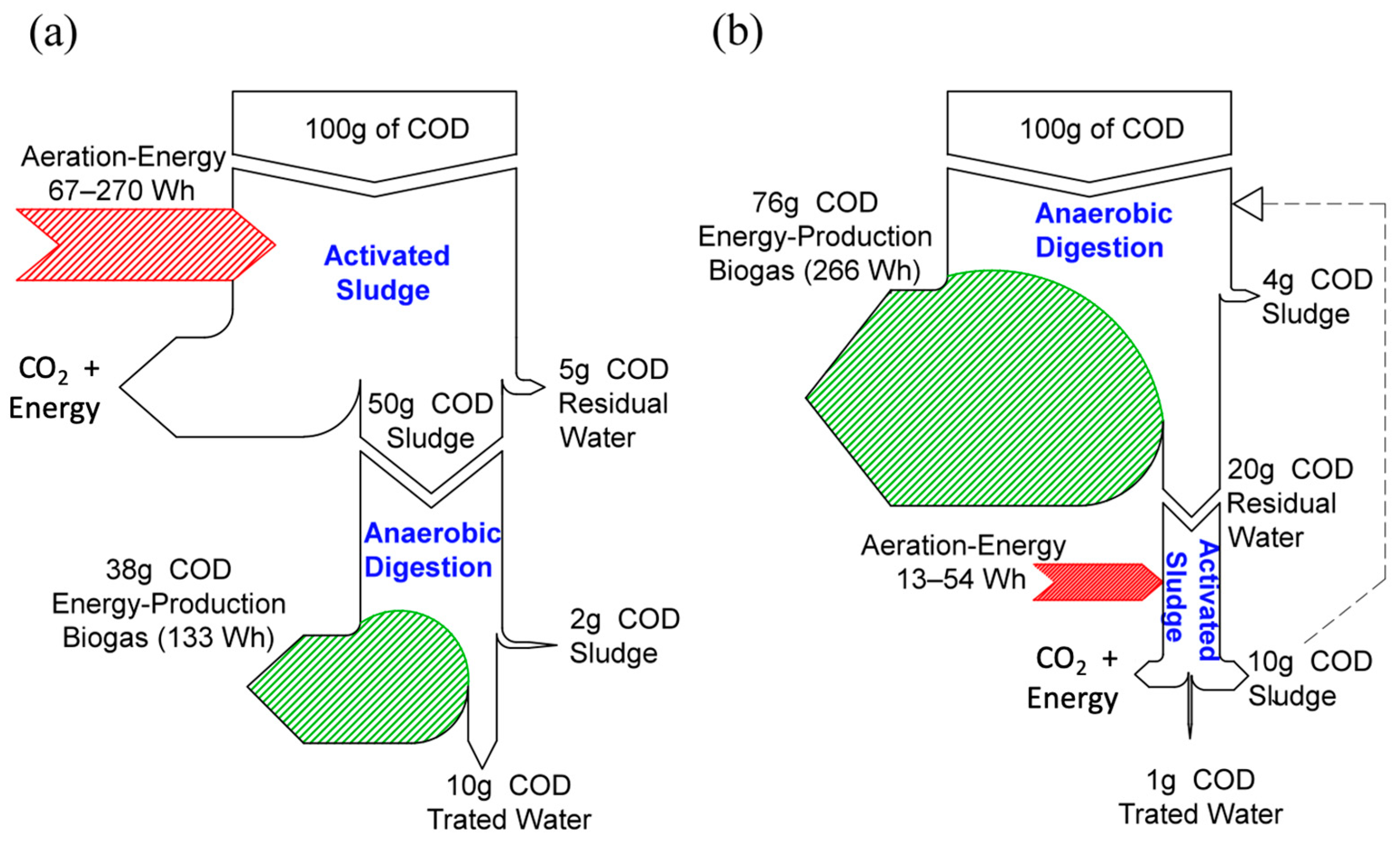
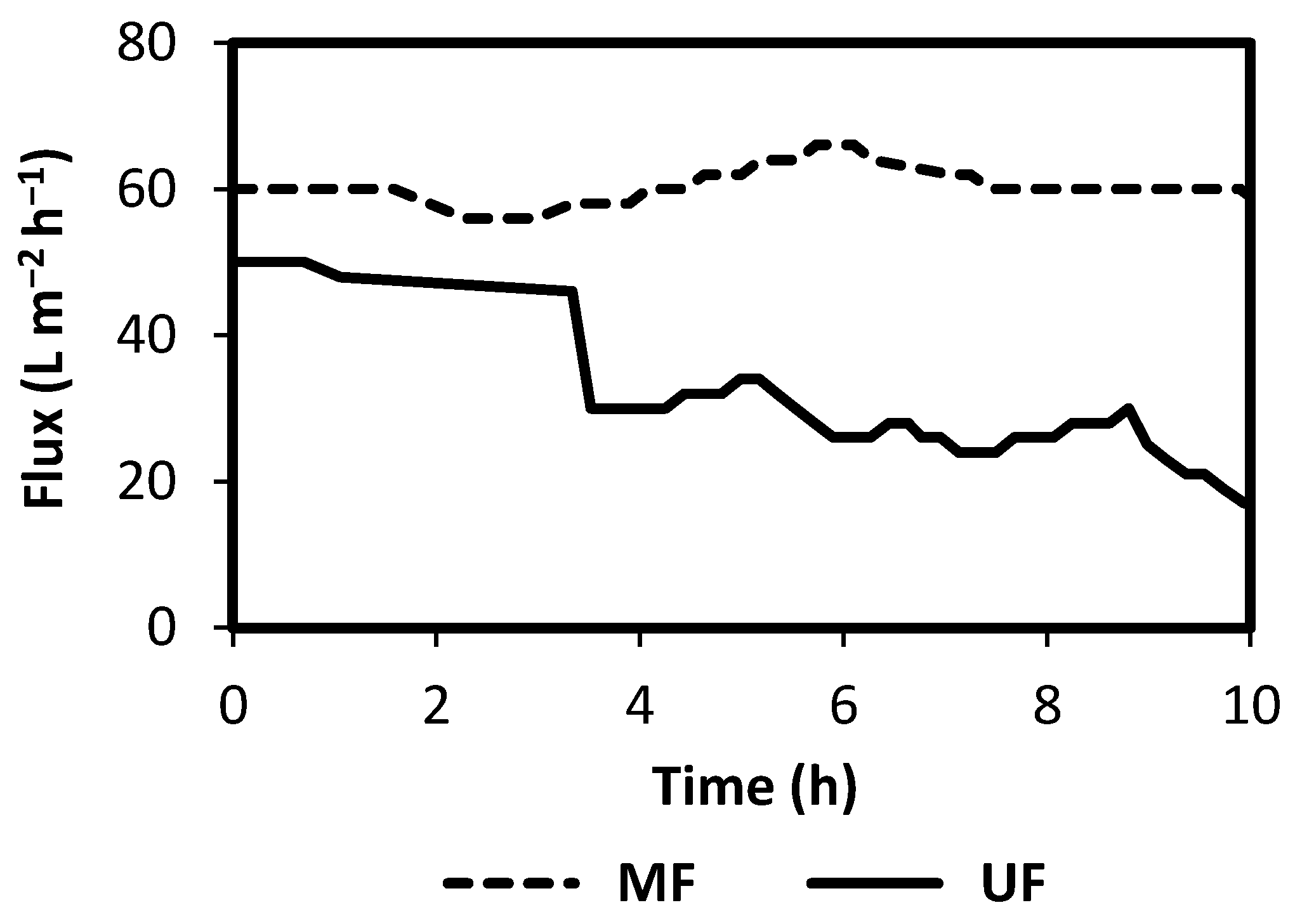
3. Results and Discussion
4. Conclusions
- Membrane concentration offers the possibility to convert organic matter contained in sewage into biogas, a source of renewable energy.
- Even though MF and UF can provide the required concentration process, enabling biogas production, rejection is normally limited to solids and colloids, so post-treatment may be required.
- Membrane fouling is a key aspect of membrane performance, and it needs to be further studied to enable the long-term operation of a direct membrane filtration operation.
- FO can provide a better rejection than MF and UF, which may result in higher methane yields. It is inferred then that FO’s sewage concentration may be technically feasible. However, some drawbacks need to be overcome, such as low water flux and potentially inhibitory salt concentration, resulting from high solute rejection and reverse salt flux from the draw solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lorain, O.; Thiebaud, P.; Badorc, E.; Aurelle, Y. Potential of freezing in wastewater treatment: Soluble pollutant applications. Water Res. 2000, 35, 541–547. [Google Scholar] [CrossRef]
- Gay, G.; Lorain, O.; Azouni, A.; Aurelle, Y. Wastewater treatment by radial freezing with stirring effects. Water Res. 2003, 37, 2520–2524. [Google Scholar] [CrossRef]
- Gao, W.; Shao, Y. Freeze concentration for removal of pharmaceutically active compounds in water. Desalination 2009, 249, 398–402. [Google Scholar] [CrossRef]
- Algehed, J.; Berntsson, T. Evaporation of black liquor and wastewater using medium-pressure steam: Simulation and economic evaluation of novel designs. Appl. Therm. Eng. 2003, 23, 481–495. [Google Scholar] [CrossRef]
- Younggreen, W.; Piazza, J. Wastewater evaporation in oil refineries. INFORM 2009, 20, 473–475. [Google Scholar]
- Shimizu, Y.; Okuno, Y.-I.; Uryu, K.; Ohtsubo, S.; Watanabe, A. Filtration characteristics of hollow fiber microfiltration membranes used in membrane bioreactor for domestic wastewater treatment. Water Res. 1996, 30, 2385–2392. [Google Scholar] [CrossRef]
- Visvanathan, C.; Ben Aim, R.; Parameshwaran, K. Membrane Separation Bioreactors for Wastewater Treatment. Crit. Rev. Environ. Sci. Technol. 2000, 30, 1–48. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.; Roest, K.; Rietveld, L.; Cornelissen, E. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Sarp, S.; Bucs, S.; Amy, G.; Vrouwenvelder, J. Forward osmosis niches in seawater desalination and wastewater reuse. Water Res. 2014, 66, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Yamakawa, M.; Hafuka, A. Direct membrane filtration (DMF) for recovery of organic matter in municipal wastewater using small amounts of chemicals and energy. Chemosphere 2021, 277, 130244. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Jin, Z.; Xu, H.; Yuan, Q.; Zuo, J.; Wu, J.; Wang, K. Enhanced membrane-based pre-concentration improves wastewater organic matter recovery: Pilot-scale performance and membrane fouling. J. Clean. Prod. 2018, 206, 307–314. [Google Scholar] [CrossRef]
- Lateef, S.K.; Soh, B.Z.; Kimura, K. Direct membrane filtration of municipal wastewater with chemically enhanced backwash for recovery of organic matter. Bioresour. Technol. 2013, 150, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Ravazzini, A.; van Nieuwenhuijzen, A.; van der Graaf, J. Direct ultrafiltration of municipal wastewater: Comparison between filtration of raw sewage and primary clarifier effluent. Desalination 2005, 178, 51–62. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Bilad, M.R.; Vankelecom, I.F. Direct sewage up-concentration by submerged aerated and vibrated membranes. Bioresour. Technol. 2012, 118, 1–7. [Google Scholar] [CrossRef]
- Wu, X.; Lau, C.; Pramanik, B.; Zhang, J.; Xie, Z. State-of-the-Art and Opportunities for Forward Osmosis in Sewage Concentration and Wastewater Treatment. Membranes 2021, 11, 305. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. Desalination by ammonia–carbon dioxide forward osmosis: Influence of draw and feed solution concentrations on process performance. J. Membr. Sci. 2006, 278, 114–123. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Cornelissen, E.; Harmsen, D.J.H.; Post, J.; Lampi, K.; Ramaekers, H.; Rietveld, L.; Roest, K. Water recovery from sewage using forward osmosis. Water Sci. Technol. 2011, 64, 1443–1449. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Z.; Wang, D.; da Costa, J.C.D. Processing municipal wastewaters by forward osmosis using CTA membrane. J. Membr. Sci. 2014, 468, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Fang, Z.; Liang, P.; Huang, X. Direct concentration of municipal sewage by forward osmosis and membrane fouling behavior. Bioresour. Technol. 2018, 247, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Jeison, D.; Van Lier, J. On-line cake-layer management by trans-membrane pressure steady state assessment in Anaerobic Membrane Bioreactors for wastewater treatment. Biochem. Eng. J. 2006, 29, 204–209. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Soler-Cabezas, J.; Mendoza-Roca, J.-A.; Vincent-Vela, M.-C.; Luján-Facundo, M.; Pastor-Alcañiz, L. Simultaneous concentration of nutrients from anaerobically digested sludge centrate and pre-treatment of industrial effluents by forward osmosis. Sep. Purif. Technol. 2018, 193, 289–296. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M. The Use of Flat Ceramic Membranes for Purification of the Liquid Fraction of the Digestate from Municipal Waste Biogas Plants. Energies 2021, 14, 3947. [Google Scholar] [CrossRef]
- Diaz, S.D.; Peña, L.V.; Cabrera, E.G.; Soto, M.M.; Cabezas, L.M.V.; Sánchez, L.R.B. Effect of previous coagulation in direct ultrafiltration of primary settled municipal wastewater. Desalination 2012, 304, 41–48. [Google Scholar] [CrossRef]
- Huang, B.-C.; Guan, Y.-F.; Chen, W.; Yu, H.-Q. Membrane fouling characteristics and mitigation in a coagulation-assisted microfiltration process for municipal wastewater pretreatment. Water Res. 2017, 123, 216–223. [Google Scholar] [CrossRef]
- Jin, Z.; Meng, F.; Gong, H.; Wang, C.; Wang, K. Improved low-carbon-consuming fouling control in long-term membrane-based sewage pre-concentration: The role of enhanced coagulation process and air backflushing in sustainable sewage treatment. J. Membr. Sci. 2017, 529, 252–262. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Hamedi, H.; Ehteshami, M.; Mirbagheri, S.A.; Rasouli, S.A.; Zendehboudi, S. Current Status and Future Prospects of Membrane Bioreactors (MBRs) and Fouling Phenomena: A Systematic Review. Can. J. Chem. Eng. 2018, 97, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebreyohannes, A.Y.; Curcio, E.; Poerio, T.; Mazzei, R.; Di Profio, G.; Drioli, E.; Giorno, L. Treatment of Olive Mill Wastewater by Forward Osmosis. Sep. Purif. Technol. 2015, 147, 292–302. [Google Scholar] [CrossRef]
- Liang, H.-Q.; Hung, W.-S.; Yu, H.-H.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y.; Xu, Z.-K. Forward osmosis membranes with unprecedented water flux. J. Membr. Sci. 2017, 529, 47–54. [Google Scholar] [CrossRef]
- Wu, X.; Tanner, J.; Ng, D.; Acharya, D.; Xie, Z. Sewage concentration via a graphene oxide modified thin-film nanocomposite forward osmosis membrane: Enhanced performance and mitigated fouling. Chem. Eng. J. 2020, 420, 127718. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.-S.; Yang, H.-Y.; Hau, N.T. Application of forward osmosis on dewatering of high nutrient sludge. Bioresour. Technol. 2013, 132, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Almoalimi, K.; Liu, Y.-Q. Fouling and cleaning of thin film composite forward osmosis membrane treating municipal wastewater for resource recovery. Chemosphere 2021, 288, 132507. [Google Scholar] [CrossRef]
- Jeison, D.; Dias, I.; Van Lier, J.B. Anaerobic membrane bioreactors: Are membranes really necessary? Electron. J. Biotechnol. 2008, 11, 1–2. [Google Scholar] [CrossRef]
- Hafuka, A.; Takahashi, T.; Kimura, K. Anaerobic digestibility of up-concentrated organic matter obtained from direct membrane filtration of municipal wastewater. Biochem. Eng. J. 2020, 161, 107692. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Liang, P.; Zhang, X.; Qiu, Y.; Kimura, K.; Huang, X. Anaerobic digestion performance of concentrated municipal sewage by forward osmosis membrane: Focus on the impact of salt and ammonia nitrogen. Bioresour. Technol. 2019, 276, 204–210. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Boles, M.A. Thermodynamics: An Engineering Approach, 8th ed.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Gu, Y.; Chen, L.; Ng, J.-W.; Lee, C.; Chang, V.W.-C.; Tang, C.Y. Development of anaerobic osmotic membrane bioreactor for low-strength wastewater treatment at mesophilic condition. J. Membr. Sci. 2015, 490, 197–208. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Cao, C.; Zhang, J.; Ng, J.-W.; Tang, C. Performance of a submerged anaerobic membrane bioreactor with forward osmosis membrane for low-strength wastewater treatment. Water Res. 2014, 50, 114–123. [Google Scholar] [CrossRef] [PubMed]

| MF | UF | FO | |
|---|---|---|---|
| Provider | Atech Innovations, Germany | X-Flow Norit, The Netherlands | Hydration Technology Innovations, USA |
| Material | Ceramic aluminum oxide (Al2O3) | Polymeric | Cellulose triacetate with embedded polyester screen flat-sheet membrane (CTA) |
| Pore size | 0.2 µm | 20 nm | - |
| Configuration | Tubular, inside/out | Tubular, inside/out | Flat sheet |
| Filtration area | 0.0094 m2 | 0.0176 m2 | 0.03 m2 |
| Sewage | Na+ (g L−1) |
|---|---|
| Original Sewage | 0.22 |
| UF concentrated | 0.20 |
| MF concentrated | 0.19 |
| FO concentrated | 1.94 |
| Pm | Rm | λ | R2 | |
|---|---|---|---|---|
| mLCH4 gSV−1 | mLCH4 gSV−1 d−1 | d | ||
| MF | 161.9 | 105.2 | 0.33 | 0.988 |
| UF | 160.5 | 146.4 | 0.36 | 0.977 |
| FO | 210.6 | 16.4 | 3.94 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Bravo, J.C.; Pavez, J.; Hidalgo, V.; Reyes-Caniupán, I.; Torres-Aravena, Á.; Jeison, D. Biogas Production from Concentrated Municipal Sewage by Forward Osmosis, Micro and Ultrafiltration. Sustainability 2022, 14, 2629. https://doi.org/10.3390/su14052629
Ortega-Bravo JC, Pavez J, Hidalgo V, Reyes-Caniupán I, Torres-Aravena Á, Jeison D. Biogas Production from Concentrated Municipal Sewage by Forward Osmosis, Micro and Ultrafiltration. Sustainability. 2022; 14(5):2629. https://doi.org/10.3390/su14052629
Chicago/Turabian StyleOrtega-Bravo, Juan Carlos, Javier Pavez, Víctor Hidalgo, Isaac Reyes-Caniupán, Álvaro Torres-Aravena, and David Jeison. 2022. "Biogas Production from Concentrated Municipal Sewage by Forward Osmosis, Micro and Ultrafiltration" Sustainability 14, no. 5: 2629. https://doi.org/10.3390/su14052629






